Acral Syringotropic Melanoma in situ with Eccrine Duct Hyperplasia
Article Information
Mitsuhiro Tachibana1*, Seiya Kitano2, Ayako Mikura3, Masakazu Fujimoto4, Kuniaki Ohara5, Miki Izumi6, Yutaka Tsutsumi1,7
1Department of Diagnostic Pathology, Shimada Municipal Hospital, Shimada, Shizuoka, Japan
2Department of Dermatology, Shimada Municipal Hospital, Shimada, Shizuoka, Japan
3Departmenty of Plastic Surgery, Shimada Municipal Hospital, Shimada, Shizuoka, Japan
4Department of Diagnostic Pathology, Kyoto University Hospital, Kyoto, Kyoto, Japan
5Dermatology Clinic, Akasaka Toranomon Clinic, Minato-ku, Tokyo, Japan
6Department of Medical Education, Showa University, Shinagawa-ku, Tokyo, Japan
7Diagnostic Pathology Clinic, Pathos Tsutsumi, Inazawa, Aichi, Japan
*Corresponding Author: Dr. Mitsuhiro Tachibana, Department of Diagnostic Pathology, Shimada Municipal Hospital, 1200-5 Noda, Shimada, Shizuoka 427-8502, Japan
Received: 12 March 2021; Accepted: 22 March 2021; Published: 31 March 2021
Citation: Mitsuhiro Tachibana, Seiya Kitano, Ayako Mikura, Masakazu Fujimoto, Kuniaki Ohara, Miki Izumi, Yutaka Tsutsumi. Acral Syringotropic Melanoma in situ with Eccrine Duct Hyperplasia. Archives of Clinical and Medical Case Reports 5 (2021): 355-360.
View / Download Pdf Share at FacebookAbstract
Acral syringotropic melanoma with eccrine duct hyperplasia (ASMEDH) is rare. We describe ASMEDH arising in the right sole of a Japanese woman aged 80’s. On a 15 x 10 mm-sized, irregular-shaped pigmented macule, the dermoscopy indicated pararrel ridges. The lesion was removed surgically. No nodal swelling was noted. Microscopically, the pigmented melanoma cells were distributed not only in the basal epidermis but also in the cutaneous sweat gland duct. The melanoma cells were positive for HMB45, melan A, S-100 protein, bcl-2, vimentin, CD5 and SOX10, but negative for cytokeratins (CKs) and adipophilin. Ki-67 labeling was around 10%. In the dermis, basal cells immunoreactive for CK 34βE12, CK5/6 and p40 surrounded the intraductally spreading melanoma cells and ductal lumina were frequently located in the center. Invasive growth was absent. The surgical margins were negative. The patient did not receive adjuvant chemotherapy, and she is doing well eight months after surgery. Our final diagnosis was ASMEDH, melanoma in situ, the third case in the world.
Keywords
Malignant melanoma in situ; Syringotropism; Eccrine duct hyperplasia
Malignant melanoma in situ articles; Syringotropism articles; Eccrine duct hyperplasia articles
Malignant melanoma in situ articles Malignant melanoma in situ Research articles Malignant melanoma in situ review articles Malignant melanoma in situ PubMed articles Malignant melanoma in situ PubMed Central articles Malignant melanoma in situ 2023 articles Malignant melanoma in situ 2024 articles Malignant melanoma in situ Scopus articles Malignant melanoma in situ impact factor journals Malignant melanoma in situ Scopus journals Malignant melanoma in situ PubMed journals Malignant melanoma in situ medical journals Malignant melanoma in situ free journals Malignant melanoma in situ best journals Malignant melanoma in situ top journals Malignant melanoma in situ free medical journals Malignant melanoma in situ famous journals Malignant melanoma in situ Google Scholar indexed journals melanoma articles melanoma Research articles melanoma review articles melanoma PubMed articles melanoma PubMed Central articles melanoma 2023 articles melanoma 2024 articles melanoma Scopus articles melanoma impact factor journals melanoma Scopus journals melanoma PubMed journals melanoma medical journals melanoma free journals melanoma best journals melanoma top journals melanoma free medical journals melanoma famous journals melanoma Google Scholar indexed journals Syringotropism articles Syringotropism Research articles Syringotropism review articles Syringotropism PubMed articles Syringotropism PubMed Central articles Syringotropism 2023 articles Syringotropism 2024 articles Syringotropism Scopus articles Syringotropism impact factor journals Syringotropism Scopus journals Syringotropism PubMed journals Syringotropism medical journals Syringotropism free journals Syringotropism best journals Syringotropism top journals Syringotropism free medical journals