Activity of Aqueous Extract of Phyllanthus niruri Linn in 2,4-Dinitrophenylhydrazine Induced Anaemic Rats
Article Information
Abubakar Amali Muhammad1*, Rukayya Bandi Ibrahim2, Chika Aminu2, Abdullahi Yahaya Abbas3, Abubakar Kabiru1, Chindo Ibrahim Bisallah4
1Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria
2Department of Pharmacology and Therapeutics, College of Health Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria
3Department of Biochemistry, Faculty of Science, Usmanu Danfodiyo University, Sokoto, Nigeria
4Ministry of Health, Niger state, Nigeria
*Corresponding Author: Abubakar Amali Muhammad, Associate Professor and Deputy Dean, Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria
Received: 08 September 2020; Accepted: 19 September 2020; Published: 28 September 2020
Citation: Abubakar Amali Muhammad, Rukayya Bandi Ibrahim, Chika Aminu, Abdullahi Yahaya Abbas, Abubakar Kabiru, Chindo Ibrahim Bisallah. Antianaemic Activity of Aqueous Extract of Phyllanthus niruri Linn in 2, 4-Dinitrophenylhydrazine Induced Anaemic Rats. Journal of Pharmacy and Pharmacology Research 4 (2020): 79-95.
View / Download Pdf Share at FacebookAbstract
Background: Phyllanthus niruri is an annual plant that is widely used in Asia and Africa traditionally for the treatment of various ailments such as Jaundice, Asthma, Hepatitis, Flu, Dropsy, Diabetes, Malaria, Hemorrhages, Diarrhea, and Anaemia.
Objective: This study evaluates the haemotological and biochemical parameters of P. niruri in 2,4-dinitrophenylhydrazine-induced haemolytic anaemia in Wister rats for possible antianemic potential.
Methods: This study evaluates the Antianaemic effect of the plant extract based on haemotological and biochemical parameters and body weight in 2,4-dinitrophenylhydrazine-induced haemolytic anaemia in Wister rats. Extract was administered in three different doses (250, 500, and 1000 mg/ kg b.wt) of the plant extract and folic acid was used as a positive control. Haematological parameters such as PCV, Hb, RBC, WBC, MCV, MCH, Reticulocyte count were evaluated. Oxidative stress markers (MAD, catalase and SOD) and bilirubin (total and unconjugated) were also analyzed.
Results: The results showed that, PCV, Hb, RBC concentration increased significantly (P<0.001) at a dose of 500 and 1000 mg/kg b.wt respectively while no significant (P>0.05) effect was observed at a dose of 250 mg/kg b.wt. WBC, MCV, MHC and reticulocytes was decreased significantly (P<0.001) at doses of 500 and 1000 mg/kg b.wt while no significant (P>0.05) effect was observed at a dose of 250 mg/kg b.wt. A significant (P<0.01) decrease in MAD and bilirubin concentration and a significant (P<0.01) increase in catalase and SOD activity was seen at doses of 500 and 1000 mg/kg b.wt, while no significant (P>0.05) effect was observed at a dose of 250 mg/kg b.wt.
Conclusion: The results suggest that the aqueous whole plant extract of P. niruri plant is relatively safe and possesses a significant anti-anaemic pr
Keywords
Phyllanthus niruri; Antianaemic; 2,4-dinitrophenylhydrazine; Haemolytic anaemia; Oxidative stress markers
Phyllanthus niruri articles, Antianaemic articles, 2,4-dinitrophenylhydrazine articles, Haemolytic anaemia articles, Oxidative stress markers articles
Article Details
Introduction
Anaemia is among the common blood associated ailments which affect people all around the globe [1]. It can emerge at all phases of the life process, but it occurs mostly in pregnant women and young children [2]. The occurrence of anaemia is higher in the tropics due to the high prevalence of malaria and other parasitic infections that could lead to decrease in circulating red blood cells or hemoglobin level [3]. The World Health Organization (WHO) categories anaemia as a dreadful public health problem (prevalence > 40%) for children of not up to five years in 69 countries and for pregnant women in 68 countries. According to National Family Health Survey (NFHS-3), the occurrence of anaemia in the developed world is 71%, in the underdeveloped world it is 84% and the global occurrence is 79% [4] The major cause of anaemia is linked to low iron levels. However, anaemia exists in association with other causes, such as malaria, parasitic infection, nutritional deficiencies, drug toxicity as well as genetic or acquired defect [5].
