A Case of Tumor Lysis Syndrome Induced by Surgery in Colon Cancer with Multiple Liver Metastases
Article Information
Manabu Yamamoto MD1, Norifumi Iseda MD1, Hiroko Yano MD1, Yohei Tominaga MD1, Ayako Sugino MD2, Hiroyuki Kobayasi MD2
1Department of Surgery, Fukuoka Sanno Hospital, Momochi-hama 3-6-45, Fukuoka 814-0001, Japan
2Department of Gastroenterology, Fukuoka Sanno Hospital, Momochi-hama 3-6-45, Fukuoka 814-0001, Japan
*Corresponding Author: Dr. Manabu Yamamoto, Department of Surgery, Fukuoka Sanno Hospital, Momochi-hama 3-6-45, Fukuoka 814-0001, Japan
Received: 22 July 2019; Accepted: 05 August 2019; Published: 13 November 2019
Citation: Yamamoto M, Iseda N, Yano H, Tominaga Y, Sugino A, Kobayasi H. A Case of Tumor Lysis Syndrome Induced by Surgery in Colon Cancer with Multiple Liver Metastases. Archives of Clinical and Medical Case Reports 3 (2019): 428-435.
View / Download Pdf Share at FacebookAbstract
Although tumor lysis syndrome is well described after chemotherapy for non-solid tumors, it is rarely seen or suspected in solid malignancies. Early recognition of this condition is paramount to reduce morbidity and mortality. We report on a case of tumor lysis syndrome induced by surgery in a patient with colon cancer with multiple liver metastases. We also review fifteen cases of diagnosed tumor lysis syndrome accompanying colorectal cancers. Though tumor lysis syndrome is rare in solid tumors, we should realize this possibility in patients with solid tumors of larger volumes, in particular, with enlarged liver metastases.
Keywords
Colon cancer; Tumor lysis syndrome; Surgery; Liver metastases
Colon cancer articles, Tumor lysis syndrome articles, Surgery articles, Liver metastases articles
Colon cancer articles Colon cancer Research articles Colon cancer review articles Colon cancer PubMed articles Colon cancer PubMed Central articles Colon cancer 2023 articles Colon cancer 2024 articles Colon cancer Scopus articles Colon cancer impact factor journals Colon cancer Scopus journals Colon cancer PubMed journals Colon cancer medical journals Colon cancer free journals Colon cancer best journals Colon cancer top journals Colon cancer free medical journals Colon cancer famous journals Colon cancer Google Scholar indexed journals cancer articles cancer Research articles cancer review articles cancer PubMed articles cancer PubMed Central articles cancer 2023 articles cancer 2024 articles cancer Scopus articles cancer impact factor journals cancer Scopus journals cancer PubMed journals cancer medical journals cancer free journals cancer best journals cancer top journals cancer free medical journals cancer famous journals cancer Google Scholar indexed journals Tumor lysis syndrome articles Tumor lysis syndrome Research articles Tumor lysis syndrome review articles Tumor lysis syndrome PubMed articles Tumor lysis syndrome PubMed Central articles Tumor lysis syndrome 2023 articles Tumor lysis syndrome 2024 articles Tumor lysis syndrome Scopus articles Tumor lysis syndrome impact factor journals Tumor lysis syndrome Scopus journals Tumor lysis syndrome PubMed journals Tumor lysis syndrome medical journals Tumor lysis syndrome free journals Tumor lysis syndrome best journals Tumor lysis syndrome top journals Tumor lysis syndrome free medical journals Tumor lysis syndrome famous journals Tumor lysis syndrome Google Scholar indexed journals Surgery articles Surgery Research articles Surgery review articles Surgery PubMed articles Surgery PubMed Central articles Surgery 2023 articles Surgery 2024 articles Surgery Scopus articles Surgery impact factor journals Surgery Scopus journals Surgery PubMed journals Surgery medical journals Surgery free journals Surgery best journals Surgery top journals Surgery free medical journals Surgery famous journals Surgery Google Scholar indexed journals Liver metastases articles Liver metastases Research articles Liver metastases review articles Liver metastases PubMed articles Liver