A New Patient of Chanarin Dorfman Syndrome in Egypt
Article Information
Zeinab el kabbany1, Ola khalifa2, Asmaa Wafeeq1*, Khushnooda Ramzan3, Lina Al Baik3, Faiqa Imtiaz3, Yasser Wageeh4, Khaled Zalata5, Alaa Mousa1
1Department of Paediatric Hepatology, Faculty of medicine, Ain Shams University, Cairo, Egypt
2Department of Genetics, Faculty of medicine, Ain Shams University, Cairo, Egypt
3Department of Genetics, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
4Department of Clinical Pathology, Ain Shams University, Cairo, Egypt
5Department of Pathology, Mansoura University, Mansoura, Dakahlia, Egypt
*Corresponding Author: Dr. Asmaa Wafeeq, Department of Paediatric Hepatology, Faculty of medicine, Ain Shams University, Cairo, Egypt
Received: 11 Sep 2019; Accepted: 08 October 2019; Published: 29 November 2019
Citation: Zeinab el kabbany, Ola khalifa, Asmaa Wafeeq, Khushnooda Ramzan, Lina Al Baik, Faiqa Imtiaz, Yasser Wageeh, Khaled Zalata, Alaa Mousa. A New Patient of Chanarin Dorfman Syndrome in Egypt. Archives of Clinical and Medical Case Reports 3 (2019): 702-707.
View / Download Pdf Share at FacebookAbstract
Chanarin Dorfman syndrome (CDS) is an autosomal recessive neutral lipid storage disorder characterized by ichthyosis, and multisystem affection. Here, we report another patient from Egypt. The clinical data from a patient and his family were collected. DNA was isolated from peripheral blood leukocytes. Coding exons and the flanking introns of ABHD5 gene were PCR amplified, purified, and sequenced. Our patient meets the major clinical symptoms of CDS. Direct sequencing of ABHD5 gene led to the identification of a homozygous single nucleotide change (c.700C>T) in our patient. The parents were both heterozygous carriers, while his sister was wild type normal for the identified variant. In conclusion, these results indicate that p.Arg234Ter is a pathogenic mutation associated with the Chanarin-Dorfman syndrome in our patient in this study.
Keywords
Chanarian-Dorfman syndrome; Cytoplasmic vacuoles; Gene mutation; ABHD5 gene
Chanarian-Dorfman syndrome articles, Cytoplasmic vacuoles articles, Gene mutation articles, ABHD5 gene articles
Chanarian-Dorfman syndrome articles Chanarian-Dorfman syndrome Research articles Chanarian-Dorfman syndrome review articles Chanarian-Dorfman syndrome PubMed articles Chanarian-Dorfman syndrome PubMed Central articles Chanarian-Dorfman syndrome 2023 articles Chanarian-Dorfman syndrome 2024 articles Chanarian-Dorfman syndrome Scopus articles Chanarian-Dorfman syndrome impact factor journals Chanarian-Dorfman syndrome Scopus journals Chanarian-Dorfman syndrome PubMed journals Chanarian-Dorfman syndrome medical journals Chanarian-Dorfman syndrome free journals Chanarian-Dorfman syndrome best journals Chanarian-Dorfman syndrome top journals Chanarian-Dorfman syndrome free medical journals Chanarian-Dorfman syndrome famous journals Chanarian-Dorfman syndrome Google Scholar indexed journals Chanarian-Dorfman articles Chanarian-Dorfman Research articles Chanarian-Dorfman review articles Chanarian-Dorfman PubMed articles Chanarian-Dorfman PubMed Central articles Chanarian-Dorfman 2023 articles Chanarian-Dorfman 2024 articles Chanarian-Dorfman Scopus articles Chanarian-Dorfman impact factor journals Chanarian-Dorfman Scopus journals Chanarian-Dorfman PubMed journals Chanarian-Dorfman medical journals Chanarian-Dorfman free journals Chanarian-Dorfman best journals Chanarian-Dorfman top journals Chanarian-Dorfman free medical journals Chanarian-Dorfman famous journals Chanarian-Dorfman Google Scholar indexed journals syndrome articles syndrome Research articles syndrome review articles syndrome PubMed articles syndrome PubMed Central articles syndrome 2023 articles syndrome 2024 articles syndrome Scopus articles syndrome impact factor journals syndrome Scopus journals syndrome PubMed journals syndrome medical journals syndrome free journals syndrome best journals syndrome top journals syndrome free medical journals syndrome famous journals syndrome Google Scholar