Phyllanthus niruri is also known as ‘Chanca piedra in Spanish, ‘quebrapiedra’ (stone breaker) in Brazil, Pitirishi or Budhatri in India [6]. In Nigeria, the Yorubas of the South-West called it Iyin-olobe, the Nupes in the Northern part called it Egi kpeye kpezuma and the Igbos of the South-East called it Ngwu ite kwowa nasu [7]. P. niruri is popularly used in Asia, Africa and South America [8] for the treatment of various ailments such as jaundice, asthma, hepatitis, flu, dropsy, diabetes, malaria [9] hemorrhages, diarrheas, dysentery, jaundice, cough and anaemia, It is also said to be effective in bronchitis, anaemia, leprosy, asthma, Urinary tract infections, stimulating liver, improving digestion, increase appetite and produce laxative effects [10]. Phyllanthus niruri is also traditionally used in treating anaemia [7], tuberculosis, vaginitis, tumors, gonorrhea, syphilis [8]. A study by Okoli et al. (2010) [8] reveals that the pharmacologically active compounds present in P. niruri consist of flavonoids, anthraquinones, alkaloids, saponins, steroids, tannins and terpenoids. Coumarins and lignans were also found to be present [6]. Harish et al., 2006 reported the high antioxidant potentials P. niruri extracts (aqueous and methanolic) in vivo by their ability to inhibit CCl4 – induced lipid peroxidation in rats. Thus, the plant is used in treating various diseases including anaemia across the globe.
Materials and Methods
Drugs and Reagents
2,4-dinitrophenylhydrazine (British drug house, UK), Folic acid (Emzor pharmaceutical industry, Lagos). Other chemicals used were of analytical grade purchased from BDH, Poole UK.
Plant Material and Extraction
Fresh P. niruri whole plant was collected from Minna in Niger state, Nigeria in the month of October 2018. The plant was identified by a Pharmacognosist from the Department of Ethnomedicine and Pharmacognosy, Faculty of Pharmaceutical Sciences, Usmanu Danfodiyo University Sokoto. A specimen was deposited at the herbarium of the faculty and a voucher number (PCG/UDUS/Euph/0011) was obtained.
The plant was carefully rinsed with water and then shed dried to a constant weight. Using a pestle and mortar the plant was grounded to a coarse powder. Using cold maceration method 2kg of the powdered plant was dissolved in 10liters of distilled water to give 200g/L. The homogenate was kept for 3 days at 40°C with daily stirring. The mixture was then filtered using a muslin cloth, then with a Whatman No. 1 filter paper. The filtrate was then concentrated to dryness using a freeze dryer and stored at 4°C until it was required for the study.
Animals Grouping and Experimental Procedure
Adult Wister albino rats of both sexes (males and females) of about 12 weeks of age weighing between 180-200g were used for the study. They were procured from the Faculty of Pharmaceutical Sciences, A.B.U Zaria, Nigeria. The animals were housed in standard cages at the animal house of Faculty of Pharmaceutical Sciences, Usmanu Danfodiyo University Sokoto and allowed to acclimatize to the new environment under optimum feeds (Standard commercial diet from Vital Feeds company, Jos, Plateau State Nigeria) with full access to drinking water for a period of 2 weeks prior to the commencement of the experiment. The animals were randomly divided using computer aided randomization (Decision analyst stats 2.0) into various groups and each rat was marked using a permanent marker for easier identification. The extract was administered orally using a feeding cannula. Each rat was weighed prior to extract administration to determine the appropriate dose in mg/kg body weight. The maintenance and handling of the animals were in line with the regulations of the Institutional Animal Ethics Committee. Weights of the rats were monitored through the period of the experiment.
Induction of Anaemia using 2,4-dinitrophenylhydrazine
A modified method described by Berger (2007) was used in this study. The experiment was performed for a period of three weeks. The animals were randomly divided into five groups of eight animals each (n=8). All the animals received 2,4-DNPH (20 mg/kg b.wt, p.o) once daily for 7 consecutive days using a feeding canula. On the 8th day, their blood sample was collected by sinus puncture at the tail veins of each rat into heparinized capillary tubes for hematological analysis. Rats with ≥30% reduction in PCV were considered anemic and were used for the study [5].
PCV was determined pre and post induction of anaemia by obtaining blood from a slight cut around the tail and allowing the blood to flow through a capillary tube to more than two-thirds of the tube. The end of the tube was then sealed off using a Bunsen burner. The samples were centrifuged for 5 min at 3,000 rpm. The capillary tubes were placed on the microhaematocrit to determine the PCV.
Treatment with the extract commenced immediately after the establishment of anaemia. Treatment groups were given different doses (250, 500 and 1000mg/kg) of plant extract, positive control group was given Folic acid (75µg/kg) while the negative control was left untreated. All treatments were administered once daily for 14 consecutive days using oral feeding cannula.
