A Review on the Phytochemicals of Parkia Speciosa, Stinky Beans as Potential Phytomedicine
Article Information
Nurul Izzah Ahmad1*, Salina Abdul Rahman1, Yin-Hui Leong2, Nur Hayati Azizul1
1Institute for Medical Research, Ministry of Health Malaysia, Kuala Lumpur, Malaysia
2National Poison Centre of Malaysia, Universiti Sains Malaysia, Penang, Malaysia
*Corresponding Author: Nurul Izzah Ahmad, Institute for Medical Research, Ministry of Health Malaysia, 50588 Jalan Pahang, Kuala Lumpur, Malaysia
Received: 28 June 2019; Accepted: 15 July 2019; Published: 22 July 2019
Citation: Nurul Izzah Ahmad, Salina Abdul Rahman, Yin-Hui Leong, Nur Hayati Azizul. A Review on the Phytochemicals of Parkia Speciosa, Stinky Beans as Potential Phytomedicine. Journal of Food Science and Nutrition Research 2 (2019): 151-173.
View / Download Pdf Share at FacebookAbstract
Plants pythochemicals are extensively known for their advantageous in health to promote biochemical benefits in the area of reactions, cofactors and inhibitors of enzyme, absorbents/sequestrants that bind to and eliminate undesirable elements in the human body. Some research findings have supported the beneficial role of phytochemicals against cancers, coronary heart disease, diabetes, high blood pressure, inflammation and many other diseases. This review discussed phytochemical compounds properties of Perkia specios (PS) in Malaysia. The plants of focus are smelly legumes/stink beans; commonly grown and cultivated in Southeast Asian countries, including Malaysia, Indonesia and in some parts of Northeastern India. The young leaves, flowers and fruits that are collected from the wild are consumed as vegetables and herbal medicines. The seeds are either eaten raw or cooked and half-ripe seeds can also be traditionally prepared as a pickle in brine. The review will be useful for future studies through current knowledge on the phytochemical elements and medicinal functions to a possible magnitude with relevant data as these plants have potential to be developed as phyto-/herbal-/botanical medicine.
Keywords
Phytochemicals, Phenolic constituents, Terpenoid, Alkaloids, Saponins, Cyclic polysulfides, Tannins, Parkia speciasa
Phytochemicals articles, Phenolic constituents articles, Terpenoid; Alkaloids articles, Saponins articles, Cyclic polysulfides articles, Tannins articles, Parkia speciasa articles
Phytochemicals articles Phytochemicals Research articles Phytochemicals review articles Phytochemicals PubMed articles Phytochemicals PubMed Central articles Phytochemicals 2023 articles Phytochemicals 2024 articles Phytochemicals Scopus articles Phytochemicals impact factor journals Phytochemicals Scopus journals Phytochemicals PubMed journals Phytochemicals medical journals Phytochemicals free journals Phytochemicals best journals Phytochemicals top journals Phytochemicals free medical journals Phytochemicals famous journals Phytochemicals Google Scholar indexed journals Phenolic constituents articles Phenolic constituents Research articles Phenolic constituents review articles Phenolic constituents PubMed articles Phenolic constituents PubMed Central articles Phenolic constituents 2023 articles Phenolic constituents 2024 articles Phenolic constituents Scopus articles Phenolic constituents impact factor journals Phenolic constituents Scopus journals Phenolic constituents PubMed journals Phenolic constituents medical journals Phenolic constituents free journals Phenolic constituents best journals Phenolic constituents top journals Phenolic constituents free medical journals Phenolic constituents famous journals Phenolic constituents Google Scholar indexed journals Terpenoid articles Terpenoid Research articles Terpenoid review articles Terpenoid PubMed articles Terpenoid PubMed Central articles Terpenoid 2023 articles Terpenoid 2024 articles Terpenoid Scopus articles Terpenoid impact factor journals Terpenoid Scopus journals Terpenoid PubMed journals Terpenoid medical journals Terpenoid free journals Terpenoid best journals Terpenoid top journals Terpenoid free medical journals Terpenoid famous journals Terpenoid Google Scholar indexed journals Alkaloids articles Alkaloids Research articles Alkaloids review articles Alkaloids PubMed articles Alkaloids PubMed Central articles Alkaloids 2023 articles Alkaloids 2024 articles Alkaloids Scopus articles Alkaloids impact factor journals Alkaloids Scopus journals Alkaloids PubMed journals Alkaloids medical journals Alkaloids free journals Alkaloids best journals Alkaloids top journals Alkaloids free medical journals Alkaloids famous journals Alkaloids Google Scholar indexed journals Saponins articles Saponins Research articles Saponins review articles Saponins PubMed articles Saponins PubMed Central articles Saponins 2023 articles Saponins 2024 articles Saponins Scopus articles Saponins impact factor journals Saponins Scopus journals Saponins PubMed journals Saponins medical journals Saponins free journals Saponins best journals Saponins top journals Saponins free medical journals Saponins famous journals Saponins Google Scholar indexed journals Cyclic polysulfides articles Cyclic polysulfides Research articles Cyclic polysulfides review articles Cyclic polysulfides PubMed articles Cyclic polysulfides PubMed Central articles Cyclic polysulfides 2023 articles Cyclic polysulfides 2024 articles Cyclic polysulfides Scopus articles Cyclic polysulfides impact factor journals Cyclic polysulfides Scopus journals Cyclic polysulfides PubMed journals Cyclic polysulfides medical journals Cyclic polysulfides free journals Cyclic polysulfides best journals Cyclic polysulfides top journals Cyclic polysulfides free medical journals Cyclic polysulfides famous journals Cyclic polysulfides Google Scholar indexed journals Tannins articles Tannins Research articles Tannins review articles Tannins PubMed articles Tannins PubMed Central articles Tannins 2023 articles Tannins 2024 articles Tannins Scopus articles Tannins impact factor journals Tannins Scopus journals Tannins PubMed journals Tannins medical journals Tannins free journals Tannins best journals Tannins top journals Tannins free medical journals Tannins famous journals Tannins Google Scholar indexed journals Parkia speciasa articles Parkia speciasa Research articles Parkia speciasa review articles Parkia speciasa PubMed articles Parkia speciasa PubMed Central articles Parkia speciasa 2023 articles Parkia speciasa 2024 articles Parkia speciasa Scopus articles Parkia speciasa impact factor journals Parkia speciasa Scopus journals Parkia speciasa PubMed journals Parkia speciasa medical journals Parkia speciasa free journals Parkia speciasa best journals Parkia speciasa top journals Parkia speciasa free medical journals Parkia speciasa famous journals Parkia speciasa Google Scholar indexed journals herbal medicines articles herbal medicines Research articles herbal medicines review articles herbal medicines PubMed articles herbal medicines PubMed Central articles herbal medicines 2023 articles herbal medicines 2024 articles herbal medicines Scopus articles herbal medicines impact factor journals herbal medicines Scopus journals herbal medicines PubMed journals herbal medicines medical journals herbal medicines free journals herbal medicines best journals herbal medicines top journals herbal medicines free medical journals herbal medicines famous journals herbal medicines Google Scholar indexed journals
Article Details
1. Introduction
Parkia speciosa Hassk is abundantly found in tropical regions, especially in South East Asian countries: Malaysia, Indonesia, Thailand and Philippines [1-4]. This rainforest tree belongs to the genus ?Parkia?, family ?Fabaceace? and is also found in India, Africa, Madagascar and Fiji [4, 5]. Parkia genus is divided into three sections namely Parkia, Platyparkia and Sphaeroparkia. The Parkia speciosa is group under Parkia section with three other species: P. cachimboensis, P. decussate and P. discolor [5]. This species thrives well in rainforest, on sandy, loamy and podzolic soils, waterlogged locations, freshwater swamp forest and areas nearby the riverbanks [3, 5].
The matured plant can grow up to 40 m height and it bears bulb shape flowers that hang oblong and twisted pods which contain 15-18 seeds. The light green seeds are encapsulated with seed coats in these pods. The seeds have foul, peculiar, but unique and distinctive smell with elliptical shape that can be eaten raw, roasted, cooked or blanched [3-5]. In Malaysia, PS seeds were mostly eaten as ?ulam? namely raw or boiled seeds consumed with chilies mixed with prawn paste as the main ingredient. Only a small portion of PS seeds were consumed after blanching or boiling [6, 7]. It is also cooked in chili paste mixed with seafood, boiled in coconut milk with varieties of vegetables or added as an ingredient in many other dishes, including fried rice or stir-fried food [5]. Matured seeds are usually pickled in brine and are among popular pickle products in the country [8]. Although PS seeds are widely used as a cooking ingredient [9], in traditional medicine, the seeds are consumed as remedies to clean and detoxify the kidneys and urinary tract, to treat diabetes and headache [10-12]. It is also a traditional remedy for itchiness [13], inflammation, oedema, liver failure and to eliminate intestinal worms [3].