metastases PubMed Central articles Liver metastases 2023 articles Liver metastases 2024 articles Liver metastases Scopus articles Liver metastases impact factor journals Liver metastases Scopus journals Liver metastases PubMed journals Liver metastases medical journals Liver metastases free journals Liver metastases best journals Liver metastases top journals Liver metastases free medical journals Liver metastases famous journals Liver metastases Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals colorectal cancers articles colorectal cancers Research articles colorectal cancers review articles colorectal cancers PubMed articles colorectal cancers PubMed Central articles colorectal cancers 2023 articles colorectal cancers 2024 articles colorectal cancers Scopus articles colorectal cancers impact factor journals colorectal cancers Scopus journals colorectal cancers PubMed journals colorectal cancers medical journals colorectal cancers free journals colorectal cancers best journals colorectal cancers top journals colorectal cancers free medical journals colorectal cancers famous journals colorectal cancers Google Scholar indexed journals colorectal articles colorectal Research articles colorectal review articles colorectal PubMed articles colorectal PubMed Central articles colorectal 2023 articles colorectal 2024 articles colorectal Scopus articles colorectal impact factor journals colorectal Scopus journals colorectal PubMed journals colorectal medical journals colorectal free journals colorectal best journals colorectal top journals colorectal free medical journals colorectal famous journals colorectal Google Scholar indexed journals Tetany articles Tetany Research articles Tetany review articles Tetany PubMed articles Tetany PubMed Central articles Tetany 2023 articles Tetany 2024 articles Tetany Scopus articles Tetany impact factor journals Tetany Scopus journals Tetany PubMed journals Tetany medical journals Tetany free journals Tetany best journals Tetany top journals Tetany free medical journals Tetany famous journals Tetany Google Scholar indexed journals Tumor articles Tumor Research articles Tumor review articles Tumor PubMed articles Tumor PubMed Central articles Tumor 2023 articles Tumor 2024 articles Tumor Scopus articles Tumor impact factor journals Tumor Scopus journals Tumor PubMed journals Tumor medical journals Tumor free journals Tumor best journals Tumor top journals Tumor free medical journals Tumor famous journals Tumor Google Scholar indexed journals
Article Details
Abbreviations:
TLS-Tumor Lysis Syndrome; CEA- Carcinoembryonic Antigen; DIC-Disseminated Intravascular Coagulation
1. Introduction
Tumor lysis syndrome (TLS) is a well described oncologic emergency. The syndrome is deemed spontaneous when it occurs before initiation of any cytotoxic or definitive treatment. Here, we report the rare case of a 70-year old man with colon cancer and extensive liver involvement manifesting with TLS following surgery. Previous investigators had demonstrated only fourteen cases with TLS associated in colorectal cancer [1-14]. Ten of the fourteen cases were induced by chemotherapy, three were spontaneous tumor lysis syndrome (STLS), and one was induced by radiofrequency ablation (RFA). This is therefore the first report of TLS induced by surgery in case of colon cancer with liver metastases.
2. Case Presentation
A 70-year old Japanese man, who had been in good health until two months prior to this admission, presented with worsening abdominal pain, accompanied by loss of appetite, fever and subjective weight loss. The patient also complained of postprandial abdominal pain in the right upper quadrant. Blood examination revealed anemia and elevated levels of the tumor marker, carcinoembryonic antigen (CEA). His past medical history detailed two previous abdominal operations, due to intestinal obstruction fifty years ago. His social history was negative for tobacco use, alcohol use and use of illicit drugs.