Haematological Assay
After two weeks of treatment with the aqueous extract, blood samples were obtained through cardiac puncture and collected in EDTA bottles to prevent the blood from clotting. The samples were used for determination of haematological parameters (RBC, PCV, Hb, WBC, MCHC, MCV and reticulocyte count). All haematological parameters except reticulocytes were carried out using the automation method using sysmex Machine (Model No KX-21N) made by Sysmex Laboratories Ltd USA. The blood samples were centrifuged or mixed and put through a machine called Automated Analyzer which counts the numbers and types of different cells within the blood. Reticulocyte count was conducted using visual method [20].
Lipid peroxidation assay (Thiobarbituric acid method)
MDA react with thiobarbituric acid (TBA) to form an MDA-TBA2 adduct that absorbs strongly at 532 nm.
Procedure
As described by (David et al., 1990) 150µL of the sample was diluted to 500µL with double deionized water. In test tubes, 250µL of 1.34% Thiobarbituric acid was added followed by the addition of equal volume of 40% trichloroacetic acid. The mixture was well shaken and incubated for 30 minutes in boiling water (temperature >900C). Tubes were allowed to cool down to room temperature and the absorbance of Malondialdehyde (MDA) formed was read at 532nm using 0 concentration as blank. The concentration of Malondialdehyde formed was calculated using the following formula.
MDA (µmol/L) = Absorbance at 532 x 1000/1.56.
Enzymatic Assay for Superoxide Dismutase (Kakkar et al., 1984)
Principle
The principle of this method is based on the competition between the pyrogallol autoxidation by O2•¯ and the dismutation of this radical by SOD.
Procedure
Test tubes were labeled as the test and blank and 0.1mL of Buffer was added into test labeled tubes while 0.15mL was added into blank labelled test tube. 0.83mL of distilled water was then added to both blank and test. Serum (0.05mL) was added into test and incubated at 240C for 10 minutes then transferred into cuvette. 0.02mL of Pyrogallol was added to both blank and test. The mixture is immediately mixed by inversion and change in absorbance at 240nm was recorded after 3 minutes approximately. The change in absorbance at 420nm/minute is calculated using the maximum linear rate for both test and blank.
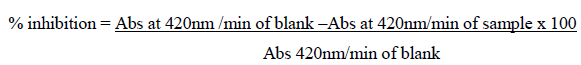
Abs – Absorbance
The number of units of SOD is calculated using the following formula:
% inhibition / (100-% inhibition) = Units/ml
One unit of SOD activity was defined as the amount of enzyme that reduces the rate of auto-oxidation of pyrogallol by 50% at standard assay conditions.
Bilirubin reacts with diazotized Sulphanilic acid to form a colored azobilirubin compound. The unconjugated bilirubin coupled with the Sulphanilic acid in d presence of caffeine produces a colour complex also. The intensity of the color is directly proportional to the concentration of Bilirubin in the specimen and is measured at 546 nm.
As described by (Olukunle et al.,2010). Test tubes were labeled as blank and sample 200µL of reagent 1 was added to both sample and blank tubes followed by the addition of 50µL of reagent 2 to sample test tubes. Reagent 3 (100µL) was added to both sample and blank tubes followed by addition of 200µL of the sample (serum) to both sample and blank tubes. The mixtures were incubated at 20-250C for 10 minutes followed by the addition of 1000µL of reagent. The content of the tubes were mixed and incubated at 25oC for 5-30minutes. The content of the tubes was transferred into a Cuvette and absorbance was recorded at 546nm. The concentration of total Bilirubin was calculated using the following formula.
Total bilirubin (µm/L) = 185 X Absorbance of total bilirubin (546nm)
Test tubes were labeled as blank and sample, 200µL of reagent 1 was pippetted into both blank and sample test tubes. Followed by the addition of 50µL of reagent 2 into sample test tubes. Addition of 0.9% NaCl (200µL) was done to both sample and blank test tubes followed by the addition of 200µL of the test sample (serum) into both sample and blank test tubes. The content of the tubes was mixed and incubated for 10min at 20-250C. Absorbance was taken at 546nm. The concentration of direct Bilirubin was calculated using the following formula.
Direct bilirubin (umo/L) = 246 X Absorbance of direct bilirubin (546nm)
Statistical Analysis
The Data were expressed as mean ± standard error and were analyzed using One-way Analysis of Variance (ANOVA). The comparison between untreated and Treatment groups was done using the Dunnet's multiple comparison tests. The level of significance was set at P < 0.05.