PS seeds contain many nutritional values such as protein, fatty acids, carbohydrate, minerals, vitamins etc. [4, 5]. The seeds also rich in bioactive compounds either essential or non-essential from the secondary metabolites that have abundant therapeutic benefits. Phytochemicals are natural plant elements occurred in the leaves or roots which are useful as defense instruments to protect them from various diseases that over time have been discovered and used by diverse groups of people for treatment of various diseases [14]. Beneficial phytochemicals in plants may enhance the needs of the human body as they are rich in antioxidants and other health benefits as well.
2. Phytochemicals of PS
Primary and secondary metabolites are bioactive phytochemicals, which produced in plant tissues and they are well known for their advantages in medicinal benefits to human beings [14, 15]. Chlorophyll, amino acids, proteins, common sugars or simple carbohydrate, membrane lipids, purines and pyrimidines of nucleic acids [16-18] are an example of the primary constituents that play recognized roles in biochemical reactions in plants for example the major activities such as photosynthesis and respiration [17]. The secondary compounds are the terpenoid, alkaloids, phenolic compounds, lignins, plants steroid, curcumines, saponins, flavonoids, glucosides [16, 18] etc. and these compounds are synthesized as part of the defense system of the plants [15, 17]. These constituents protected plants from diseases and damages subsequently enhanced the plant?s color, aroma and flavor. Phytochemicals also serve as a defense mechanism to protect plant cells from unfavorable conditions such as environmental pollution, climate change, UV and pathogens exposure [16]. These compounds accumulated in the whole parts of the plant, especially the three main parts which are the roots, stems and leaves. The pigment molecules are abundance in the chloroplasts of the plant. Their levels vary from plant to plant depending upon the variety, growing conditions, processing and cooking methods [16].
Literature review indicated that phenolic compounds (45%) are the most numerous and structurally diverse plant phytoconstituents, followed by the terpenoids and steroids (27%), alkaloids (18%) and others (10%) [16]. Harborne identified and delineated the major classes of plant chemicals of recently recognized as health benefits specific phytochemicals [19]. These compounds were cited by Dillard et al. and listed in Table 1 [20]. These divisions of categories were constructed from their biosynthetic origin [15]. Alkaloids, tannins, flavonoids and phenolic compounds are among the most important bioactive constituents that are still being studied under phytochemistry or natural product chemistry [21]. Among phytochemicals, flavonoids, polyphenols, stilbenes, carotenoids and anthocyanins are several compounds that are vital for health promoting effects [22]. Several of these elements have been extracted from PS and researched for pharmacological activity. Terpenoids [23-25], polyphenols and flavonoids [23-29], alkaloids [23-25, 27-28], saponins [23, 24, 27, 28], steroids [23, 25, 28], tannins [23, 27] and phytosterol [30, 31].
|
No |
Classes |
Compounds |
|
1 |
Terpenoids |
monoterpenoids, iridoids, sesquiterpenoids, sesquiterpene lactones, diterpenoids, triterpenoidsaponins, steroid saponins, cardenolides and bufadienolides, phytosterols, cucurbitacins, nortriterpenoids, other triterpenoids and carotenoids. |
|
2 |
Phenolic metabolites |
anthocyanins, anthochlors, benzofurans, chromones, coumarins, minor flavonoids, flavonones and flavonols, isoflavonoids, lignans, phenols and phenolic acids, phenolic ketones, phenylpropanoids, quinonoids, stilbenoids, tannins and xanthones. |
|
3 |
Alkaloids |
amaryllidaceae, betalain, diterpenoid, indole, isoquinoline, lycopodium, monoterpene, sesquiterpene, peptide, pyrrolidine and piperidine, pyrrolizidine, quinoline, quinolizidine, steroidal, and tropane. |
|
4 |
Nitrogen-containing plant constituents |
non-protein amino acids, amines, cyanogenic glycosides, glucosinolates, and purines and pyrimidines. |
Table 1: Harborne [19] identified and delineated the major classes of plant phytochemicals as cited by Dillard and German [20].
2.1 Phenolic constituents
Phenolics consist of the aromatic ring (C6) bonded directly to at least one (phenol) or more (polyphenol) hydroxyl group (-OH) and other substituents such as methoxyl (CH3O?) or carboxyl (COOH) groups. These substituents either give the hydrophilic or hydrophobic character to the compounds [32]. These compounds occurred in conjugated forms with one or more sugar residues linked to hydroxyl group inclusive direct linkages of sugar to an aromatic carbon. The association forms with other compounds as well, for example carboxylic and organic acids, amines, lipids and other phenol [33]. Such structural diversity resulted of these compounds often referred to as polyphenols. These compounds are classified based on the number of phenol rings where the structural elements bind these rings to one another [34]. In the wide range, phenolic compounds can be categorized into several classes as shown in Table 2 and were divided into four classes (Figure 1) [34]. Of these, phenolic acids, flavonoids and tannins are the main dietary phenolic compounds [35]. Plant phenolic compounds arise from phenylalanine or shikimic acid [33] where the aromatic ring of phenolic compounds is synthesized in the shikimic acid pathway from amino acid phenylalanine. The initial step is simple detachment of the amino group (-NH2) from phenylalanine which convert to cinnamic acid. This reaction is catalyzed by enzyme phenylalanine ammonia-lyase [33].
|
Class |
Structure |
|
Simple phenolic, benzoquinones |
C6 |
|
Hydroxybenzoic acids |
C6-C1 |
|
Acethophenones, phenylacetic acids |
C6-C2 |
|
Hydroxycinnamic acids, phenylpropanoids (coumarins, isocoumarins, chromones, chromenes) |
C6-C3 |
|
Naphtoquinones |
C6-C4 |
|
Xanthones |
C6-C1-C6 |
|
Stilbenes, anthraquinones |
C6-C2-C6 |
|
Flavonoids, isoflavonoids |
C6-C3-C6 |
|
Lignins, neolignans |
(C6-C3)2 |
|
Biflavonoids |
(C6-C3-C6)2 |
|
Lignins |
(C6-C3)n |
|
Condensed tannins (proanthocyanidins or flavolans) |
(C6-C3-C6)n |
Table 2: Classes of phenolic compounds in plants [35].
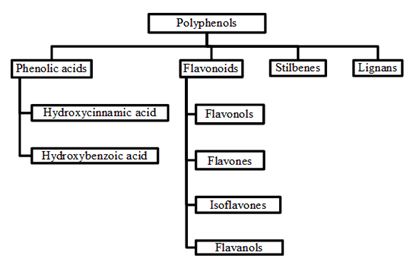
Figure 1: Classification of major plant polyphenols [34].
Phytochemicals screening of PS revealed the presence of pharmacologically active compound in many parts of the plants especially the empty pods. A study conducted by Ko et al. [29] indicated that the antioxidant activities of empty pods of PS extracts vary with the type of assays due to different responses of bioactive compounds towards these assays. Results showed that ethanol extracts (EE) of PS possessed good DPPH (1, 1-diphenyl-2-picrylhydrazyl) radical scavenging activity than an aqueous extracts (AE) due to higher total phenolic and flavonoid contents. Specific analysis tracked total flavonoids was almost five times higher in EE compared to AE [29]. The most abundant phenolic constituents in EE were gallic acid while this compound with the other three compounds, the catechin, ellagic acid and quercetin were found in a moderate amount in the AE. Other phenolics detected with significant amounts were chlorogenic acid, vanillic acid, caffeic acid, epicathecin and kaempferol [29]. In earlier studies, phenolics (gallic acid) and flavonoids have also found in AE and EE of PS seeds [36, 37].
Hasim et al. [23] conducted a qualitative phytochemical screening of PS Hassk peels extract and reported that the n-hexane extract contained saponins, flavonoids, tannin and steroids. The ethyl acetate and ethanol extracts contained similar compounds of alkaloids, saponins, flavonoids, tannins and triterpin except for flavonoids, these compounds were not detected in the ethanol extract [23]. Ghasemzadeh et al. [24] reported that PS seeds collected from different locations in Malaysia contain alkaloids, terpenoids, flavonoids and phenolic compounds. Six flavonoid compounds (quercetin, rutin, kaempferol, catechin, luteolin and myricetin) were identified from PS seed extracts. Five phenolics (gallic acid, caffeic acid, ferulic acid, trans-cinnamic acid and p-coumaric acid) were also identified. The study indicated that the phytochemical composition and biological activity of PS seeds vary significantly and it depends on where the PS tree is grown. Their results showed highest antioxidant and antibacterial activities in PS seeds grown in Perak followed by Negeri Sembilan and Johor states of Malaysia [24].