Syringotropism famous journals Syringotropism Google Scholar indexed journals environment articles environment Research articles environment review articles environment PubMed articles environment PubMed Central articles environment 2023 articles environment 2024 articles environment Scopus articles environment impact factor journals environment Scopus journals environment PubMed journals environment medical journals environment free journals environment best journals environment top journals environment free medical journals environment famous journals environment Google Scholar indexed journals Eccrine duct hyperplasia articles Eccrine duct hyperplasia Research articles Eccrine duct hyperplasia review articles Eccrine duct hyperplasia PubMed articles Eccrine duct hyperplasia PubMed Central articles Eccrine duct hyperplasia 2023 articles Eccrine duct hyperplasia 2024 articles Eccrine duct hyperplasia Scopus articles Eccrine duct hyperplasia impact factor journals Eccrine duct hyperplasia Scopus journals Eccrine duct hyperplasia PubMed journals Eccrine duct hyperplasia medical journals Eccrine duct hyperplasia free journals Eccrine duct hyperplasia best journals Eccrine duct hyperplasia top journals Eccrine duct hyperplasia free medical journals Eccrine duct hyperplasia famous journals Eccrine duct hyperplasia Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Intraepithelial lymphocytes articles Intraepithelial lymphocytes Research articles Intraepithelial lymphocytes review articles Intraepithelial lymphocytes PubMed articles Intraepithelial lymphocytes PubMed Central articles Intraepithelial lymphocytes 2023 articles Intraepithelial lymphocytes 2024 articles Intraepithelial lymphocytes Scopus articles Intraepithelial lymphocytes impact factor journals Intraepithelial lymphocytes Scopus journals Intraepithelial lymphocytes PubMed journals Intraepithelial lymphocytes medical journals Intraepithelial lymphocytes free journals Intraepithelial lymphocytes best journals Intraepithelial lymphocytes top journals Intraepithelial lymphocytes free medical journals Intraepithelial lymphocytes famous journals Intraepithelial lymphocytes Google Scholar indexed journals Colon Cancer articles Colon Cancer Research articles Colon Cancer review articles Colon Cancer PubMed articles Colon Cancer PubMed Central articles Colon Cancer 2023 articles Colon Cancer 2024 articles Colon Cancer Scopus articles Colon Cancer impact factor journals Colon Cancer Scopus journals Colon Cancer PubMed journals Colon Cancer medical journals Colon Cancer free journals Colon Cancer best journals Colon Cancer top journals Colon Cancer free medical journals Colon Cancer famous journals Colon Cancer Google Scholar indexed journals Microscopic colitis articles Microscopic colitis Research articles Microscopic colitis review articles Microscopic colitis PubMed articles Microscopic colitis PubMed Central articles Microscopic colitis 2023 articles Microscopic colitis 2024 articles Microscopic colitis Scopus articles Microscopic colitis impact factor journals Microscopic colitis Scopus journals Microscopic colitis PubMed journals Microscopic colitis medical journals Microscopic colitis free journals Microscopic colitis best journals Microscopic colitis top journals Microscopic colitis free medical journals Microscopic colitis famous journals Microscopic colitis Google Scholar indexed journals
Article Details
1. Introduction
Malignant melanomas on the volar skin (the glabrous skin of palms and soles) are most often encountered on the foot, especially the heel being the most common site. In the majority of cases, the melanomas have a distinct lentiginous pattern of growth reminiscent of a lentigo maligna pattern [1]. The average age of the patients of acral melanoma is between 60 and 70. The characteristic dermoscopic finding of acral melanoma is termed as the parallel ridge pattern [1]. Microscopically, the most common form of melanoma on the volar skin belongs to acral lentiginous melanoma [2]. Only two cases have been reported as acral syringotropic melanoma with florid eccrine duct hyperplasia [3]. Herein, we report a rare case of acral melanoma in situ arising on the sole in a Japanese female patient aged 80’s, showing a prominent pattern of syringotropism and eccrine duct hyperplasia.