Results
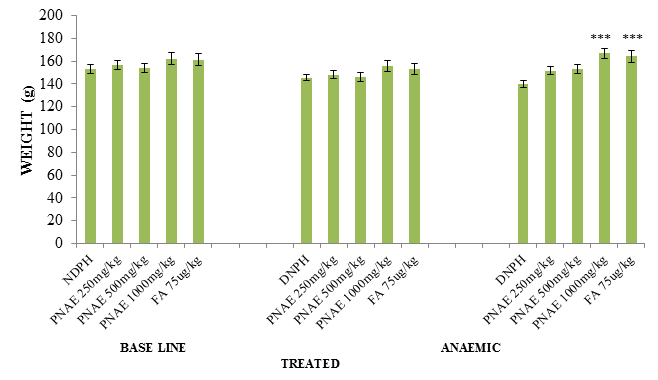
Following the induction of anaemia, body weights of the rats pre and post induction of anaemia with PHZ was evaluated. There was a significant (P<0.05) reduction in weight in the PHZ induced animals and a significant (P<0.01) increase in weight the groups with various concentration of aqueous extract of P. niruri when compared with the untreated group as seen in Figure 1 below.
Figure 1: Effect of P. niruri Aqueous Extract on weight of Rats pre and post induction of anaemia 14 days after treatment with PNAE. Values represent the means ± SE (n= 8 rats each for all the groups). P<0.05 and P<0.001 was considered significant when compared with untreated group. PNAE = P niruri aqueous extract, DNPH = 2, 4-dinitrophenylhydrazine, FA = Folic acid.
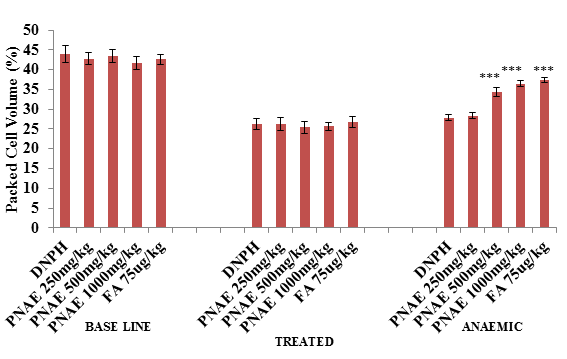
Following induction of anaemia treatment, the result of PCV shows a significant (P>0.001) decrease in PCV in PHZ treated groups (at day 7) but a dose-dependent increase in PCV was observed after 14 days of treatment with P. niruri extract when compared with the untreated control group compared with the untreated normal control group as illustrated in Figure 2 below.
Figure 2: Effect of P. niruri aqueous extract on PCV in 2,4-DPHZ-induced anaemic rats at baseline, after induction of anaemia and after 14 days treatment with PNAE. Values represent the means ± SE (n= 8 rats each for all the groups). One-way ANOVA with Dunnet’s posttest was used to arrive at the P values. * signifies P<0.05 when compared with untreated group. PNAE = Phyllanthus niruri aqueous extract, DNPH = 2,4-dinitrophenylhydrazine, FA = Folic acid.
The treatment of PHZ induced anemic rats with PNAE for 14 days shows a significant (P>0.001) and dose-dependent increase in Hb concentration and also a significant (P<0.001) decrease in the untreated control group as illustrated in the Figure 3 below.
The result of RBC concentration following treatment of PHZ induced anemic rats with PNAE Produces a significant (P<0.001) and dose-dependent increase in RBCs when compared with the untreated group. There was also a significant (P<0.001) decrease when PHZ group was compared to the control after treatment as shown in Table 1 below.
The result of WBC concentration following treatment of PHZ induced anemic rats with PNAE Produces a significant (P<0.02) and dose-dependent decrease in WBCs when compared with the untreated group. There was also a significant (P<0.001) increase in WBC level when PHZ group was compared to the control after treatment as shown in Table 1 below.
Table 1: Effect of treatment of anemic rats with aqueous extract of P. niruri on HB, RBCs, and WBCs
|
Treatments |
Indices |
||
|
Dose (mg/kg) |
HB (g/dl) |
RBC (×106µl) |
WBC (×103µl) |
|
DNPH PNAE |
7.79±0.34 |
3.19±0.29 |
19.16±0.38 |
|
250 |
8.21±0.27ns |
3.51±0.21ns |
17.58±0.24* |
|
500 |
10.43±0.54* |
4.83±0.27* |
12.65±0.44* |
|
1000 |
11.31±0.28* |
5.33±0.10* |
11.81±0.32* |
|
Folic acid (7.5µg/100g) |
11.55±0.36* |
5.74±0.16* |
10.95±0.39* |
Values are expressed as mean ± SEM, n=8. P<0.05 is considered as significant. Values with * are significant when compared to untreated groups.