Physical examination revealed right upper quadrant tenderness on deep palpation with no signs of peritonitis, and bilateral pitting edema up to the knees. Cardiovascular, pulmonary, and neurological examinations were grossly normal. Laboratory findings at the time of presentation included: white blood cells: 9.35 × 103/μL, hemoglobin: 7.7 g/dL, platelets: 353 × 103/μL, potassium: 4.5 mmol/L, serum creatinine: 0.77 mg/dL, albumin: 2.9 g/dL, total bilirubin: 0.7 mg/dL, alanine aminotransferase: 1037 U/L, aspartate aminotransferase: 40 U/L, alkaline phosphatase: 47 U/L and lactate dehydrogenase levels: 979 U/L. Elevated CEA was observed at 283.6 ng/UL. Colonoscopy revealed a mass in the transverse colon: biopsy of the mass confirmed a moderately- to well-differentiated invasive adenocarcinoma (Figure 1). Computed tomography scan of the abdomen and pelvis revealed hepatomegaly with multiple liver lesions alongside with mural thickening of the transverse colon, with ascites across the whole body (Figure 2). The patient underwent right hemicolectomy as the palliative surgery to prevent anemia or colon obstruction. On the first postoperative day, the patient experienced tumor lysis, with lactate dehydrogenase levels: 20072 U/L, uric acid: 8.7 mg/dL, serum potassium: 6.8 mEq/dL, serum calcium: 7.4 mg/dl, and serum phosphate: 6.3 mg/dL (Table 1). Kidneys ultrasound showed normal renal parenchyma with no evidence of obstructive uropathy. We diagnosed this as TLS. All available supportive measures, including continuous hemodiafiltration, were initiated from 1 to 7 postoperative days. Then, the patient also experienced liver failure and disseminated intravascular coagulation (DIC) from 3 to 11 postoperative days (Table 1). The patient subsequently recovered from TLS, and was administered the anticancer-drugs (FOLFOX) on postoperative day 21.
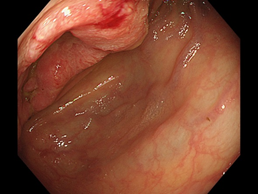
Figure 1: Colonoscopy showing a Bormann II type tumor at the transverse colon (arrow).
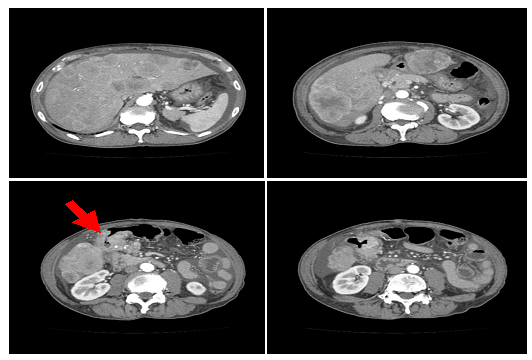
Figure 2: Computed tomography scan revealing multiple liver metastasis and ascites across the whole peritoneal cavity. The arrow represents the tumor at the transverse colon.
|
1POD |
7POD |
14POD |
21POD |
|
|
WBC (µl) |
10220 |
23760 |
17130 |
10320 |
|
Hemoglobin (g/dl) |
13.5 |
9.2 |
7 |
8 |
|
Platelets (x104/µl) |
33 |
12.2 |
21.7 |
29.1 |
|
BUN (mg/dl) |
18 |
15 |
9 |
6 |
|
Cre (mg/dl) |
1.35 |
0.88 |
0.62 |
0.56 |
|
K (mEq/l) |
6.8 |
3 |
4 |
4.3 |
|
Ca (mg/dl) |
7.4 |
7.4 |
7 |
7.9 |
|
P (mg/dl) |
6.3 |
2.4 |
3.1 |
3 |
|
T-Bil (mg/dl) |
2.3 |
8.6 |
3.2 |
2.3 |
|
GOT (U/l) |
1190 |
48 |
39 |
33 |
|
GPT (U/l) |
286 |
38 |
24 |
22 |
|
LDH (U/l) |
20072 |
591 |
643 |
530 |
|
Uric Acid (mg/dl) |
8.7 |
2.8 |
2.9 |
5.3 |
|
CK (U/l) |
1609 |
68 |
98 |
95 |
|
CRP (mg/dl) |
11.31 |
15.63 |
5.49 |
4.09 |
|
Continuous hemodiafiltration 1POD~7POD |
||||
|
DIC 3POD~11POD |
||||
POD: postoperative day; DIC: disseminated intravascular coagulation
Table 1: Laboratory trends in the patient presented.