1. Case Presentation
An 80’s-year-old Japanese woman fell down in her living room. She was admitted to Shimada Municipal Hospital, Shimada, Shizuoka, Japan, and treated with right femoral head replacement for right femoral neck fracture. She had a medical history of hypertension, diabetes mellitus, hyperlipidemia, and Alzheimer’s dementia, but without any life, familial, social and environmental histories. During surgery, the orthopedic surgeon noticed a melanotic lesion of her right sole. On a 15 × 10 mm-sized, irregular-shaped pigmented macule (Figure 1a), the dermoscopy indicated pararrel ridges (Figure 1b). The lesion was later excised surgically. The patient complained of no symptoms related to the skin lesion. No nodal swelling was noted. Microscopically, the pigmented melanoma cells were distributed not only in the basal epidermis but also in the cutaneous sweat gland duct (Figure 2a, b). The nucleoli were inconspicuous. In the dermis, basal cells immunoreactive for cytokeratin (CK) 34bE12, CK5/6 (Figure 2c) and p40 surrounded the intraductal melanoma cells and ductal lumina were often recognized in the center of the involved sweat gland. No stromal invasion was revealed by immunostaining for laminin (data not shown). The surgical margins were negative. The melanoma cells were immunoreactive for HMB45, melan A (Figure 2d), S-100 protein, bcl-2, vimentin, CD5 (Figure 2e), SOX10 (Figure 2f) and CD117 (c-kit), but negative for CKs, BRAFV600E and adipophilin (Figure 2g). Ki-67 labeling was around 10%. Our final diagnosis was ASMEDH, melanoma in situ, pTis cN0 cM0: pStage 0. The patient did not receive adjuvant chemotherapy, and she remained well without recurrence eight months after the treatment.
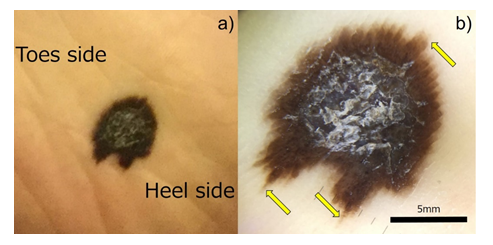
Figure 1: Macroscopic and dermatoscopic images of the asymptomatic melanocytic lesion on the right sole. a) a 15x10 mm-sized irregular-shaped pigmented lesion is seen. b) Dermatoscopy illustrates the pararrel ridge (yellow arrows). bar=5 mm.
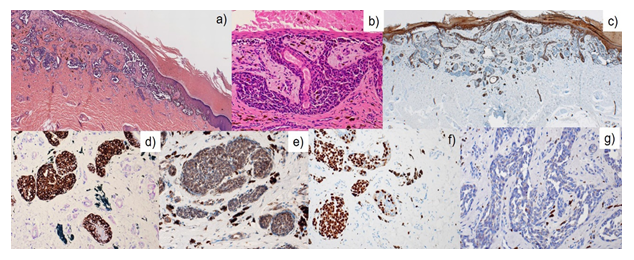
Figure 2: Microscopic findings. The pigmented melanoma cells were distributed not only in the basal epidermis but also along the cutaneous sweat gland duct (a, b). In the dermis, basal cells are immunoreactive for CK5/6 (c), and the melanoma cells are positive for melanA (d), CD5 (e) and SOX10 (f), but negative for adipophilin (g).
