A Case of Hemophagocytic Lymphohistiocytosis Induced by Regorafenib
Article Information
Marion Jaffrelot MD1*, Noémie Gadaud MD2, Jean-Pierre Delord MD, PHD3, Carlos Gomez-Roca MD3, Sarah Bétrian MD3
1Departments on digestive oncology, Centre Hospitalier Universitaire Toulouse, France
2Departments of hematology, Institut Claudius Regaud, Institut Universitaire du Cancer, Toulouse Oncopole, France
3Departments of Oncology and Clinical Research, Institut Claudius Regaud, Institut Universitaire du Cancer, Toulouse Oncopole, France
*Corresponding Author: Dr. Marion Jaffrelot, Departments on digestive oncology, Centre Hospitalier Universitaire Toulouse, France
Received: 07 November 2020; Accepted: 21 December 2020; Published: 13 January 2021
Citation: Marion Jaffrelot, Noémie Gadaud, Jean-Pierre Delord, Carlos Gomez-Roca, Sarah Bétrian. A Case of Hemophagocytic Lymphohistiocytosis Induced by Regorafenib. Archives of Clinical and Medical Case Reports 5 (2021): 96-100.
View / Download Pdf Share at FacebookAbstract
Hemophagocytic lymphohistiocytosis (HLH) is a rare and severe condition of immune dysregulation characterized by severe organ damage induced by a hyperinflammatory response and uncontrolled T-cell and macrophage activation. Secondary HLH, also known as macrophage activation syndrome, commonly presents in adulthood and characterized by acquired immune dysfunction in response to infections, malignancies, or autoinflammatory/autoimmune disorders. Secondary HLH induced by regorafenib has not been yet described. Here we report a case of HLH induced by regorafenib, confirmed by clinical, laboratory and histopathological findings, with typical results on bone marrow aspiration.
Keywords
Hemophagocytic lymphohistiocytosis; Regorafenib
Hemophagocytic lymphohistiocytosis articles; Regorafenib articles
Hemophagocytic lymphohistiocytosis articles Hemophagocytic lymphohistiocytosis Research articles Hemophagocytic lymphohistiocytosis review articles Hemophagocytic lymphohistiocytosis PubMed articles Hemophagocytic lymphohistiocytosis PubMed Central articles Hemophagocytic lymphohistiocytosis 2023 articles Hemophagocytic lymphohistiocytosis 2024 articles Hemophagocytic lymphohistiocytosis Scopus articles Hemophagocytic lymphohistiocytosis impact factor journals Hemophagocytic lymphohistiocytosis Scopus journals Hemophagocytic lymphohistiocytosis PubMed journals Hemophagocytic lymphohistiocytosis medical journals Hemophagocytic lymphohistiocytosis free journals Hemophagocytic lymphohistiocytosis best journals Hemophagocytic lymphohistiocytosis top journals Hemophagocytic lymphohistiocytosis free medical journals Hemophagocytic lymphohistiocytosis famous journals Hemophagocytic lymphohistiocytosis Google Scholar indexed journals lymphohistiocytosis articles lymphohistiocytosis Research articles lymphohistiocytosis review articles lymphohistiocytosis PubMed articles lymphohistiocytosis PubMed Central articles lymphohistiocytosis 2023 articles lymphohistiocytosis 2024 articles lymphohistiocytosis Scopus articles lymphohistiocytosis impact factor journals lymphohistiocytosis Scopus journals lymphohistiocytosis PubMed journals lymphohistiocytosis medical journals lymphohistiocytosis free journals lymphohistiocytosis best journals lymphohistiocytosis top journals lymphohistiocytosis free medical journals lymphohistiocytosis famous journals lymphohistiocytosis Google Scholar indexed journals Regorafenib articles Regorafenib Research articles Regorafenib review articles Regorafenib PubMed articles Regorafenib PubMed Central articles Regorafenib 2023 articles Regorafenib 2024 articles Regorafenib Scopus articles Regorafenib impact factor journals Regorafenib Scopus journals Regorafenib PubMed journals Regorafenib medical journals Regorafenib free journals Regorafenib best journals Regorafenib top journals Regorafenib free medical journals Regorafenib famous journals Regorafenib Google Scholar indexed journals lymphadenopathy articles lymphadenopathy Research articles lymphadenopathy review articles lymphadenopathy PubMed articles lymphadenopathy PubMed Central articles lymphadenopathy 2023 articles lymphadenopathy 2024 articles lymphadenopathy Scopus articles lymphadenopathy impact factor journals lymphadenopathy Scopus journals lymphadenopathy PubMed journals lymphadenopathy medical journals lymphadenopathy free journals lymphadenopathy best journals lymphadenopathy top journals lymphadenopathy free medical journals lymphadenopathy famous journals lymphadenopathy Google Scholar indexed journals Cholestasis articles Cholestasis Research articles Cholestasis review articles Cholestasis PubMed articles Cholestasis PubMed Central articles Cholestasis 2023 articles Cholestasis 2024 articles Cholestasis Scopus articles Cholestasis impact factor