Mustafa, et al. [38] identified quercetin in the hexane and ethyl acetate extract at 5.84 mg/100g dried empty pods of PS but none of flavonoid compounds identified in the methanol extracted samples in study conducted by Miean, et al. [39]. Notwithstanding, a preliminary phytochemical screening revealed the methanolic extract of PS empty pods contained alkaloid, flavonoids, tannins, phenolic compounds and saponin with flavonoids and tannins been the most prominent compounds [27]. Santha Maria, et al. [40] reported on the presence of flavonoids in ethanol extract of PS pods. Recent study by Sonia et al. [26] identified alkaloid, saponin, steroid, flavonoid, tannin, coumarins, quinines, phenols, terpenoid and other compounds as well in the extract of methanol, hydromethanolic and aqueous of PS seeds. The chromatographic profile of polyphenol compounds which comprising of gallic acid, ellagic acid, catechin and quercetin were detected by Kamisah and co-workers at 254 nm [4]. These compounds were identified in the methanolic extract of PS pods and the extract contained 65.39 mg quercetin per 100 g dry PS pods extract. They also reported on 17 peaks detected from the LC-MS chromatogram where the major compounds were epigallocatechin gallate, quercetin, epicatechin/cathecin, coutaric acid, tangeritin and apigenin. However, two other major peaks could not be identified. Recent study by Mustafa, et al. [38] reported on identification of quercetin alone at concentration of 5.84 mg/100 g dry weight of ethyl acetate extract of PS pods.
Phenolic acids account for about a third of the polyphenolic compounds in our diet and in all other plant materials inclusive in pods and seeds of PS, but are particularly abundant in acidic-tasting fruits [33]. Phenolic acid consists of two subgroups; the hydroxybenzoic acids, which in common to have C6-C1 structure (gallic, p-hydroxybenzoic, protocatechuic, vanillic and syringic acids) while hydroxyl cinnamic acids, the aromatic compounds with a three-carbon side chain (C6-C3) (cafeic, ferulic, p-coumaic and sinapic acids) [35, 41]. Eight phenolic acids were identified in PS (gallic acid (GA), ellagic acid (EA), ferulic acid (FA), chlorogenic acid (CGA), vanillic acid (VA), Caffeic acid (CA), trans-cinnamic acid and p-coumaric acid) are some common phenolic acids [33] and structures of these compounds are shown in Figure 2.
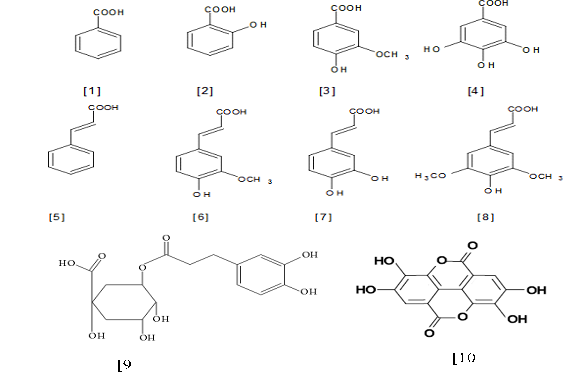
Figure 2: Structures of the important naturally occurring phenolic acids in PS. (Hydroxybenzoic acid are 1-Benzoic acid, 2-Salicyclic acid, 3-Vaininilic acid, 4-Gallic acid; Hydroxycinnamic acid are 5-Cinnamic acid, 6-Ferulic acid, 7-Sinapic acid, 8-Caffeic cid) [16], 9-chlorogenic acid [45], 10-ellagic acid [91]).
GA, (3,4,5-trihydroxybenzoic acid), a naturally occurring low molecular weight tri-phenolic compound, has been suggested to possess strong antioxidant activities. This compound provides effective protection against oxidative damage caused by reactive species often encountered in biological systems. These species include hydroxyl (HO?), superoxide (O2??), and peroxyl (ROO?) and the nonradicals, hydrogen peroxide (H2O2) and hypochlorous acid (HOCl). In addition, GA derivatives (GADs) can exhibit both antioxidant and prooxidant characteristics, displaying a dual edge sword behavior [42]. Another compound, EA is an increasingly popular dietary supplement and exhibits powerful anticarcinogenic and antioxidant properties [43]. The constituent is an active agent to induce vasorelaxation, oxygen scavenging, hypolipidemic, antiinflamatory and anticarcinogenic activities either in-vitro or in-vivo in various animal preparation [43]. FA, 4-hydroxy-3-methoxycinnamic acid, is widely distributed in beverages, fruits, vegetables, cereals, flowers and nuts. It is a caffeic acid derivative formed by the action of the enzyme caffeate O-methyltransferase [41]. FA is recognized for its ability to remove free radicals. As a result, its? potential therapeutic effects cover in the medication in either infectious or noninfectious diseases such as cancer, diabetes and cardiovascular diseases, hepatic and antimicrobial and anti-inflammatory activities [41, 44] and great attention is now directed toward its incorporation into cosmetic emulsions for topical application [41].
CGA is another important phenolic acid identified in PS and it is produced from the esterification of caffeic acid and L-quinic acid (Figure 2). It is also extensively scattered in coffee and fruits (apples, pears, etc.) and is among the important hydroxycinnamic acid derivatives in plants [41]. This compound exhibits many biological properties and reported in numerous publications that support its potential as antibacterial, antioxidant, and anticarcinogenic activities, particularly hypoglycemic and hypolipidemic effects, anti-inflammatory, antiviral, and anti-tyrosinase agentmetabolic disordered conditions [41, 45]. While VA is a widely used as flavoring and perfumery that corresponds to the oxidized form of the aldehyde vanillin [46]. CA is one of the most commonly determined in plants, especially in fruits, vegetables, mushrooms and herbs. It is biosynthesized by hydroxylation of p-coumaric acid. This compound covers a wide range of medicinal properties inclusive antioxidant, antitumor, anti-inflammatory, antimicrobial and antidiabetic activity [41]. This compound is also actively involved in plant physiology and mechanisms of stress tolerance primarily utilized by plants for the synthesis of lignin. The process ultimately caused cell walls thickening and increase the resistance to ion toxicity sodium and heavy metal stress. It also resolves the absorption of high energy radiations in mesophyll cells under drought stress. The mechanism involves the production of FA through the ethylation of CA catalyzed by O-methyltransferase [47].
Cinnamic acid and its derivatives were shown in Figure 3 and the most common are cinnamic acid, CA, FA, isoferulic acid, and p-hydroxycinnamic acid [48]. Among various biological activities, cinnamic acid and its derivatives are associated with the diabetes and its complications. These compounds act on various actions, including to promote insulin secretion, to improve pancreatic β-cell function, to inhibit hepatic gluconeogenesis, to enhance glucose uptake, to increase insulin signaling pathway, to delay carbohydrate digestion and glucose absorption, and to inhibit protein glycation and insulin fibrillation ([48]. While, hydroxycinnamic acids and their derivatives display antioxidant, anti-collagenase, anti-inflammatory, antimicrobial and anti-tyrosinase activities and act as a UV protective tool. These suggested that they can be utilized as anti-aging and anti-inflammatory agents, preservatives and hyperpigmentation-correcting ingredients [41]. p-Coumaric acid is a phenolic acid, which is synthesized mainly from tyrosine and phenylalanine. It is the main pathway in the synthesis of other phenolic acids, CA, CGA, rosmarinic and FA. p-coumaric acid and its conjugated forms showed properties, such as antioxidant, antimicrobial, antitumor, anti-inflammatory, antiplatelet aggregation and many other interesting health benefits. Their major properties are the potential for depigmentation, antioxidant, anti-collagenase, antimicrobial and anti-inflammatory activities. These were among the most important properties in the cosmeceutical industries [41].
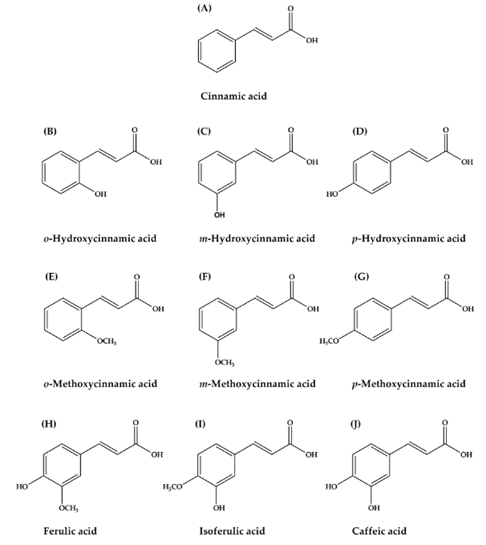
Figure 3: The chemical structure of cinnamic acid and its derivatives. (A) Cinnamic acid; (B) o-Hydroxycinnamic acid; (C) m-Hydroxycinnamic acid; (D) p-Hydroxycinnamic acid; (E) o-Methoxycinnamic acid; (F) m-Methoxycinnamic acid; (G) p-Methoxycinnamic acid; (H) Ferulicacid (I) Isoferulic acid; (J) Caffeic acid [48].