indexed journals Cytoplasmic vacuoles articles Cytoplasmic vacuoles Research articles Cytoplasmic vacuoles review articles Cytoplasmic vacuoles PubMed articles Cytoplasmic vacuoles PubMed Central articles Cytoplasmic vacuoles 2023 articles Cytoplasmic vacuoles 2024 articles Cytoplasmic vacuoles Scopus articles Cytoplasmic vacuoles impact factor journals Cytoplasmic vacuoles Scopus journals Cytoplasmic vacuoles PubMed journals Cytoplasmic vacuoles medical journals Cytoplasmic vacuoles free journals Cytoplasmic vacuoles best journals Cytoplasmic vacuoles top journals Cytoplasmic vacuoles free medical journals Cytoplasmic vacuoles famous journals Cytoplasmic vacuoles Google Scholar indexed journals vacuoles articles vacuoles Research articles vacuoles review articles vacuoles PubMed articles vacuoles PubMed Central articles vacuoles 2023 articles vacuoles 2024 articles vacuoles Scopus articles vacuoles impact factor journals vacuoles Scopus journals vacuoles PubMed journals vacuoles medical journals vacuoles free journals vacuoles best journals vacuoles top journals vacuoles free medical journals vacuoles famous journals vacuoles Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals Gene mutation articles Gene mutation Research articles Gene mutation review articles Gene mutation PubMed articles Gene mutation PubMed Central articles Gene mutation 2023 articles Gene mutation 2024 articles Gene mutation Scopus articles Gene mutation impact factor journals Gene mutation Scopus journals Gene mutation PubMed journals Gene mutation medical journals Gene mutation free journals Gene mutation best journals Gene mutation top journals Gene mutation free medical journals Gene mutation famous journals Gene mutation Google Scholar indexed journals ABHD5 gene articles ABHD5 gene Research articles ABHD5 gene review articles ABHD5 gene PubMed articles ABHD5 gene PubMed Central articles ABHD5 gene 2023 articles ABHD5 gene 2024 articles ABHD5 gene Scopus articles ABHD5 gene impact factor journals ABHD5 gene Scopus journals ABHD5 gene PubMed journals ABHD5 gene medical journals ABHD5 gene free journals ABHD5 gene best journals ABHD5 gene top journals ABHD5 gene free medical journals ABHD5 gene famous journals ABHD5 gene Google Scholar indexed journals gene articles gene Research articles gene review articles gene PubMed articles gene PubMed Central articles gene 2023 articles gene 2024 articles gene Scopus articles gene impact factor journals gene Scopus journals gene PubMed journals gene medical journals gene free journals gene best journals gene top journals gene free medical journals gene famous journals gene Google Scholar indexed journals Tendinitis articles Tendinitis Research articles Tendinitis review articles Tendinitis PubMed articles Tendinitis PubMed Central articles Tendinitis 2023 articles Tendinitis 2024 articles Tendinitis Scopus articles Tendinitis impact factor journals Tendinitis Scopus journals Tendinitis PubMed journals Tendinitis medical journals Tendinitis free journals Tendinitis best journals Tendinitis top journals Tendinitis free medical journals Tendinitis famous journals Tendinitis Google Scholar indexed journals
Article Details
Abbreviations:
CDS-Chanarian-Dorfman syndrome
1. Introduction
Chanarin -Dorfman syndrome (CDS) is an autosomal recessive neutral lipid storage disorder, which is characterized by congenital ichthyosis, lipid vacuoles in peripheral leucocytes, and multisystem affection [1]. At birth, affected individuals usually present with dry, scaly skin. Additional features include hepatomegaly, ataxia, hearing loss, short stature, muscle weakness, nystagmus, and mild intellectual disability [2]. This condition is caused by mutations in the ABHD5 which is broadly expressed in different tissues, including skeletal muscle, lymphocytes, skin, liver, and brain. Therefore, any impairment in ABDH5 expression may lead to many complications in various tissues [3]. We have reported previously a patient from Egypt in 2003, and here we present another patient of CDS from Egypt.