3. Discussion
We present here the first case of TLS as a complication after surgery for metastatic colon adenocarcinoma. TLS is an oncological emergency precipitated by massive release into the circulation of intracellular tumor contents, manifesting in distinct laboratory abnormalities including hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia. While TLS is most commonly observed in hematological cancers, it can also be seen in solid malignancies with large tumor burdens [15]. These include breast cancer, small cell lung cancer, germ cell tumor, melanoma, Merkel cell carcinoma, head and neck cancer, non-small cell lung cancer, ovarian cancer, vulva cancer, prostate cancer, hepatocellular carcinoma, colorectal cancer, gastric cancer, sarcoma, neuroblastoma, medulloblastoma, hepatoblastoma, gestational trophoblastic neoplasia, renal cell carcinoma, transitional cell carcinoma, and thymoma [6].
In most patients, TLS occurs as a result of chemotherapy, however, it may also be induced by other anti-neoplastic treatments, including radiotherapy, hormone therapy and immunotherapy, or may develop spontaneously before initiation of therapy [16, 17]. STLS is defined as the TLS occurring in the absence of any definitive treatment [18]. The incidence of STLS in solid tumor is rare. The first step for TLS diagnosis is based on the laboratory findings in an appropriate clinical context. Hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia are the primary indicators of TLS [15, 16]. Clinical TLS is considered when traditional laboratory findings are accompanied by diarrhea, nausea, vomiting, lethargy, cardiac arrhythmias, seizures, or sudden death [17, 18]. Patient care when TLS has been established should include astute monitoring of uric acid, potassium, phosphorous, calcium, lactate dehydrogenase and serum creatinine levels, alongside cardiac monitoring and monitoring urine output. Allopurinol may be a useful prophylactic to lower uric acid production in patients at risk of TLS. However, allopurinol use may result in elevated xanthine levels by inhibiting xanthine oxidase in patients with TLS [19-21]. Xanthine may also precipitate in the renal tubules, contributing to obstructive uropathy in a similar manner as uric acid [20, 21]. As a result, rasburicase, which converts uric acid to soluble allantoin, is the recommended drug for hyperuricemia in TLS [15, 16, 18, 19]. The dialysis need to be initiated for hyperkalemia, hyperphosphatemia, or hypocalcemia [15]. The present case was not treated with rasburicase, due to a sufficient decrease in uric acid by the second postoperative day through continuous hemodiafiltration.
The first reported case of TLS in a nonhematological malignancy occurred in 1977 [22]. Approximately 120-140 reported cases of tumor lysis in solid tumors have since been reported, mostly after treatment, between 1977 and 2018 [6, 19]. Although there was different origins of primary tumors, extensive liver involvement has been noted in 82.8% of all cases. When the liver was not involved, patients inevitably had a large tumor burdens accompanied by necrosis. As noted by Gemici et al. [16], liver involvement seems to predispose patients to the TLS. This may be due to a high purine pool in the liver, which may be released during necrosis, or due to impaired uric acid metabolism if hepatic function is impaired by a high tumor burden [16]. TLS in solid tumors is unpredictable and carries a worse prognosis when compared to hematological malignancies [16]. Mortality rates are 20%–50% across all cases of TLS in solid tumors if undiagnosed or if diagnosed too late [19, 23].