journals Cholestasis Scopus journals Cholestasis PubMed journals Cholestasis medical journals Cholestasis free journals Cholestasis best journals Cholestasis top journals Cholestasis free medical journals Cholestasis famous journals Cholestasis Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals Sclerosis articles Sclerosis Research articles Sclerosis review articles Sclerosis PubMed articles Sclerosis PubMed Central articles Sclerosis 2023 articles Sclerosis 2024 articles Sclerosis Scopus articles Sclerosis impact factor journals Sclerosis Scopus journals Sclerosis PubMed journals Sclerosis medical journals Sclerosis free journals Sclerosis best journals Sclerosis top journals Sclerosis free medical journals Sclerosis famous journals Sclerosis Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals phagocytosis articles phagocytosis Research articles phagocytosis review articles phagocytosis PubMed articles phagocytosis PubMed Central articles phagocytosis 2023 articles phagocytosis 2024 articles phagocytosis Scopus articles phagocytosis impact factor journals phagocytosis Scopus journals phagocytosis PubMed journals phagocytosis medical journals phagocytosis free journals phagocytosis best journals phagocytosis top journals phagocytosis free medical journals phagocytosis famous journals phagocytosis Google Scholar indexed journals tomography articles tomography Research articles tomography review articles tomography PubMed articles tomography PubMed Central articles tomography 2023 articles tomography 2024 articles tomography Scopus articles tomography impact factor journals tomography Scopus journals tomography PubMed journals tomography medical journals tomography free journals tomography best journals tomography top journals tomography free medical journals tomography famous journals tomography Google Scholar indexed journals
Article Details
1. Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a rare and severe condition of immune dysregulation characterized by severe organ damage induced by a hyperinflammatory response and uncontrolled T-cell and macrophage activation. HLH can be caused by genetic mutations affecting cytotoxic functions (familial HLH) or be secondary to infectious, rheumatologic, malignant, or metabolic conditions (acquired HLH) [1, 2]. In rare cases, HLH has been described to be induced by drugs, for example following the use of lamotrigine [3]. In this case report, we describe a HLH induced by Regorafenib. Regorafenib (Stivarga®) is an oral multikinase inhibitor with a distinct and wide-ranging profile of tyrosine kinase inhibition involved in angiogenesis (VEGFR1-3, TIE-2), oncogenesis (c-KIT, RET, RAF1, BRAF) and maintenance of the tumor microenvironment (PDGFβ-FGFR-1) [4]. It has been shown to improve overall or progression-free survival in patients with colorectal cancer and gastrointestinal stromal tumors, respectively [5, 6]. Regorafenib is also evaluated in clinical trials, as monotherapy or combination therapy (with chemotherapy or immune checkpoint inhibitors), for advanced solid tumors or myeloid malignancies.
2. Case Report
A 65-year-old woman presenting a heavily pretreated (4 lines) colic adenocarcinoma with pulmonary metastasis was enrolled in a prospective open-labeled trial with regorafenib in combination with a checkpoint inhibitor. In this trial, patients received an induction treatment with regorafenib as single-agent with the introduction of the checkpoint inhibitor at D15C1 (human monoclonal antibody targeting the protein programmed death-ligand 1). The patient received regorafenib orally once daily for three weeks on, one week off (160 mg per day). At day 15 of the first cycle, she developed a grade II fever up to 39.5 degrees, a grade III maculo-papular rash and grade II fatigue. Physical examination did not find hepatosplenomegaly or lymphadenopathy. Laboratory studies showed pancytopenia (hemoglobin: 9.4 g/dl, platelets: 31000/mm3 and neutrophil count: 1200/mm3), hepatic cytolysis (aspartate aminotransferase (AST) = 5 X ULN, alanine aminotransferase (ALT) = 2 X ULN), and bilirubin elevation up to 4 X ULN), elevation of lactate deshydrogénase LDH (3 X ULN) and hyperferritenemia (5478 ng/ml, normal range lab 11-306.8 ng/ml). Fibrinogen, triglycerides, serum electrolytes were in the norms. Cholestasis values (alcaline phosphatases (PAL) and gamma-glutamyl transpeptidase (GGT) were similar with the screening blood test (PAL: 1.5 X ULN, GGT: 5 X ULN). Bone marrow aspiration revealed hyperactivity with some morphologically benign macrophages with an evidence of hemophagocytosis (Figure 1).
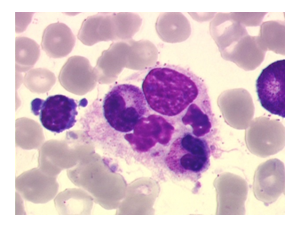
Figure 1: Bone marrow aspiration showing hemophagocytosis: phagocytosis by histiocytes of erythrocytes, leukocytes, platelets, and their precursors.