2. Subjects and Methods
2.1 Demographic and clinical data
A 15-month-old male presented with generalized erythematous scaling since birth, with no bullous lesions, associated with gradual abdominal swelling. There was no history of visual, hearing or any focal neurological deficits. He was the first child of first cousin consanguineous marriage, with no previous pregnancy loss and no other family member had been similarly affected. Skin examination revealed generalized erythematous fine scaly patches with peeling of hands and trunk (Figure 1A). On examination of his abdomen a 10 cm firm, non tender hepatomegaly was found. The nervous system examination was normal. His anthropometric measurements (length-for-age, weight-for-age, head circumference-for-age, and weight-for-length) were all below the third percentile according to the CDC (Centre for Disease Control) growth charts. A small ASD was discovered accidentally during his echocardiographic examination. Complete blood film, liver functions, lipid profile, liver biopsy, bone marrow aspiration was done for the affected child and genetic testing was ordered for the boy and his family
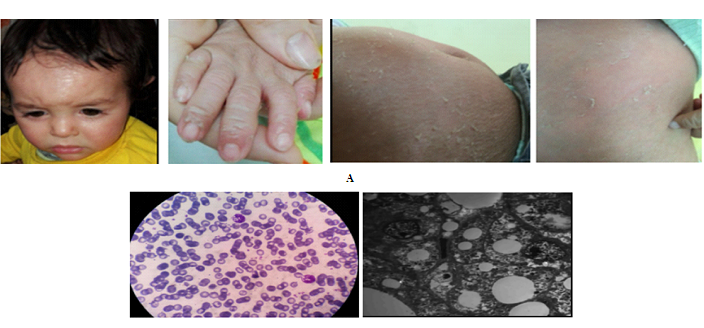
Figure 1: Clinical findings and investigations of the patient. A: generalized erythematous fine scaly patches associated with peeling of hands and trunk. B: the peripheral smear shows leucocytic vacuolations in the neutrophils (Jordan's anomaly). C: The electron microscopic examination shows the excess number of non membrane bound lipid droplets in the hepatocytes.
2.2 Genotyping
Following informed consent, blood samples were taken from the index case (II:1), healthy parents (I:1 and I:2) and healthy sister (II:2, Figure 2A). DNA was isolated from peripheral blood leukocytes. Coding exons and the flanking introns of ABHD5 genes (GenBank sequence accession numberNM_016006.5) were PCR amplified by primer pairs designed by using Primer3 v.0.4.0 program (http://bioinfo.ut.ee/primer3-0.4.0, then PCR was purified and sequenced with ABI PRISM Big Dye Terminator v3.1 Cycle Sequencing Kit on an ABI PRISM 3730 automated DNA Analyzer (Applied Biosystems, Inc., Foster City, CA, USA). Sequence analysis was performed using Laser gene (DNA Star Inc., Madison, WI, USA) software package, and then compared to the standard sequence.