Other than phenolic acids, flavonoids are another group of phenolic constituents identified among major phytochemicals in PS. Eight flavonoids were identified in its pod and seed extracts; catechin, epicathecin, quercetin, kaempferol, rutin, luteolin and myricetin [24, 29, 38] (Figure 4). Flavonoids are low molecular weight compounds arranged in a C6-C3-C6 or C15, configuration consist of two aromatic rings join by a three carbon bridges usually in the form of three heterocyclic rings [35] or heterocyclic skeleton of flavan (2-phenylbenzopyrane) [22, 49]. Variation in these heterocyclic rings resulted in variety of flavonoid classes. The heterocyclic rings condensed with the benzene ring, is either flavones and flavonols or flavanones and flavan-3-ols. These position divides the flavonoids into two classes, which are flavone and isoflavone. Most natural flavonoids associated with sugar in conjugated form and may be characterized as monoglycosidic, diglycosidic, etc. The glycosidic linkage is normally located at position 3 or 7. While the carbohydrate unit can exist in L-rhamnose, D-glucose, glucorhamnose, galactose or arabinose [16].
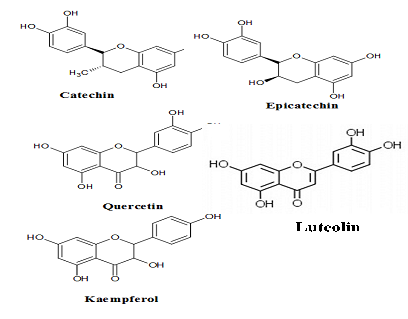
Figure 4: Some flavonoid compounds identified in PS seeds and pods.
Major classification of flavonoids were chalcones, flavones, flavonols, flavandiols, proanthocyanidins and their derivatives anthocyanidins, condensed tannins and aurones [16, 22, 32, 50] (Figure 5). Flavones and flavonols are major compounds occurred in plants and were structurally diverse [32, 35]. These compounds are widely distributed in nearly all plant species. Recently, more than 9,000 flavonoid compounds have been identified. Majority of them have been reported to possess many useful properties, including anti-inflammatory, oestrogenic, enzyme inhibition, antimicrobial, antiallergic, vascular and cytotoxic antitumor activity [22, 32, 49, 51, 52]. For later properties, flavonoids could prevent through blocking of several points in the progression of carcinogenesis. Those points include cell transformation, invasion, metastasis, and angiogenesis. This carcinogenesis is progressing through inhibiting kinases, reducing transcription factors, regulating the cell cycle, and inducing apoptotic cell death [53].
Epicathecin is also reported as major flavonoid compounds in green tea (Camellia sinensis) and comprises of about 30-40 % of extractable solid from dried green tea leaves [54, 55]. Major catechins identified are epicatechin, epicatechin-3-gallate, epigallocatechin, and epigallocatechin-3-gallate [54]. Bitter flavor of tea is attributed from the presence of compounds such as oligomeric quinines which are products of catechin derivatives during tea fermentation and also from the quercetin, kaempferol and myrecetin, the non-water-soluble flavonoids [54]. Epicatechins reduces the risks of diabetes mellitus and cardiovascular diseases. It also reduced the pharmalogical effect such as anti-hyperlipidemic, anti-inflammatory, antioxidative effects, anticarcinogenic, and cytoprotective [54, 55]. These flavonoids can be used individually or in combination with other synthetic drugs and antibiotics as therapeutic agents. These will produce cost effective, highly biocompatible and low toxicity of new generation phytopharmaceuticals [55]. On the other hand, luteolin is a glycosylated in plants, and the glycoside is hydrolysed to form free luteolin during adsorption. Plants rich in luteolin have been used as Chinese traditional medicine for hypertension, inflammatory diseases and cancer [53]. While, rutin (3,3?,4?,5,7-pentahydroxyflavone-3-rhamnoglucoside), is a glycoside comprising of flavonolic aglycone quercetin together with disaccharide rutinose. It has been reported in many pharmacological activities, for example, antioxidant, cytoprotective, vasoprotective, anticarcinogenic, neuroprotective and cardioprotective activities [56, 57]. Rutin is also used in the treatment of chylothorax in dogs [57].
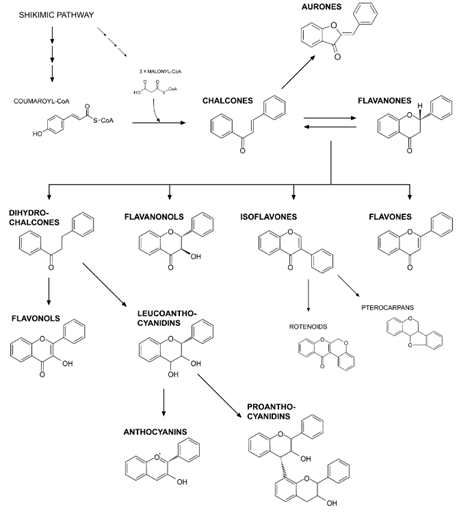
Figure 5: Their biosynthesis pathway is initiated with the condensation of one p-coumaroyl-CoA molecule with three molecules of malonyl-CoA. This is to produce chalcone (4', 2', 4', 6'-tetrahydroxychalcone) and catalyzed by chalcone synthase (CHS). The next step is isomerization of chalcone to flavanone through enzymatic reaction (chalconeisomerase-CHI). Onwards, the pathway branches to form several different flavonoid classes. These classes include aurones, dihydrochalcones, flavanonols, isoflavones, flavones, flavonols, leucoanthocyanidins, anthocyanins and proanthocyanidins [51].
Quercertin give colors to plants, most often the brilliant citron yellow and this compound is insoluble in water but quite soluble in alcohol [32, 58]. Quercetin is 3,3?,4?,5,7-pentahydroxyflavanone or its synonym 3,3?,4?,5,7-pentahydroxy-2-phenylchro- men-4-one. It has hydroxyl group (OH) attached at positions 3,5,7,3?, and 4?. Kaempferol is different from quercetin as it lacks of the OH group at position 3?. While, myricetin has an extra OH group at position 5? [58]. Furthermore, quercetin is an aglycone which is lacking of attached sugar [58]. In addition, quercetin is an aglycone which is lacking of attached sugar [58]. In food, quercetin is bounded with sugars, phenolic acids, alcohols etc. [58]. This compound with its derivatives has been studied for their pharmacological properties including, antiviral, antioxidant, anticancer, antimicrobial, anti- inflammatory, neurological effects, cardiovascular, and hepatoprotective [58]. While, kaempferol supplementing body?s antioxidant defense against free radicals, which promote the development of cancer. At the molecular level, this compound could modulate a number of key elements in cellular signal transduction pathways. These are linked to apoptosis, angiogenesis, inflammation, and metastasis [59]. Kaempferol also significantly inhibits cancer cell growth and angiogenesis and induces cancer cell apoptosis. On the other hand, kaempferol appear stop reserve normal cell viability and in some cases exerting a protective effect [59].
2.2 Terpenoids
Santha Maria and co-workers [40] reported on the presence of terpenoid active compounds when conducting a screening on the ethanol extract of PS pods. In earlier specific study, terpenoid compounds were identified in PS seeds at different temperature and pressure using gas chromatography/time of flight mass spectrometry from the extraction by supercritical carbon dioxide: ?-sitosterol and squalene were identified in all conditions studied, while campesterol, stigmasterol, stigmasterolmethylether and lupeol were identified in certain condition of temperature and pressure of supercritical fluid extraction (SFE) system [60]. In addition, the highest extracted yield was achieved at the smallest percentage of oil yield extracted at low pressure and high temperature conditions [60, 61]. Later, squalene, campesterol, stigmasterolmethylether, stigmasterol, stigmastan-6, 22-dien, 3, 6, 3dehidro-(3-sitosterol) were also identified in PS seeds by SFE in hexane extract [31]. In earlier study, stigmast-4-en-3-one was identified in PS empty pod andthis compound is assumed as a necessary intermediate in the metabolism of ?-sitosterol [30]. Similar study also showed that the hypoglycaemic effect of PS seed is due to the synergistic action of ?-sitosterol and stigmasterol [62]. Some of terpenoids identified in PS were shown in Figure 6.