Also, a significant (P<0.05) decrease in MCV, MCH and reticulocytes was observed at 500 and 1000mg/kg after 14 days treatment with 500 and 1000mg/kg body weight when compared to the 2,4-DNPH induced anemic group of the extract. No significant (P>0.05) effect was observed at a dose of 250 mg/kg body weight as shown in Table 2 below.
Table 2: Effect of treatment of anemic rats with aqueous extract of P. niruri on MCV, MHC, and Retics
|
Treatments |
Indices |
||
|
Dose (mg/kg) |
MCV (fl) |
MHC (pg) |
RETICULOCYTES (%) |
|
DNPH PNAE |
93.50±7.14 |
25.38±1.78 |
8.30±0.36 |
|
250 |
84.54±3.69ns |
23.06±0.79ns |
7.44±0.30ns |
|
500 |
71.59±1.78* |
21.61±0.36* |
5.67±0.35* |
|
1000 |
68.63±0.598* |
21.00±0.24* |
5.35±0.26* |
|
Folic acid (7.5µg/100g |
65.35±1.61* |
20.05±0.53* |
5.11±0.29* |
Values are expressed as mean ± SEM, n=8. P<0.05 is considered as significant. Values with * are significant when compared to untreated group.
Biochemical Parameters
Malondialdehyde
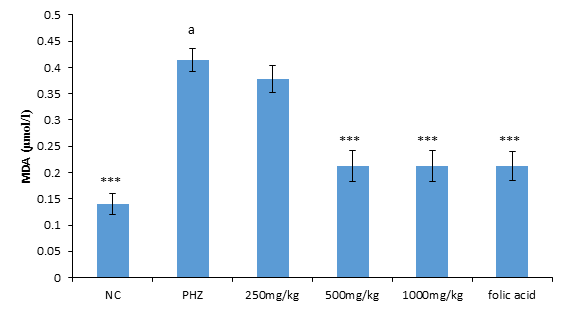
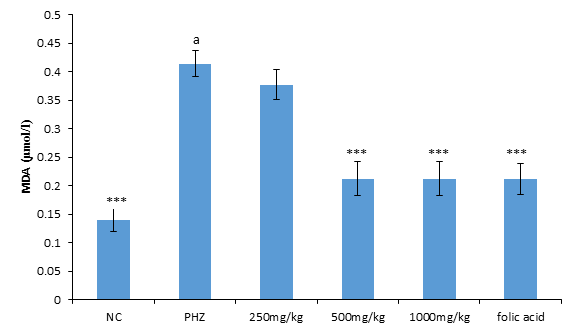
The result of malondialdehyde following treatment of PHZ induced anemic rats with PNAE produces a significant (P<0.05) and dose-dependent decrease in malondialdehyde when compared with the untreated group (PHZ). There was also a significant (P<0.001) increase in MDA level when PHZ group was compared to the control after treatment as shown in Figure 3 below.
Figure 3: Effect of PNAE on MDA in PHZ-induced Anaemic Rats after 14 days treatment with PNAE. Values represent the means ± SD (n= 8 rats each for all the groups). Oneway ANOVA with Dunnet’s post-test was used to arrive at the P values. *, ** and *** signifies P<0.005, P<0.02 and P<0.001 respectively when compared with PHZ-treated group while a signifies P<0.001when compared with normal control.
Superoxide Dismutase Stimulatory Activity of PNAE
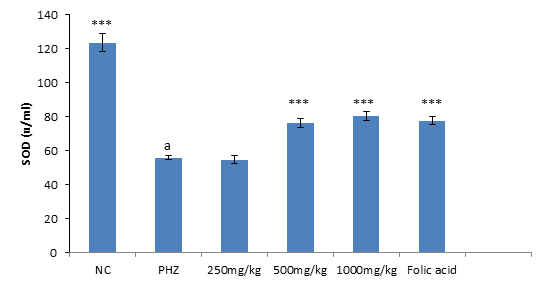
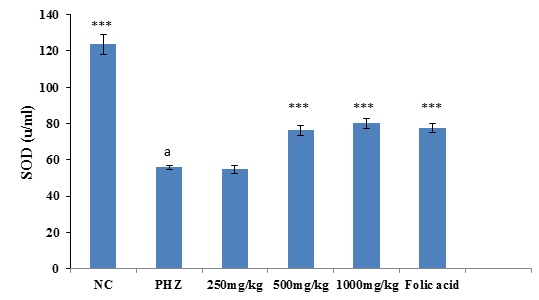
The result of SOD activity following treatment of PHZ induced anemic rats with PNAE shows a significant (P<0.01) and dose-dependent increase in SOD activity when compared with the untreated group (PHZ). There was also a significant (P<0.001) decrease in SOD activity level when PHZ group was compared to the control after treatment as shown in Figure 4 below.