As etiological review, serological tests were negative for cytomegalovirus (CMV), Epstein-Barr virus (EBV), human immunodeficiency virus (HIV), B and C-hepatitis virus, human herpes virus 6 and 8, and Herpes simplex virus-1. Blood culture and urine analysis were also negative. Abdominal ultrasound and chest X-rays were normal. At the same time a CT scan was performed in order to exclude a disease progression. Because of these clinical and biological arguments, we concluded to the diagnosis of a hemophagocytic lymphohistiocytosis syndrome.
Regorafenib was stopped and management consisted of intravenous methylprednisolone (1.5 mg/kg) leading to a rapid regression of the rash, fever and the cytopenia once twenty-four hours. Prednisone was continued orally with a rapid decrease and was stopped after 15 days. All clinical and biological symptoms were resolved when the prednisone was stopped. Regorafenib was definitively stopped.
3. Discussion
HLH, is a hyperinflammatory condition caused by a highly stimulated, but ineffective immune response. Primary HLH usually presents in childhood, and is associated with mutations in genes such as MUNC 13-4, perforin 1, Syntaxin-122, affecting lymphocyte cytotoxicity and immune regulation. Secondary HLH, also known as macrophage activation syndrome, commonly presents in adulthood and characterized by acquired immune dysfunction in response to infections, malignancies, or autoinflammatory/autoimmune disorders. EBV, CMV and HIV are the most frequent infections involved in HLH, while lymphoma is the most commonly associated malignancy. The clinical presentation of HLH could be non-specific. It is generally acute, with a high-grade fever, splenomegaly, lymphadenopathies, hepatomegaly. Among the laboratory abnormalities, serum ferritin level is commonly elevated (≥500 µg/L), associated with cytopenia, bone marrow hemophagocytosis and hypertriglyceridemia. Other common signs consistent with the diagnosis include edema, rash, hyponatremia, hypoalbuminemia, elevated LDH. The diagnosis of HLH is based on clinical, laboratory and histopathological findings. Diagnosis criteria were revised by the Histiocyte Society in 2004. A major obstacle in diagnosing is the overlap of symptoms with a variety of other illness; it is especially challenging to differentiate between sepsis and HLH [7, 8].
There are no specific HLH treatment guidelines based on randomized trials. The American Society of Hematology had recently published recommendations for the management of hemophagocytic lymphohistiocytosis in adults [9]. Based on HLH-94 and 2004 protocols, high-dose glucocorticoids, etoposide, methotrexate and cyclosporine are most commonly recommended treatments. In addition to supportive therapy, specific treatment is necessary. Prompt recognition is paramount, but often challenging and prognosis remains poor. Less than 50% of adults with HLH received HLH-directed therapy because of lack of awareness and missed diagnosis of this condition. High morbidity and mortality due to HLH is partially attributed to delay in diagnosis. The rarity of the syndrome, non-specific clinical and biological symptoms are among the contributing factors. However, early diagnosis and treatment are critical to avoid progressive tissue damage, organ failure and possibly death.
A variety of malignancies could be associated with HLH in adults, especially hematologic neoplasms. HLH associated with solid tumor remains rare. However, disease progression must always be excluded [9]. HLH induced by drugs are described. We report here the first case of HLH induced by regorafenib. Secondary HLH has been reported after treatment with chimeric antigen receptor (CAR) T-cells due to its immunomodulatory effects, and there are increasing reports of treatment-induced HLH in patients treated with CTLA4- and PD1/PDL1-directed checkpoint antibodies. Treatment interruption or corticosteroids have been used with meaningful responses [10].
This case suggests that HLH should be a differential diagnosis for patients treated with regorafenib presenting with fever, cytopenia and any other signs of a hyperinflammatory status, to avoid missed or delayed diagnosis.
References
- Sepulveda FE, de Saint Basile G. Hemophagocytic syndrome: primary forms and predisposing conditions. Curr Opin Immunol. déc 49 (2017): 20-26.
- Brisse E, Wouters CH, Matthys P. Hemophagocytic lymphohistiocytosis (HLH): A heterogeneous spectrum of cytokine-driven immune disorders. Cytokine Growth Factor Rev 26 (2015): 263-280.
- Kim T, Kulick CG, Kortepeter CM, et al. Hemophagocytic lymphohistiocytosis associated with the use of lamotrigine. Neurology 92 (2019): e2401 5.
- Wilhelm SM, Dumas J, Adnane L, Lynch M, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 129 (2011): 245-255.
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381 (2013): 303-312.
- Demetri GD, Reichardt P, Kang Y-K, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381 (2013): 295-302.
- Otrock ZK, Eby CS. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol 90 (2015): 220-224.
- Henter J-I, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. févr 48 (2007): 124-131.
- La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 133 (2019): 2465-2477.
- Sadaat M, Jang S. Hemophagocytic lymphohistiocytosis with immunotherapy: brief review and case report. Journal for ImmunoTherapy of Cancer 6 (2018): 49.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