Terpenes/terpenoids constitute one of the largest classes of natural products that can arise from the biochemical transformation of the prenyldiphosphate, a polymer chain of prenyl units. [63]. Various lengths of this starter units are the backbones of all terpenes/terpenoids. The ionization of the diphosphate group, caused the remaining allylic carbocation intermediates to be coaxed into complex chemical cascades. Thus, will lead to diverse linear and cyclized hydrocarbon backbones [64]. These can then be further modified with a wide range of functional groups, for example, alcohol and ketones; and other substituent additions as well; sugars and fatty acids). Terpenoids can be classified as hemiterpenes, monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), sesterpenes (C25), triterpenes (C30), tetraterpenes (C40) and polyterpenes (>C40) based on the basis of C5 units [63, 65, 66]. As these chemicals are diverse, they are widely used in the industry for flavors, fragrances, high grade lubricants, biofuels, agricultural chemicals and medicines [64].
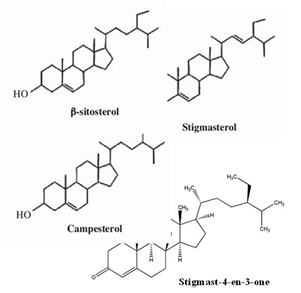
Figure 6: Sterols identified in PS seed and pods.
Terpenoids has numerous therapeutic properties including anticancer, antimicrobial, antifungal, antiparasitic, antiallergenic, antihyperglycemic, anti-inflammatory and immune-modulatory properties [57]. ?-sitosterol which identified in different extraction conditions in PS is among several terpenes understudies of clinical trials, to prevent and relieve prostate symptoms, to lower cholesterol and also suggested to help strengthening the immune system [66]. It is also usually used for treatment of rheumatoid arthritis, tuberculosis, cervical cancer and hair loss [68]. Another compound, the squalene is considered a potent chemo preventive and chemotherapeutic agent. This compound could inhibit the tumor growth in the colon, skin, lung and breast. It can also stimulate the immune system for the application of drugs in the treatment of diseases such as HIV, H1N1, leukemia, papilloma and herpes [69]. These compounds are dispensable for plant growth and development, but are indispensable for plant survival [63]. Those terpenoids have huge structural diversity, and intraspecific variability within plant taxa, even among genotypes. This enables the plants to secure individual habitats from natural enemies, competitors and friends [65].
2.3 Alkaloids
Alkaloids are other compounds identified when researchers screened phytochemicals in either the seeds, seed coat or pods/bark of PS [23-28]. Liliwirianis and co-workers [25] reported on the present of alkaloid in the acidic aqueos phase of the PS seed, bark (pods) and its seed coat when conducted a screening on phytochemical in ulam and fruits from Malaysia. While Sihombing and co-workers [28] following similar methodology and reported the presence of alkaloids in PS peel samples. Current study by Ghasemzadeh and co-workers [24] reported the present of alkaloids in the ethanol extract of PS seeds collected from three states of Malaysia (Perak, Negeri Sembilan and Johor). Alkaloids were also determined in the methanolic extract [23, 27], ethanol and ethyl acetate extracts [23] of PS pods. While, Sonia and co-workers [26] analyzed seeds of PS in water, methanol and hydromethanolic extracts and denotes an average of alkaloids presence in the later extract compared to the other two extracts.
All analysis of alkaloids in PS identified currently was at screening or qualitative stage, none was conducted quantitatively. This is due to various chromatography methods need to be involved to isolate and purified the extract. In addition, identification of the isolated alkaloids was based on their spectral data and various spectroscopic methods [70]. Unless the study aimed to characterize these compounds in specific/selected plants is conducted as alkaloids vary in polarity and thermal stability. Their separation is still remaining a big challenge for the process of identification and characterization of the compounds. Their efficient isolation, determination and characterization process may need a combination of conventional chromatography methods (thin layer chromatography, column chromatography, etc.) and instrumentation technologies (CTLC, HLPC, LCMS etc.) [71]. Major methods for characterization of alkaloids involved spectroscopic with MS and NMR or in combination with chromatographic methods [70]. Besides that, non-chromatography techniques alone, such as immunoassay, screening assay and fourier-transform infrared spectroscopy (FTIR) is been used to obtain and facilitate the identification process [72].
Nitrogen atom in the heterocyclic ring of alkaloids are derived from amino acids. The main role of alkaloids in plants toxicity is to protect against predators and pathogens [73]. These compounds have been classified into different categories based on their biosynthetic precursor and heterocyclic ring system. Different types of alkaloids are inclusive indole, tropane, piperidine, purine, imidazole, pyrrolizidine, pyrrolidine, quinolizidine and isoquinoline alkaloids [70, 73, 74]. Different alkaloids have their own specific properties and act useful for the medicinal purposes. For example, indole alkaloids associated with about 2,000 compounds inclusive vinblastine and vincristine, which are often used as anticancer drugs. Scopolamine, hyoscyamine, cocaine and atropine are among tropane alkaloids are known to have anticholinergic activities. Whereas, quinoline possessed important biological activities viz. antimalarial, antibacterial, antifungal anthelmintic, cardiotonic, anticonvulsant, anti-inflammatory and analgesic activity. Isoquinoline exhibit biological activities like antihyperglycemic, antitumor and antibacterial activity and etc. [70]. Pure extracted plant alkaloids and their synthetic derivatives are utilized as common medicinal ingredients for their analgesic, antispasmodic, bactericidal effects and some alkaloids are used as an antiseptic due to its antibiotic activity [74]. Recent review focused on the potential efficacy of plant-derived alkaloids which possesses therapeutic effects against several neurodegeratives disorder (NDDs) by attenuating the development of NDDs through their vast mode of action. These actions may inhibit the activity of acetyl-cholinesterase (AchE) enzyme or increasing the level of gamma-aminobutyric acid (GABA) or acting as antagonist of NMDA and many more [75].
2.4 Saponins
Determination of saponins in PS also involved only screening or qualification phases in all studies identified. Phytochemical screening of PS revealed saponins present in peels/pods of PS [23, 27, 28], seed extract [26, 67] and its seed coats [25]. In most studies, saponin was present in methanolic extracts, only few studies reported on positive identification of these compounds in ethanolic, n-hexane and ethyl acetate extracts [23, 26, 27, 28, 66].
Foam test is a qualitative standard protocol for detection of saponins [26]. This natural detergent is made from a combination of hydrophobic aglycone backbone and hydrophilic sugar molecules that allows foaming and emulsifying properties of the compounds [75, 76]. Saponins with one sugar chain have the best foaming characteristics compared to with two or three chains, though saponins without foaming character have also been observed [76]. Saponins consist of a glycan moiety linked to aglycon or genin or sapogenin (triterpenoid saponins with 30 carbon atoms) and steroidal saponins (27 carbon atoms with a 6-ring spirostane or a 5-ring furostane skeleton) (Figure 7) [75]. Based on the carbon skeleton of the aglycon, saponins are sometimes further classified into 12 main classes (dammaranes, tirucallanes, lupanes, hopanes, oleananes, 23-nor oleananes, taraxasteranes, ursanes, cycloartanes, lanostanes, cucurbitanes, and steroids) [75]. Plant-derived triterpenoid and steroidal saponins have been used in the production of steroid hormones in the pharmaceutical, food additives, fire extinguishers and other industries. Other interesting biological applications include their use in anti?inflammatory, hypo?cholesterolemic, immune?stimulating remedies, cytotoxicy, antitumor, antimutagenic, antiviral, cardiac activities, hyperglycemic/hypolipidaemic activities, antifungal/antimicrobial, etc. [76-78].
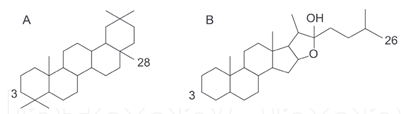
Figure 7: Structures of (A) triterpenoid and (B) steroidal saponins [76].
2.5 Cyclic polysulfides/organosulfur compounds
Cyclic polysulfides/organosulfur compounds which were found in plant seeds are accountable for a significant pungent smell and taste. Earlier studies indicated that 1,2,4-trithiolane, 1,2,4,6-tetrathiane, lenthionene, 1,2,4,5,7,8-hexathionine and 1,2,4,6,7- or 1,2,4,5,7-pentathiocanewere the major constituentsfrom a chloroform extract of PS crushed beans that incubated in water [9] (Figure 8). Later, Suvachittanont and co-workers [79] reported on the formation of thiazolidine-4-carboxylic acid, a thioproline was significantly increased after boiling of PS seeds. In year 2001, Miyazawa and Osman [80] reported on the abundance of hydrogen sulfide in the headspace constituent of PS. Salman and co-workers [60] also reported on the present of 1,2,4,5-tetrathiane and 1,2,3,5,6-pentathiepane (lenthionene) in PS seeds.