Figure 4: Effect of PNAE on SOD in PHZ-induced Anaemic Rats after 14 days of treatment with PNAE. Values represent the means ± SD (n= 8 rats each for all the groups). One-way ANOVA with Dunnet’s post- test was used to arrive at the P values. *, ** and *** signifies P<0.005, P<0.02 and P<0.001 respectively when compared with PHZ-treated group while a signifies P<0.001when compared with normal control.
Effect of treatment on catalase activity
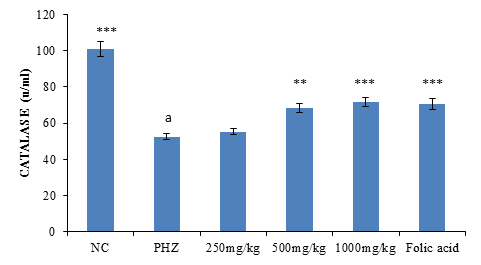
The result of Catalase activity following treatment of PHZ induced anemic rats with PNAE shows a significant (P<0.01) and dose-dependent increase in catalase activity when compared with the untreated group (PHZ). There was also a significant (P<0.001) decrease in catalase activity when PHZ group was compared to the control after treatment as shown in Figure 5 below.
Figure 5: Effect of PNAE on Catalase in PHZ-induced Anaemic Rats after 14 days of treatment with PNAE. Values represent the means ± SD (n= 8 rats each for all the groups). One-way ANOVA with Dunnet’s post-test was used to arrive at the P values. *, ** and *** signifies P<0.005, P<0.02 and P<0.001 respectively when compared with PHZ-treated group. while a signifies p<0.001when compared with normal control.
Biochemical Parameters
Oxidative Stress Markers
The result of malondialdehyde following treatment of PHZ induced anemic rats with PNAE produces a significant (P<0.05) and dose-dependent decrease in malondialdehyde when compared with the untreated group (PHZ). There was also a significant (P<0.001) increase in MDA level when PHZ group was compared to the control after treatment as shown in Figure 6 below.
Figure 6: Effect of PNAE on MDA in PHZ-induced Anaemic Rats after 14 days treatment with PNAE. Values represent the means ± SD (n= 8 rats each for all the groups). One-way ANOVA with Dunnet’s post-test was used to arrive at the P values. *, ** and *** signifies P<0.005, P<0.02 and P<0.001 respectively when compared with PHZ-treated group while a signifies p<0.001when compared with normal control.
Superoxide Dismutase Stimulatory Activity of PNAE
The result of SOD activity following treatment of PHZ induced anemic rats with PNAE shows a significant (P<0.01) and dose-dependent increase in SOD activity when compared with the untreated group (PHZ). There was also a significant (P<0.001) decrease in SOD activity level when PHZ group was compared to the control after treatment as shown in Figure 7 below.
Figure 7: Effect of PNAE on SOD in PHZ-induced Anaemic Rats after 14 days of treatment with PNAE. Values represent the means ± SD (n= 8 rats each for all the groups). One-way ANOVA with Dunnet’s post-test was used to arrive at the P values. *, ** and *** signifies P<0.005, P<0.02 and P<0.001 respectively when compared with PHZ-treated group. While a signifies P<0.001when compared with normal control.
Effect of Treatment on Bilirubin
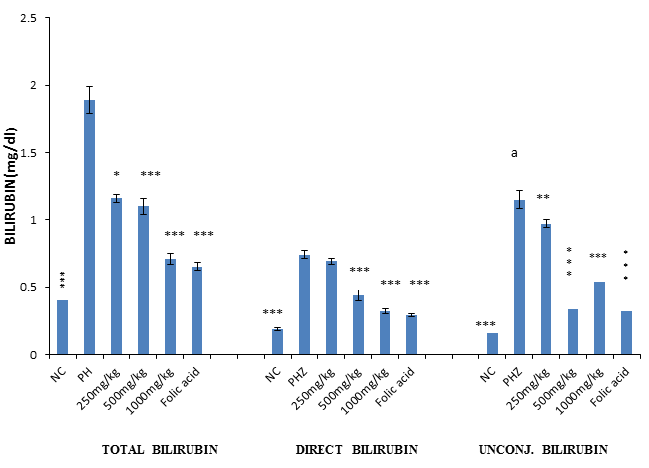
The result of bilirubin assay revealed a significant (P<0.001) and dose-dependent reduction in total, direct and unconjugated bilirubin when PHZ induced anaemic rats were treated with PNAE compared with the untreated group. There was also a significant (P<0.001) increase in bilirubin when PHZ group was compared to the control after treatment as shown in Figure 8 below.