Our literature review of TLS in patients with colon cancer revealed only three cases of STLS, ten of tumor lysis induced by a chemotherapy, one of tumor lysis induced by RFA, and the present case of tumor lysis induced by surgery [1-14] (Table 2). This case may represent a pre-process to STLS during surgery, though it is difficult to understand why TLS was induced by surgery. Of the fifteen cases, including the present case identified, most cases had multiple liver metastasis, with larger tumor volumes, and only five (33.3%) recovered from TLS. Early diagnosis and treatment are crucial if patients are to survive TLS.
|
No. |
Age |
Gender |
Tumor sites |
Treatment |
Onset of TLS |
Prognosis |
Ref |
|
1 |
27 |
M ? |
Liver, lung, pleura |
STLS |
Died |
[1] |
|
|
2 |
82 |
F |
Extensive liver involvement |
STLS |
Recovery |
[2] |
|
|
3 |
49 |
F |
Liver mass |
STLS |
Died |
[3] |
|
|
4 |
49 |
F |
Multiple liver |
Etoposide+cisplatin |
1 days |
- |
[4] |
|
5 |
83 |
F |
Peritoneal dissemination |
FOLFIRI+cetuximab |
5 days |
Died |
[5] |
|
6 |
59 |
M |
Numerous liver |
FOLFOX |
3 days |
Recovery |
[6] |
|
7 |
51 |
F |
Enlarger liver |
FOLFOX |
- |
Recovery |
[7] |
|
8 |
64 |
M |
Liver |
FOLFIRI+bevacizumab |
7 days |
Died |
[8] |
|
9 |
62 |
M |
Lung |
FOLFIRI+bevacizumab |
2 days |
Died |
[9] |
|
10 |
66 |
M |
Liver, lung |
FOLFIRI |
3 days |
Died |
[10] |
|
11 |
64 |
M |
Lung |
Cetuximab |
18 hr |
Recovery |
[11] |
|
12 |
38 |
F |
Liver |
Irinotecan |
6 days |
Died |
[12] |
|
13 |
42 |
F |
Multiple liver |
Irinotecan |
8 days |
Died |
[13] |
|
14 |
- |
- |
Liver |
RFA |
- |
- |
[14] |
|
Present case |
70 |
M |
Extensive liver involvement |
Surgery |
18 hr |
Recovery |
STLS, spontaneous tumor lysis syndrome; RFA, radiofrequency ablation;
FOLFIRI, 5-fluorouracil, leucovorin and irinotecan; FOLFOX: 5-fluorouracil, leucovorin and oxaliplatin.
Table 2: Fifteen cases induced tumor lysis syndrome in colorectal cancers.
In conclusion, we reported the first documented case of TLS induced by surgery in a colon cancer with multiple liver metastases. This patient recovered from TLS by early diagnosis and treatment, including continuous hemodiafiltration due to resolve hyperuricemia and hyperkalemia.
Conflict of Interest
The authors declared that they have no competing interests.
Consent for Publication
The informed consent for publication and presentation was obtained from the patient.
Funding
This work was no supported from any funding.
Authors’ Contribution
MY drafted the manuscript. NI, HY, YT, AS, and HK acquired data and revised the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
We thank Dr. Masafumi Ohya for pathological diagnosis.
References
- Frestad D, Perner A, Pedersen UG. Acute onset and rapid progression of multiple organ failure in a young adult with undiagnosed disseminated colonic adenocarcinoma. BMJ Case Rep (2014) doi: 10.1136/bcr-2014-205002.
- Vaisban E, Braester A, Mosenzon O, et al. Spontaneous tumor lysis syndrome in solid tumors: really a rare condition? Am J Med Sci 325 (2003): 38-40.
- Sommerhalder D, Takalkar AM, Shackelford R, Peddi P. Spontaneous tumor lysis syndrome in colon cancer: a case report and literature review. Clin Case Rep 5 (2017): 2121-2126.
- El Mesbahi O, Beaudoin A, Louvet D, De Gramont A. Tumor lysis syndrome after chemotherapy for colon small cell carcinoma. Rev Med Interne 25 (2004): 768-769.