(1) 1,2,4-trithiolane; (2) 1,3,5-trithiane; (3) 3,5-dimethyl-1,2,4-trithiolane; (4) 1,2,4,5-tetrathiane; (5) 1,2,4,6-tetrathiepane; (6) 1,2,4,5-tetrathiocane; (7) 1,2,3,5,6-pentathiepane (lenthionine); (8) 1,2,3,4,5,6-hexathiepane; (9) 1,2,4,5,7,8-hexathionane.
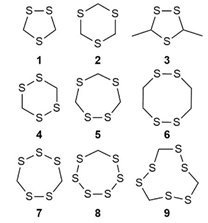
Figure 8: Cyclic organosulfide from PS [92].
Fr?rot and co-workers [81] reported that sulfur compounds make up the vast majority of the identified compounds and was classified into three categories: cyclic polysulphides, linear polysulphides and hetero sulphur compounds. 1,2,4-trithiolane was the major component for cyclic polysulphides followed by 1,2,4,6-tetrathiepane, 1,2,3,5-tetrathiane and lenthionine. They also reported for the first identification of 2,4-dithiapentane, 2,3,5-trithishexane and 2,4,6-trithiaheptane in cooked beans of PS (Figure 8). Other cyclic polysulfides that have been identified in PS were 1,2,3,4,5-pentathiane, 1,3,5,7-tetrathiocane, 1,2,3,4,6-pentathiepane, 1,2,3,4,5,6-hexathiepane, 1,2,3,4,6,7-hexathiocane or 1,2,3,5,6,7-hexathiocane, isomerix hexathionanes, 1,2,4,6-tetrathiepane, methyl1-1,2,4,5-tetrathiane and 3-methyl-1,2,4-trithiolane [81]. Linear polysulphides identified in PS seeds were 1,3-dithiabutane, 1,3,5-trithiahexane, 2, 4-dithiapentane, 2,5,7-trithiaheptane 2,4,5-trithiahexane. 1, 3-dithiabutane is a strong odorant that possessing an incredible strong alliaceous note formed via unstable methanedithioles [81, 82]. Sulphur compounds containing other heteroatoms identified in this study were 4,5-dihydrothiazole, 2,5-dihydrothiazole and 2,3-dihydrothiazole [81].
In a current study, thirteen sulphurous compounds were identified in unripe, mid-ripe and ripe PS, where during ripening these compounds increased from 7.1 to 20.13%. Major components are 1,2,4-trithiolane, methanethiol and hydrogen sulfide [83]. Djenkolic acid identified in PS is formed from the dithiocetal (S-C-S), might be formed from formaldehyde and two molecules of cycteine [81]. Blockage of urinary tubules is connected to low solubility of djenkolic acid in acid condition subsequently precipitate as crystals which can cause pain, haematuria and sometimes death to human [84]. This is especially so when ingestion of djenkol beans that contain large amount of the djenkolic acid, in the range of 0.3-1.3 g/100g wet weight or 93% of acid exist in a free state [85]. In traditional medicine, PS has been used for antibacterial and antifungal effects on kidney, ureter and urinary bladder and later was found to be the effects from cyclic polysulfides [84]. PS is also suggestd as potential functional food with H2S donation property where 1,2,4-trithiolane slowly release H2S donor through reaction with glutathione. During this reaction, the thiolate attacks the carbon which lead to an opening of the trithiolane ring. This resulting in conjugation of H2S and several glutathione-methylene-sulphides [86].
2.6 Tannin
Tannins are undesirable compounds when plant-parts are consumed as food but they are useful as a protection from biotic-stress and are also important in reinforcing plant tissues [87]. Tannins are polyphenolic secondary metabolites of higher plants. They were divided into four groups based on their structural characteristic: Gallotannins, ellagitannins, complex tannins and condensed tannins. Gallotannins is comprises of polyphenolic and a polyol residue, the simplest hydrolysable tannins. Most of these compounds contain a polyol residue derived from D-glucose. Ellagitannins are derived from gallotannins by the oxidative coupling of at least two galloyl units. Complex tannins are a combination of gallotannin or an ellagitannin unit and a catechin unit. While, condensed tannins are oligomeric and polymeric proanthocyanidins that consist of coupled flavan-3-ol (catechin) units [88] (Figure 9).
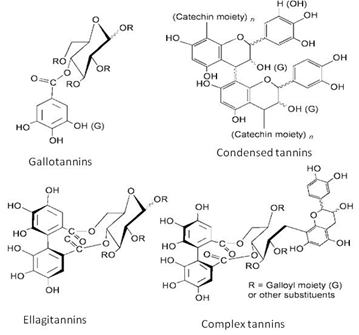
Figure 9: Classification of tannins [88].
In PS, phytochemicals inclusive tannins were screened from the plant extracts using standard procedure, the ferric chloride test [26]. This is a simple method for tannin quantifications as these compounds are differ in their sensitivity and specificity. Due to this fact, condensed tannins are the easiest and safest tannin group to be quantified, since the spectrophotometric HCl?butanol assay is highly specific to this group. The test depolymerised plant condensed tannins into colourful monomeric units that are then detected and quantified at approximately 550 nm [89]. Balaji and co-workers [27] reported on positive identification of tannins in methanolic extracts of PS, which is contradicted to findings from Chanu and co-workers [67] where identification of tannins were in aqueous extracts only. Hasim and co-workers [23] reported on positive identification of tannins in all three extracts tested (n-hexane, ethyl acetate and ethanol) with abundance identification in 70% of ethanol extracts. While Sonia and co-workers [26] denoted an average identification of tannins in hydromethanolic extract compared to the aqueous and methanol extract.
Higher amount of tannins was identified in antimicrobial herbal which selected from the vegetation in Northern Brazil compared to antidiarrheal and hypoglycemia and/or antidiabetic activities [90]. Tannins have been a target compound in many studies on antioxidant and anti carcinogens activities and there is ample proof of their anti-inflamatory, cicatrizant and anti-HIV functions [87].
3. Conclusion
Parkia speciosa, or stink bean, is a Malaysian favourite culinary ingredient. The seed of PS contains important chemical and medicinal properties including its pods that always been discarded as a waste. In this present paper, we have reviewed the relevant literature to congregate the phytochemicals secondary metabolites and pharmacological information on PS. PS seeds and its pods are rich in biologically active compounds such as phenolic acids, flavonoids, alkaloids, saponins, terpenoids, cyclic polysulfides and tannins. Phenolic constituents specifically, the gallic acid is the most abundant phytochemicals identified in PS pods. This is followed by the catechin, ellagic acid and quercetin. The terpenoids, ?-sterol, squalene, campesterol and stigmasterol were identified in PS seeds. Cyclic polysulfides, compounds that responsible for a strong pungent smell and taste in PS were also identified in PS seeds, with 1,2,4-trithiolane being the most abundant compound identified.
Other cyclic polysulfides which were also among most identified in PS seeds were 1, 2, 4, 6-tetrathiepane, 1, 2, 3, 5-tetrathiane and lenthionine. Alkaloids, saponins and tannins were only reported in qualitative phytochemical screening of PS in all articles reviewed. The alkaloids and saponins were reported to be abundant in the methanolic extracts but tannins were in ethanol extracts. These phytochemicals are beneficial in terms of antioxidant activity, hypoglycemic activity, antitumor/antimutagenicity, antimicrobial activity and current research showed some evidence of their effects on the cardiovascular system. Many scientific studies have been performed to prove on antioxidant, hypoglycemic activity as well as antitumor/antimutagenicity by different model either in vitro or in vivo. The studies also revealed that plants with high antioxidant activity contained high total phenolic and flavonoid compounds while for hypoglycemic effect is from the synergistic effect of terpenoid compounds; the ?-sterol and stigmasterol. The antimicrobial properties is possesses from both the seed and pod of PS, however, the spectrum of the activity depends on the type of the extract.
Acknowledgement
We would like to thank the Director General of Health Malaysia for his permission to publish this Chapter. We are also grateful to the Director of the Institute for Medical Research (IMR), Kuala Lumpur, for the continuous support and encouragement. We thank Ms Sumarni Mohd Ghazali from Epidemiology and Bio-Statistic Unit, IMR for editing and proof reading the text.