Figure 8: Values represent the means ± SD (n= 8 rats each for all the groups). One-way ANOVA with Dunnet’s posttest was used to arrive at the P values. *, ** and *** signifies P<0.005, P<0.02 and P<0.001 respectively when compared with PHZ-treated group while signifies P<0.001 when compared with normal control.
Discussion
The present study evaluated the anti-anaemic activity of P. niruri in phenylhydrazine-induced hemolytic anaemia in rats. The results of the study revealed a significant reduction of the weight of rats after induction of anaemia which could be due to the effect of oxidative damages (erythrocytes haemolysis) induced by 2,4-DPH. The reduction in body weight was seen to improve in the group of rats treated with P. niruri aqueous extract after 14 days probably due to reversal of the oxidative damage by the extract. This finding is in agreement with some studies reported by [2,4,11]. 2015 where the body weights of rats significantly reduced after induction of anaemia with PHZ but were improved after treatment with 200mg/kg body weight of ethanolic extract of J. repens and C. fascicularis L and 400mg/kg body weight Z. jujuba fruits aqueous extract for 2 weeks respectively.
Berger in 2007 reported that, PHZ injected at a dose of 90 mg/kg body weight to 8 weeks old rats produced a reduction of normal RBC by 45% and PCV by 53% on day 3; reticulocytes by 47% on day 7; and a high MCV of 170% as well as a 60% increase in MCV on day 3. More than 30%, reduction in HB and RBCs was also reported by [4,5,12-16]. In the present study, administration of PHZ in rats produces a decreased PCV level which agreed with previous reports by [17], Maria Claro et al., 2006; [5,13] that reported reduction in PCV in hemolytic anaemia induced by 2, 4-DPHZ caused that leads to oxidation of HB and sulfhydryl groups of the erythrocytes membrane and enzymes leading to haemolysis of erythrocytes [17]. However, Oral administration of P. niruri whole plant aqueous extract produces a dose-dependent increase in PCV, RBC, HB (P<0.05) and a significant (P<0.05) decrease in WBC, MCV, MCH and reticulocytes after 14 days treatment. This agreed with earlier findings by [13] where oral administration of 250 and 500mg/kg body weight aqueous extract of P. erinaceus Stem Bark significantly increased PCV, Hb, RBC and significantly decreased WBC, MCH and MCHC in PHZ induced anaemic rats probably due to the ability of PNAE to protect the RBC against oxidative haemolysis induced by 2,4-DNPH. The increase in WBC level seen after induction of anaemia might be due to immune stimulatory ability of the chemical similar to what was reported by [18] following administration of ethanolic extract of P. kurroa leaves extracts at 100 mg/kg and 200 mg/kg, to PHZ induced anaemic rats which caused increased the level of RBC, PCV and HB in rats. Similarly, treatment of 2,4-DPHZ induced anemic rabbits with Hibiscus sabdarifa anthocyanin extract produces a significant (P<0.05) increase in, RBC counts, PCV and Hb and a decrease in WBC counts [19].
Conclusion
The results of the present study revealed a significant anti-anemic property of whole plant aqueous extract of P. niruri in 2,4-dinitrophenylhydrazine-induced haemolytic anaemia in experimental rats. The anti-anaemic activity of the plant could be due to the presence of important phytochemicals such as flavonoids and alkaloids which are known to possess erythrocytes protective abilities hence, reversing the anaemia induced in the rats.
References
- Priya MS, Anbu N, Parthibhan P. Kanakavalli: Preclinical Evaluation of Hematinic Potential of the Siddha Formulation Sarakondrai Chooranan using Phenylhydrazine Induced Anaemia in rats. Int. J. Curr. Res. Med. Sci 3 (2017): 48-52.
- Shende VS, Shaikh FH, Medhekar SK, et al. Protective effects of ethanolic leaf extracts of Corchorus fascicularis in phenylhydrazine induced anemic rats. Therapy 5 (2017): 6.
- Toma I, Victory NC, Kabir Y. The effect of aqueous leaf extract of fluted pumpkin on some hematological parameters and liver enzymes in 2, 4-dinitrophenylhydrazine-induced anemic rats. African Journal of Biochemistry Research 9 (2015): 95-98.