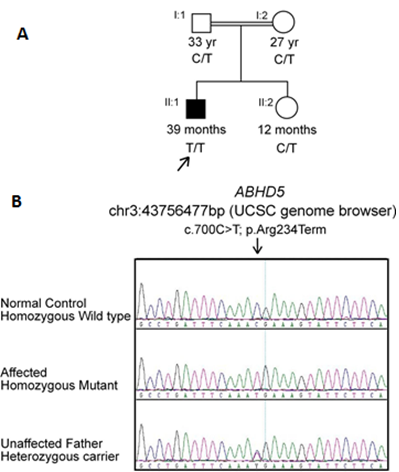
Figure 2: (A) Pedigree of the families studied with Chanarin-Dorfman syndrome demonstrating the recessive inheritance pattern. Filled symbols indicate affected individual. (B) Identification of a mutation in ABHD5. Sequencing chromatogram indicates the homozygous wild type, homozygous mutant and heterozygous carrier forms. Homozygous c.700C>T (p.Arg234Ter) mutation is identified in our patient (II:1). Nucleotide and amino acid numbering correspond to NM_016006.5 for the cDNA and NP_057090.2 for the protein. Nucleotides were numbered using the A of the ATG translation initiation codon as +1 nucleotide of the coding sequence.
3. Results and Analysis
On investigation, complete blood counts showed mild normocytic normochromic anemia and the peripheral smear showed leucocytic vacuolations in the neutrophils (Jordan's anomaly) that became clear on Giemsa-Wright staining (Figure 1B). Regarding liver functions tests, there was mildly elevated alanine aminotransferase (51 IU/L) with normal serum albumin, bilirubin and INR. Lipid profile was normal including serum triglycerides (72 mg) and serum cholesterol (171 mg). Creatine phosphokinase was normal. His ultrasound abdomen revealed coarse bright hepatomegaly. The light microscopic examination of his liver biopsy showed micronodular cirrhosis with macrovesicular steatosis and steatohepatitis with bridging fibrosis, while the electron microscopic analysis of his biopsy showed that the hepatocytes contained an excess number of non membrane bound lipid droplets (Figure 1C). Also, his bone marrow examination showed normocellular marrow with vacuolated myeloid series.
Direct sequencing of ABHD5 gene led to the identification of a homozygous single nucleotide change (c.700C>T) in the index case (II:1 from pedigree, Figure 2A). At the protein level, this transition introduced a stop codon at amino acid position 234 (p.Arg234Ter) (Figure 2B). In order to confirm this mutation and to examine its segregation through the family, Sanger sequencing was performed for the parents and sibling who were all healthy and show no signs or symptoms related to the disorder. The parents were both heterozygous carriers, while his sister was wild type normal for the identified variant.
4. Discussion
Chanarin- Dorfman syndrome (CDS) is one of the Neutral lipid storage diseases (NLSDs) characterized by an impairment in the triacylglycerol degradation and its abnormal accumulation in cytoplasmic lipid droplets present in most tissues [4]. Our patient meets the major clinical symptoms of CDS, which are non-bullous congenital ichthyosiform erythroderma [5], hepatomegaly and liver steatosis [6]. Clinical evidence of myopathy usually appears late [7] which could explain that our patient doesn’t have clinical muscle affection, supported by normal CPK. Also, he doesn’t have any neurological impairment, ataxia, nystagmus, or neurosensory deafness; these are late manifestations of the disease as triglyceride storage does not affect the central nervous system before middle age [8]. Hepatic involvement is known to be variable in CDS and liver biopsy is of great importance in reaching a diagnosis. Although our patient had liver function tests within normal range except for slightly elevated ALT, liver biopsy showed steatosis, in addition to the characteristic lipid droplets. The liver is clinically affected in 64% of CDS patients; however the increased liver enzymes and histological steatohepatitis are found in the 100% CDS patients with or without hepatomegaly [9].
Serum lipid pro?le has been inconsistent in various reports. Some patients had elevated triglyceride levels and very low density lipoproteins [10], while our patient had normal serum triglycerides similar to what was previously reported by [4, 11]. The clinical diagnosis of CDS is based on observation of Jordans’ bodies [12], which is also described in our patient’s peripheral neutrophils and their myeloid precursors in bone marrow.
Apart from the features described above, our patient had a congenital heart disease (ASD) which is an association not reported before with CDS. The identified homozygous stop gain, mutation (p.Arg234Ter) inABHD5gene, which encodes for Abhydrolase Domain-Containing protein, is predicted to result in a premature stop codon and an extremely truncated protein. To date, there are 38 different mutations in ABHD5, which cause Chanarin-Dorfman syndrome (The Human Gene Mutation Database, HGMD; http://www.hgmd.cf.ac.uk/). The variant mutation identified in our study (HGMD: CM052822) has been previously reported [13].