Author Contributions
Nurul Izzah Ahmad and Salina Abdul Rahman drafted the manuscript; Leong Yin Hui and Nur Hayati Azizul edited and commented on the manuscript. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Gui JS, Jalil J, Jubri Z, et al. Parkia speciose empty pod extractr exerts anti-inflamatory properties by modulating NFkB and MAPK pathways in cardiomyocytes exposed to tumor necrosis factor-?. Cytotechnol 71 (2019): 79-89.
- Kamisah Y, Zuhair JSF, Juliana AH, et al. Parkia speciose empty pod prevents hypertension and cardiac damage in rats given N(G)-nitro-L-arginine methyl ester. Biomedicine and Pharmacotherapy 96 (2017): 291-298.
- Zaini NA, Mustaffa F. Review: Parkia speciose as valuable, miracle of nature. Asian J Med Health 2 (2017): 1-9.
- Kamisah Y, Othman F, Qodriyah HMS, et al. Parkia speciose Hassk.: A Potential Phytomedicine. Evidence-Based Complement Altern Med (2013).
- Chhikara N, Devi HR, Jaglan S, et al. Bioactive compounds, food application and health benefits of Parkia speciosa (stinky beans): a review. Agric and Food Secur 7 (2018): 46.
- Nurul Izzah A, Aminah A, Pauzi A, et al. Patterns of fruits and vegetable consumption among adults of different ethnics in Selangor, Malaysia. Inter Food Res J 19 (2012): 1095-1107.
- Nurul Izzah A, Aminah A, MD Pauzi A, et al. Tabiat pengambilan ulam-ulaman di kalangan orang dewasa pelbagai ethnic di Selangor. J Sains Kes Malaysia 8 (2010): 27-35.
- Norhashila I. Preliminary study on the marketing of pickles in Malaysia. J of Agribusiness Marketing 7 (2015): 60-82.
- Gmelin R, Susilo R, Fenwick GR. Cyclic polysulphides from Parkia speciosa. Phytochemistry 20 (1981): 2521-2523.
- Azliza MA, Ong HC, Vikineswary S, et al. Ethno-medicinal resources used by the Temuanin Ulu Kuang Village. Studies on Ethno-Medicine 6 (2012): 17-22.
- Milow P, Ghazali, NH, Mohammad NS, et al. Characterization of plant resource at Kampung Parit Tok Ngah, Perak, Malaysia. Scientific Research and Essays 6 (2011): 2606-2618.
- Samuel AJSJ, Kalusalingam A, Chellappan DK, et al. Ethnomedical survey of plants used by the Orang Asli in KampungBawong, Perak, West Malaysia. J Ethnobiol Ethnomed 6 (2010): 5.
- Fatimah I. Green synthesis of silver nanoparticles using extract of Parkia speciosa Hassk pods assisted by microwave irradiation. J Advanced Res 7 (2016): 961-969.
- Egamberdieva D, Mamedov N, Ovidi E, et al. Phytochemical and pharmacological properties of medicinal plants from Uzbekistan: A review. J Med Active Plants 2 (2016): 1-4.
- Chew YL, Chan EWL, Tan PL, et al. Assessment of phytochemical content, polyphenolic composition, antioxidant and antibacterial activities of Leguminosae medicinal plants in Peninsular Malaysia. BMC Complementary and Alternative Med 11 (2011): 12.
- Saxena M, Saxena J, Nema R, et al. Phytochemistry in medicinal plants. J Pharmacognosy and Phytochem 1 (2013): 168-182.
- Tariq AL, Reyaz AL. Significances and importance of phytochemical present in Terminalia chebula. Int J Drug Dev and Res 5 (2013): 256-262.
- Wadood A, Ghufran M, Jamal SB, et al. Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem and Anal Biochem 2 (2013): 144.
- Harborne JB. Classes and functions of secondary products from plants, in Chemicals from Plants?Perspectives on Plant Secondary Products. In Eds.: Walton JN and Brown DE. Imperial College Press, London, UK (1999): 1-25.
- Dillard CJ, German JB. Review phytochemicals: nutraceuticals and human health. J Sci Fd Agric 8 (2000): 1744-1756.
- Sikolia SF, Omondi S. Phytochemical analysis of some selected plants and families in the University Botanic Garden of Maseno, Kenya. IOSR J Pharma Biol Sci 12 (2017): 31-38.
- Kamboh AA, Arain MA, Mughal MJ, et al. Flavonoids: Health promoting phytochemicals for animal production-a review. J Animal Health and Prod 3 (2015): 6-13.
- Hasim, Faridah DN, Kurniawati DA. Antibacterial actviry of Perkia speciosa Hassk peel to Escherichia coli and Staphylococcus aureus bacteria. J Chem Pharma Res 7 (2015): 239-243.
- Ghasemzadeh A, Jaafar HZE, Mohamad Bukhori MF, et al. Assessment and comparison of phytochemical constituents and biological activities of bitter bean (Parkia speciosa Hassk.) collected from different locations in Malaysia. Chem Central J 12 (2018): 12.
- Liliwirianis N, Musa NLW, Wan Mohd Zain WZ, et al. Preliminary studies on phytochemical screening of ulam and fruit from Malaysia. E-J Chem 8 (2011): 285-288.
- Sonia N, Dsouza MR, Alisha. Pharmacological evaluation of Parkia speciosa Hassk. For antioxidant, anti-inflammatory, anti-diabetic and anti-microbial activities in vitro. Inter J Life Sci Special issue A 11 (2018): 49-51.
- Balaji K, Nedumaran SA, Devi T, et al. Phytochemical analysis and in vitro antioxidant activity of Parkia speciosa. Inter J Green Pharmacy 9 (2015): 50-54.
- Sihombing JR, Dharma A, Chaidir Z, et al. Phytochemical screening and antioxidant activities of 31 fruit peel extract from Sumatera, Indonesia. J Chem and Pharma Res 7 (2015): 190-196.
- Ko HJ, Ang LH, Ng LT. Antioxidant activities and polyphenolic constituents of bitter bean Parkia speciosa. Int J Food Prop 17 (2013): 1977-1986.
- Fathaiya J, Suhaila M, Md Nordin L. Hypoglycemic effect of Stigmast-4-en-3-one, from Parkia speciosa empty pods. Food Chem 54 (1995): 9-13.
- Nik Norulaini NAR, Salman Z, Sarker MZI, et al. Profile of Parkia speciosa Hassk metabolites extracted with SFE using FTIR-PCA method. J Chin Chem Soc 58 (2011): 1-9.
- Kulbat K. The role of phenolic compounds in plant resistence. Biotech and Food Sci 80 (2016): 97-108.
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med and Cellular Longevity 2 (2009): 270-278.
- Kumar H, Choudhary N, Varsha, et al. Phenolic compounds and their health benefits: A review. J Food Res Technol 2 (2014): 45-59.
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by- products: Antioxidant activity, occurance and potential uses. Food Chem 99 (2006): 191-203.
- Reihani SFS, Azhar ME. Antioxidant activity and total phenolic content in aqueous extracts of selected traditional Malay salads (Ulam). Int Food Research J 19 (2012): 1439-1444,
- Maisuthisakul P, Pasuk S, Ritthiruangdej P. Relationship between antioxidant properties and chemical composition of some Thai plants. J Food Compos Anal 2 (2008): 229-240.
- Mustafa NH, Ugusman A, Jalil J, et al. Anti inflammatory property of Parkia speciosa empty pod extract in human umbilical vein endothelial cells. J Applied Pharma Sci 8 (2018): 152-158.
- Miean KH, Suhaila M. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem 49 (2001): 3106-3112.
- Santha-Maria M, Devarakonda S, Ajay Kumar TV, et al. Anti-ulcer activity of ethanol extract of Perkia speciosa against indomethacin induced peptic ulcer in albino rats. IJPSR 6 (2015): 895-902.
- Toufiq O, Gonzalez-Paramas AM, Barreiro MF, et al. Hydroxycinnamic acids and their derivatives: cosmeceutical significance, challenges and future perspectives, a review. Molecules 22 (2017): 281.
- Badhani B, Sharma N, Kakkar R. Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv 5 (2015): 27540-27557.
- Usta C, Ozdemir S, Schiariti M, et al. The pharmacological use of ellagic acid-rich pomegranate fruit. Int J Food Sci Nutr 64 (2013): 907-913.
- De Paiva LB, Goldbeck R, dos Santos WD, et al. Ferulic acid and derivatives: molecules with potential application in the pharmaceutical field. Brazillian J Pharma Sci 49 (2013): 398-411.
- Meng S, Cao J, Feng Q, et al. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evidence-Based Comple Altern Med (2013): 11.