- Matsuyama S, Kuramoto T, Tanaka R, Hashiguchi K. Tumor lysis syndrome after FOLFIRI+cetuximab for ascending colon cancer. Nihon Shoukakibyo Gakkai Zasshi 112 (2015): 714-720.
- Kim HD, Ha KS, Woo IS, et al. Tumor lysis syndrome in a patient with metastatic colon cancer after treatment with 5-fluorouracil/leucovorin and oxaliplatin: case report and literature review. Cancer Res Treat 46 (2014): 204-207.
- Gouveia HS, Lopes SO, Faria AL. Management of tumor lysis syndrome during first-line palliative chemotherapy for high volume colorectal cancer. BMJ Case Rep (2018) Doi:10.1136/bcr-2017-223474.
- Krishnan G, D’Silva K, Al-Janadi A. Cetuximab-related tumor lysis syndrome in metastatic colon carcinoma. J Clin Oncol 26 (2008): 2406-2408.
- Hentrick M, Schiel X, Scheidt S, et al. Fatal tumor lysis syndrome after irinotecan/5-FU/folic acid/bevacizumab-containing therapy in a patient heavily pretreated for metastatic colon cancer. Acta Oncol 47 (2018): 155-156.
- Oztop I, Demarkan B, Yaren A, et al. Rapid tumor lysis syndrome in a patient with metastatic colon cancer as a complication of treatment with 5-fluorouracil/leucovorin and irinotecan. Tumori 90 (2004): 514-516.
- Kalmkerian GP, Darwish B, Varterasian L. Tumor lysis syndrome in small cell carcinoma and other solid tumors. Am J Med 103 (1997): 363-367.
- Nikolic-Tomasevic Z, Jelie S, Popv I. Irinotecan as second-line treatment in metastatic colorectal cancer: dilemmas regarding patient selection and toxicity prediction. J Chemother 12 (2000): 244-251.
- Boisseau M, Bugat R, Mahjoubi M. Rapid tumour lysis syndrome in a metastatic colorectal cancer increased by treatment (CPT11). Eur J Cancer 32A (1996): 737-738.
- Barry BD, Kell MR, Redmond HP. Tumor lysis syndrome following endoscopic radiofrequency interstitial thermal ablation of colorectal liver metastases. Surg Endosc 16 (2002): 1109.
- Coiffier B, Altman A, Pui CH, et al. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence?based review. J Clin Oncol 26 (2008): 2767-2778.
- Gemici C. Tumour lysis syndrome in solid tumours. Clin Oncol (R Coll Radiol) 18 (2006): 773-780.
- Woo IS, Kim JS, Park MJ, et al. Spontaneous acute tumor lysis syndrome with advanced gastric cancer. J Korean Med Sci 16 (2001): 115-118.
- Nagaiah G, Truong Q, Monga M. Oncologic emergencies and paraneoplastic syndromes in Abraham J, editor, Gulley JL, editor, Allegra CJ, editor, eds. The Bethesda handbook of clinical oncology, 5th ed. Lippincott Williams & Wilkins, Philadelphia (2014).
- Vodopivec DM, Rubio JE, Fornoni A, Lenz O. An unusual presentation of tumor lysis syndrome in a patient with advanced gastric adenocarcinoma: case report and literature review. Case Rep Med (2012) Doi.10.1155/2012/468452.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol 127 (2004): 3-11.
- LaRosa C, McMullen L, Bakdash S, et al. Acute renal failure from xanthine nephropathy during management of acute leukemia. Pediatr Nephrol 22 (2007): 132-135.
- Crittenden DR, Ackerman GL. Hyperuricemic acute renal failure in disseminated carcinoma. Arch Intern Med 137 (1977): 97-99.
- Coiffier B. Acute tumor lysis syndrome-a rare complication in the treatment of solid tumors. Oncol Res Treat 33 (2010): 498-499.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