- Das A, Ghosal S, Chakraborty I, et al. Haematinic potential of Jussiaea repens L–a search for antianaemic herb. Journal of Pharmaceutical Research International (2015): 1-10.
- Sani HL, Malami I, Hassan SW, et al. Effects of standardized stem bark extract of Mangifera indica L. in wistar rats with 2, 4-dinitrophenylhydrazine-induced haemolytic anaemia. Pharmacognosy Journal 7 (2015).
- Bagalkotkar G, Sagineedu SR, Saad MS, et al. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: a review. Journal of Pharmacy and Pharmacology 58 (2006): 1559-1570.
- Okolo SC, Okoh-Esene RU, Ikokoh PP, et al. Phytochemicals, mineral content and antimicrobial screening of Phyllanthus amarus Schum and Thonn in Abuja, Nigeria. Journal of Microbiology and Biotechnology Research 2 (2012): 17-22.
- Okoli CO, Ibiam AF, Ezike AC, et al. Evaluation of antidiabetic potentials of Phyllanthus niruri in alloxan diabetic rats. African Journal of Biotechnology 9 (2010): 248-259.
- Nakweti RK, Ndiku SL, Doumas P, et al. Phytochemical analysis of Phyllanthus niruri L.(Phyllanthaceae) extracts collected in four geographical areas in the Democratic Republic of the Congo. African Journal of Plant Science 7 (2013): 9-20.
- Satya AK, Narendra K, Sowjanya KM. Phyllanthus niruri: A Review on its Ethno Botanical, Phytochemical and Pharmacological Profile Identification and Functional analysis of novel blast resistance genes in rice View project HPLC Method development for organic acids Journal of Pharmacy Research 5 (2012): 4681–4691.
- Pitchaiah G, Sravani K, Vasanthi C, et al. Evaluation of Anti-Anaemic and Anti-Allergic Activity of Aqueous and Methanolic Extracts of ZiziphusJujuba Fruits in Rodents. IJEP Journal 5 (2015): 91-95.
- Idu M, Igbafe G, Erhabor J. Anti-anaemic activity of Jatropha tanjorensis Ellis & Saroja in Rabbits. Journal of Medicinal Plants 2 (2014).
- Nadro MS, Modibbo AA. Effects of Pterocarpus erinaceus stem bark aqueous extract on anaemic rats. Scientific Research Journal (scrip) 2 (2014): 1-15.
- Iwalewa EO, Omisore NO, Daniyan OM, et al. Elemental compositions and antianemic property of Harungana madagascariensis stem bark. Bangladesh Journal of Pharmacology 4 (2009): 115-121.
- Saravanan VS, Manokaran S. Anti-anaemic activity of some plants in Cucurbitaceae on phenylhydrazine-induced anaemic rats. Thai J Pharm Sci 36 (2012): 150.
- Turaskar AS, More SA, Sheikh RI, et al. Inhibitory potential of Picrorhiza kurroa Royle Ex. Benth extracts on phenylhydrazine induced reticulocytosis in rats. Asian J Pharm Clin Res 6 (2013): 215-216.
- Berger J. Phenylhydrazine haematotoxicity. J Appl Biomed 5 (2007): 125-130.
- Turaskar A, More S, Sheikh R, et al. Antianaemic potential of Swertia chirata on phenylhydrazine induced reticulocytosis in rats. American Journal of Phytomedicine and Clinical Therapeutics 1 (2013): 37-41.
- Ologundudu A, Ologundudu AO, Oluba OM, et al. Effect of Hibiscus sabdariffa anthocyanins on 2, 4-dinitrophenylhydrazine-induced hematotoxicity in rabbits. African Journal of Biochemistry Research 3 (2009): 140-144.
- Nwaka AC, Ikechi-Agba MC, Okechukwu PU, et al. The effects of ethanol extracts of Jatropha curcas on some hematological parameters of chloroform intoxicated rats. American-Eurasian Journal of Scientific Research 10 (2015): 45-49.
- Viana KA, Martins Filho OA, Dusse LM, et al. Reticulocyte count: comparison among methods. Jornal Brasileiro de Patologia e Medicina Laboratorial 50 (2014): 339-345.
- Thaker RU, Vyas BA, Joshi SV, et al. Effect of dehydrated water extract of fruits of opuntia ficus indica on experimentally induced hemolytic anemia in rats. Int J Pharm Res Dev 4 (2012): 185-191.
- Nigeria: Index - AHO. Retrieved (2018).
- Yashoda KM. Phenylhydrazine anæmia in rats. Current Science Association 11 (1942): 360-363.


 Impact Factor: * 2.5
Impact Factor: * 2.5 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 