- Almeida IV, Cavalcante FML, Vicentini VEP. Different responses of vanillic acid, a phenolic compound, in HTC cells: cytotoxicity, antiperoliferative activity and protection from DNA-induced damage. Genetics Molecular Res 15 (2016).
- Riaz U, Kharal MA, Murtaza G, et al. Prospective roles and mechanisms of caffeic acid in counter plant stress: A mini review. Pakistan J Agric Res 32 (2019): 8-19.
- Adisakwattana S. Cinnamic acid and its derivatives: Mechanisms for preventive and management of diabetes and its complication. Nutrients 9 (2017): 163.
- Asif M, Khodadadi E. Medicinal uses and chemistry of flavonoid contents of some common edible tropical plants. J Paramedical Sci 4 (2013): 119-138.
- Ferreyra ML, Rius SP, Casati P. Flavonoids: biosynthesis, biological functions and biotechnological applications. Frontier Plant Sci 28 (2012): 222.
- Mierziak J, Kostyn K, Kulma A. Flavonoids as important molecules of plant interactions with the environment. Molecules 19 (2014): 16240-16265.
- Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: An overview. The Scientific World J (2013).
- Lin Y, Shi R, Wang X, et al. Luteolin, a flavonoid with potentials for cancer prevention and therapy. Curr Cancer Drugs Targets 8 (2008): 634-646.
- Gupta V, Bansal P, Niazi J, et al. Phytochemistry and pharmacology of Camilla sinensis- A review. Annals Biol Res 1 (2010): 91-102.
- Rekshmyd?dharan S, Roy A. Epicathecin- nature extraordinary therapeutic agent: A review. Inter J Pharm Tech Res 5 (2013): 1816-1822.
- Ganeshpurkar A, Saluja AK. The pharmacological potential of Rutin. Saudi Pharma J 25 (2017): 149-156.
- Atanassova M, Bagdassarian V. Rutin content in plant products. J University Chem Technol Metallurgy 44 (2009): 201-203.
- Shah PM, Priya VV, Gayathri R. Quercetin-a flavonoid: A systematic review. J Pharma Sci and Res 8 (2016): 878-880.
- Chen AY, Chen YC. A review of the dietary flavonoid, kaempherol on human health and cancer chemoprevention. Food Chem 138 (2013): 2099-2107.
- Salman Z, MohdAzizi CY, NikNorul Aini NA, et al. Gas chromatography/time-of-flight mass spectrometry for identification of compounds from Parkiaspeciosa seeds extracted by supercritical carbon dioxide. (In) Proceedings of the First International Conference on Natural Resources Engineering and Technology (2006).
- Mohd Azizi CY, Salman Z, Nik Norul ain NA, et al. Extraction and identification of compounds from Parkia speciosa seeds by supercritical carbon dioxide. J Chem and Nat Resources Engine 2 (2008): 153-163.
- Fathaiya J, Suhaila M, Nordin L. Hypoglycemic effect of Parkia speciosa seeds due to synergistic action of B-sitosterol and stigmasterol. Food Chem 49 (1994): 339-345.
- De las Heras B, Rodriguez B, Bosca L, et al. Terpenoids: Sources, structure elucidation and therapeutic potential in inflammation. Current Topics Med Chem 3 (2003): 53-67.
- Jiang Z, KempinskiC, Chappell J. Extraction and Analysis of Terpenes/Terpenoids. Curr Protoc Plant Biol 1 (2016): 345-358.
- Yazaki K, Arimura G, Ohnishi T. Hidden terpenoids in plants: Their biosynthesis, localization and ecological roles. Plant Cell Physiol 58 (2017): 1615-1621.
- Singh B, Sharma RA. Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 5 (2015): 129-151.
- Chanu KV, Devi LG, Srivastava SK, et al. Phytochemical analysis and evaluation of anticancer activity of Parkia javanica seeds. The Pharma Innovation J 7 (2018): 5305-5311.
- Bishnoi VK, Kaushal K, Sharma AK, et al. Potential of beta sitosterol in medicinal plants used in BPH: A review. Inter J Chem Sci 1 (2017): 65-68.
- Lozano-Grande MA, Gorinstein S, Espitia-Rangel E, et al. Plant sources, extraction and uses of squalene. Inter J Agronomy (2018).
- Kaur R, Arora S. Alkaloids-Important therapeutic secondary metabolites of plant origin. J Crit Rev 2 (2015): 1-8.
- Poh-Yen K. Isolation and characterization of alkaloids extracted from medicinal plant in Malaysia: Alstonia macrophylla. Ann Chromatogr Sep Tech 2 (2016): 1024.
- Sasidharan S, Chen Y, Saravanan D, et al. Extraction, isolation and characterization of bioactive compounds from plants? extract. Afr J Tradit Complement Altern Med 8 (2011): 1-10.
- Matsuura HN, Fett-Neto AG. Plant alkaloids: Main features, toxicity and mechanism of action. Plant Toxin (2015).
- Roy A. A review on the alkaloids an important therapeutic compound from plants. Inter J Plant Biotech 3 (2017).
- Faizal A, Geelen D. Saponins and their role in biological processes in plants. Phytochem Rev 12 (2013): 877-893.
- Kregiel D, Berlowska J, Witonska I, et al. Saponins-based, biological-active surfactant from plants. In Eds.: Najjar R. Application and characterization of surfactants, 1st Edn, Saponin-based, Biological-active Surfactants from Plants, Publisher: In Tech (2017).
- Kareru PG, Keriko JM, Gachanja AN, et al. Direct detection of triterpenoid saponins in medicinal plants. Afr J Trad CAM 5 (2008): 56-60.
- Desai SD, Desai DG, Kaur H. Saponins and their biological activities. Pharma Times 41 (2009): 3.
- Suvachittanont W, Kurashima Y, Esumi H, et al. Formation of thiazolidane-4-carboxylic acid (thioproline), an effective nitrite-trapping agent in human body, in Parkia speciosa seeds and other edible leguminous seeds in Thailand. Food Chem 4 (1996): 359-363.
- Miyazawa M, Osman F. Headspace constituents of Parkia speciosa seeds. Natural Product Letters 15 (2001): 171-176.
- Frerot E, Velluz A, Bagnoud A, et al. Analysis of the volatile constituents of cooked petai beans (Parkia speciosa) using high-resolution GC/ToF-MS. Flavour Fragrance J 23 (2008): 434-440.
- Holzman GR, Susilo R, Gmelin R. Collisional activation study of cyclic polysulfides. Org. Mass Spectro 17 (1982): 165-172.
- Asikin Y, Kusumiyati, Shikanai T, et al. Volatile aroma components and MS-based electronic nose profiles of dogfruit (Pithecellobium jiringa) and stink bean (Parkia speciosa). J Advanced Res 9 (2018): 79-85.
- Fathaiya J, Suhaila M. Hypoglycemic effect of extracts of Petai Papan (Parkia speciosa, Hassk). Pertanika J. Trop. Agric. Sci 16 (1993): 161-165.
- Bunawan NC, Rastegar A, White KP, et al. Djenkolism: Case report and literature review. Inter Med Case Reports J 7 (2014): 79-84.
- Liang D, Bian J, Deng LW, et al. Cyclic polysulphide 1, 2, 3-trithiolane from stinky beans (Parkia speciosa Seeds) ia a slow releasing hydrogen sulphide (H2S) donor. J Funct Foods 35 (2017): 197-204.
- Furlan CM, Motto LB, dos Santos DYAC. Tannins: What do they represent in plant life?. In Eds.: Petridis GK. Tannin: Types, Food Containing and Nutrition. Nova Science Publisher, Inc (2010).
- Khanbabaee K, van Ree T. Tannins: Classification and Definition. Nat. Prod. Rep 18 (2001): 641-649.
- Salminen JP, Karonen M. Evolitionary ecology of plant defences, Chemical ecology of tannins and other phenolics: we need a change in approach. Funct Ecol 25 (2011): 325-338.
- De Queroz Siqueira CF, Cabral DLV, da Silva Peixoto Sobrinho TJ, et al. Levels of tannins and flovonoids in medical plants evaluating bioprospecting strategies. Evidence-Based Compl Alter Med (2012).
- Lin D, Xiao M, Zhao J, et al. An Overview of plant phenolic compounds and their importance in human nutrition and management of yype 2 diabetes. Molecules 21 (2016): 1374.
- Tocmo R, Liang D, Wang C, et al. Organosulfide profile and hydrogen sulfide-releasing capacity of stinky bean (Parkia speciosa) oil: Effects of pH and extraction methods. Food Chem 190 (2016): 1123-1129.


 Impact Factor: * 3.8
Impact Factor: * 3.8 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 