2. Discussion
We report herein a case of ASMEDH on the sole of right foot. The acral lentiginous melanoma in situ may proliferate along the eccrine duct [4]. To the best of our knowledge, only two cases of ASMEDH have been reported by Kubba, et al. 2017 [3]. An increase in the density of eccrine glands is described in palmar and plantar areas. In ASMEDH, significant increase of the gland is seen in the area with the syringotropic tumor spread, and the hyperplastic sweat glands are not distributed in the area adjacent to the tumor. These findings suggest a syringoma-like hyperplasia of the eccrine glands in reaction to the tumor growth. Eccrine duct hyperplasia, or syringofibroadenoma-like change, usually occurs as a reactive process: it has been described in association with a “hamartomatous” nasal glioma and two ASMEDHs [3, 5]. A recent report has indicated that the niche of the sweat gland maintains melanocyte-melanoma precursors, and it thus explains the preferential distribution of early melanoma cells in the sweat gland of human acral skin [6].
Significant prognostic factors of the malignant melanoma include adipophilin expression [7], CD5 expression [8], pT factor, pathological staging, mitotic activity and the association of conspicuous nucleoli. In the current case, low-adipophilin expression, low-pathological stage (pTis), low-mitotic count and inconspicuous nucleoli, except for high-CD5 expression, suggest an indolent clinical behavior.
3. Conclusion
The current report describes the third case of ASMEDH. In order to reach the correct histopathological diagnosis of this rare type of in situ malignancy, careful clinicopathological evaluations, including immunostaining with multiple antibodies, are requested. A further study pursuing the mechanism of syringotropism in ASMEDH should be conducted.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure
This case was presented at the 110th Annual Meeting of the Japanese Society of Pathology at Tokyo, Japan, 2021.
Patient consent statement
All the procedures were in accordance with the ethical standards of the responsible institutional committee on human experimentation and with the Helsinki Declaration of 1964 and later versions. The patient’s daughter gave a written informed consent to publication as a case report.
Acknowledgments
We cordially thank Naoki Ooishi, M.T., Department of Diagnostic Pathology, Shimada Municipal Hospital, for his excellent technical assistance and secretarial help.
We also deeply acknowledge Toshiaki Manabe, M.D., Sakai-machi Oike Diagnostic Pathology Clinic, Kyoto, Kyoto, Japan, for his advice and suggestions for the differential diagnosis of the present lesion. There were no funding sources for reporting the present case.
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of the present case.
Authors’ Contributions
Each author has sufficiently participated in the work to take public responsibility for appropriate portions of the content. MT performed histopathological diagnosis of the resected sample, analyzed the data, drafted the figure, and made a major contribution to writing the manuscript. SK made the clinical diagnosis as malignant melanoma by analyzing with a dermatoscope. AM performed clinical evaluations, surgical treatment, and clinical follow-up. MF, MI and KO provided valuable advice and suggestions as the histopathologic consultant. YT analyzed histopathological features and brushed the manuscript up. All authors agreed with the content of the manuscript submitted for publication.
References
- Massi G, LeBoit PE. Melanoma on Acral Skin. In: Massi G, LeBoit PE. Histological Diagnosis of Nevi and Melanoma. Second edition. Heidelberg: Springer (2014): 633-634.
- Izumi M, Ohara K, Hoashi T, et al. Subungual melanoma: histological examination of 50 cases from early stage to bone invasion. J Dermatol 35 (2008): 695-703.
- Kubba F, Fouchardière ADL, Scott A, et al. Acral syringotropic melanomas with florid eccrine duct hyperplasia, a report of two cases. Histopathology 70 (2017): 316-317.
- Onodera H, Mayama S, Akasaka T, et al. A case of acral lentiginous melanoma in situ with proliferation along the eccrine duct. Skin Cancer 12 (1997): 34-36.
- Patterson JW. Tumors of cutaneous appendages. In: Patterson JW. Weedon’s SKIN PATHOLOGY. Fifth edition. Amsterdam: ELSEVIER (2021): 983.
- Okamoto N, Aoto T, Uhara H, et al. A melanocyte-melanoma precursor niche in sweat glands of volar skin. Pigment Cell Melanoma Res 27 (2014): 1039-1050.
- Fujimoto M, Matsuzaki I, Nishitsuji K, et al. Adipophilin expression in cutaneous malignant melanoma is associated with high proliferation and poor clinical prognosis. Lab Invest 100 (2020): 727-737.
- THE HUMAN PROTEIN ATLAS https://www.proteinatlas.org/ENSG00000110448-CD5/pathology/melanoma (cited 2020-08-28).


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