5. Conclusion
Collectively, these results indicate that p.Arg234Ter is pathogenic mutation associated with the Chanarin-Dorfman syndrome found in our patient in this study.
Authors’ Contributions
Dr. Zeinab el kabbany contributed to the acquisition of data and clinical followup. Dr. Asmaa Wafeeq contributed to the analysis and interpretation of data, clinical follow up and editing the manuscript. Dr. Ola Khalifa, Dr. Khushnooda Ramzan, Dr. Lina Al Baik, Dr. Faiqa Imtiaz performed the molecular studies and contributed to the interpretation of the results, and editing of the manuscript. Dr. Yasser, Dr. Khaled performed the histo-pathological investigations and Dr. Alaa contributed to clinical evaluation of the patient.
Compliance with Ethical Standards
The study was approved by the Institutional ethical committee of Ain Shams University Hospital, Egypt.
Financial Disclosure
No financial relationships relevant to this article.
Informed Consent
Written informed consent was obtained from the parent of the participating children.
Conflicts of Interest
All the authors declare that they have no conflicts of interest.
References
- Srinivasan R, Hadzic N, Fischer J, et al. Steatohepatitis and unsuspected micronodular cirrhosis in dorfman-chanarin syndrome with documented ABHD5 mutation. J Pediatr 144 (2004): 662-665.
- Schweiger M, Lass A, Zimmermann R, et al. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. American journal of physiology-endocrinology and metabolism 297 (2009): E289-E296.
- Yamaguchi T, Osumi T. Chanarin-Dorfman syndrome: deficiency in CGI-58, a lipid droplet-bound coactivator of lipase. Biochimica et biophysica acta 1791 ): 519-523.
- Missaglia S, Ribeiro Valadares E, Moro L, et al. Early onset of Chanarin-Dorfman syndrome with severe liver involvement in a patient with a complex rearrangement of ABHD5 promoter BMC Med Genet 15 (2014): 32.
- Ronchetti N, Prati D, Pezzotta MG, et al. Severe steatohepatitis in a patient with a rare neutral lipid storage disorder due to ABHD5 mutation. J Hepatol 15 (2008): 474-477.
- Bruno C, Bertini E, Di Rocco M, et al. Clinical and genetic characterization of Chanarin-Dorfman syndrome. Biochem Biophys Res Commun 15 (2008): 1125-1128.
- Hays AP, Miranda AF, Johnson W, et al. Lipid myopathy and congenital ichthyosis: Anew disorder, probably genetic. (Abstract) J Neuropath Exp Neurol 35 (1976): 346.
- Dorfman ML, Hershko C, Eisenberg S, et al. Ichthyosiform dermatosis with systemic lipidosis.Arch Dermatol 15 (1974): 2661-2666.
- Pena-Penabad C, Almagro M, Martinez W, et al. Dorfman- Chanarin syndrome (neutral lipid storage disease): new clinical features. Br J Dermatol 144 (2001): 430-432.
- Tullu MS, Muranjan MN, Save SU, et al. Dorfman–Chanarin syndrome: A rare neutral lipidstorage disease. Indian Pediatr 37 ((2000): 88-93.
- Arslansoyu Çamlar S, Gençp?nar P, Makay B, et al. Chanarin dorfman syndrome with multisystem affection in two siblings. Turk J Haematol 30 (2013): 72-75.
- Tavian D, Colombo R. Improved cytochemical method for detecting Jordans’ bodies in neutral-lipid storage diseases. J Clin Pathol 15 (2007): 956-958.
- Schleinitz N, Fisher J, Sanchez A, et al. Two new mutations of ABHD5 gene in a new adult case of Chanarin–Dorfman syndrome: an uncommon lipid storage disease. Arch Dermatol 141 (2005): 798-800.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
