Ameloblastic Carcinoma: Presentation of Two Case Reports
Article Information
Shruti Singh1*, Jaya Singh1, Shaleen Chandra2, Fahad M. Samadi3
1Senior Resident, Department of Oral Pathology and Microbiology, Faculty of Dental Sciences, KGMU, Lucknow, India
2Professor and Head, Department of Oral Pathology and Microbiology, Faculty of Dental Sciences, KGMU, Lucknow, India
3Associate Professor, Department of Oral Pathology and Microbiology, Faculty of Dental Sciences, KGMU, Lucknow, India
*Corresponding Author: Shruti Singh, Senior Resident, Department of Oral Pathology and Microbiology, Faculty of Dental Sciences, King George’s Medical University, Lucknow, India
Received: 27 April 2020; Accepted: 11 May 2020; Published: 14 May 2020
Citation: Shruti Singh, Jaya Singh, Shaleen Chandra, Fahad M. Samadi. Ameloblastic Carcinoma: Presentation Of Two Case Reports. International Journal of Applied Biology and Pharmaceutical Technology 11 (2020): 60-70.
View / Download Pdf Share at FacebookAbstract
Abstract
Ameloblastic Carcinoma is a rare malignant odontogenic neoplasm that exhibits features of ameloblastoma along with features of cytological atypia. Owing to its varied clinical presentations and its histologic resemblance with Ameloblastoma, it is often misdiagnosed. Since few cases have been reported due to its rare frequency, there is paucity in the literature and there is no distinct recommendations regarding the same. The current paper reported two cases of Ameloblastic Carcinoma with different clinical presentations, one reported in a nineteen year old male patient with features of benign ameloblastoma in the superficial lesion and deeper aspect revealed features of ameloblastic carcinoma with lymph node metastasis while the second case reported in a forty-five year old female patient with classic features of Ameloblastic Carcinoma.
Keywords
Ameloblastic carcinoma; Malignant odontogenic tumor; Lymph node metastasis; Cytological atypia
Article Details
Introduction:
Odontogenic malignancies are in rarity and accounts for only 1% of all the cysts and tumors of the jaw [1]. Owing to its rare frequency and the variable clinical presentations, odontogenic carcinoma have faced substantial transformations in its terms and WHO classification over the years. In 1972 and 1992, WHO published classification of odontogenic malignant tumors, which do not include Ameloblastic carcinoma [3]. The term “Ameloblastic Carcinoma” was first termed by Elzay in the year 1982 for a malignant epithelial odontogenic tumor that histologically retains the features of ameloblastic differentiation and exhibits cytological features of malignancy in a primary or recurrent tumor [4]. Malignant (metastasizing) ameloblastoma and ameloblastic carcinoma are two distinct malignant variants of ameloblastoma. The term “Malignant ameloblastoma” is used for ameloblastoma that metastasize without any histological features of malignancy in both the primary and the metastatic foci and the term “Ameloblastic carcinoma” for tumors with ameloblastomatous differentiation showing cytological features of malignancy with or without metastasis [5]. According to the WHO 2005 classification, Ameloblastic Carcinoma was further divided as primary-type and secondary-type (intraosseous and peripheral dedifferentiated). Primary-type Ameloblastic Carcinoma has some histological characteristics of ameloblastoma, but it is obviously characterized by cytologic atypia, poor differentiation, and high mitotic index. Secondary-type Ameloblastic Carcinoma developed from a previously existing Ameloblastoma and shows aggressive proliferation [6]. According to the new WHO 2017 classification; there is a single diagnostic entity of Ameloblastic Carcinoma [7] .
The origin of ameloblastic carcinoma is still unknown. It originates from preceeding Ameloblastoma or it’s a separate entity is still debatable [8]. Ameloblastic carcinomas are locally aggressive lesions showing rapid growth that can be accompanied with pain, paresthesia, trismus and dysphonia [1]. Ameloblastic carcinomas have been reported with local recurrences and metastasis to sites like the lungs, brain, liver and bones [1].
Materials And Method:
Case Report 1:
1.1 Clinical Findings:
A male patient aged 19-year-old complaints of swelling on left side of face. History of present illness revealed the onset of swelling present since 5 years and the lesion is gradual progressive in nature and reached to the present size. No significant family history and habit history were noted. Extra oral examination revealed swelling on lower left side of face. Lymph nodes were palpable on left lower border of mandible. Intraoral examination showed that the lesion extend for left side of mandibular canine to left posterior border of mandible. The lesion was soft in consistency.
1.2 Radiographic Findings:
Radiographic examination showed mixed radiolucent lesion over left angle region of mandible. CT images revealed ill defined multilocular radiolucent lesion on left side of mandible, crossing midline anteriorly and posteriorly extends upto ramus and coronoid process. Marked expansion of both buccal and cortical plate is also seen. Inferiorly alveolar canal is not traceable on diseased site. Based on clinical and radiological examination a provisional diagnosis odontogenic tumors most probably Ameloblastoma was made. A decision of hemi-madibulectomy along with lymph node resection was made and the excised specimen was sent for histopathological evaluation.
1.3 Gross Findings:
On gross examination of the tissue, whole hemimandibulectomy along with Level 1 lymph nodes were received. The excised specimen extended from the condylar and coronoid process to lower Central Incisor of the opposite side. Specimen showed expansion in both buccal and lingual side with thinning of cortical plates, which were almost paper-thin. Teeth present were permanent right mandibular incisors till permanent left mandibular molars, surface hard, creamish white in color, glistening surface. A total six bits of tissue were taken from lingual side of the lesion, distal part of ramus, deeper part of the main lesion, buccal side of 35, and lingual side of 36 respectively and kept for routine processing and hematoxylin and eosin staining. While removing deeper part of the main lesion, oily fluid was found which was sent for biochemical evaluation. Lymph nodes were also kept for routine processing and staining done with hematoxylin and eosin stain.
1.4 Histopathological Findings:
Histopathological examination of H and E stained sections of superficial lesions reveal long anastomosing chords of odontogenic epithelium. The chords are bounded by tall columnar ameloblast like cell surrounding the central stellate reticulum like cells. The surrounding stroma was loosely arranged and vascular displaying the features of Plexiform Ameloblastoma while the deeper part of the lesion exhibit strands and sheet in moderately dense connective tissue stroma. The tumor islands show peripheral palisading cells with hyperchromatism, abnormal mitotic figures with increased nuclear cytoplasmic ratio. The connective tissue also showed endothelial-lined blood vessels along with cross section of muscles. H and E stained sections of resected lymph node sections showed individual dysplastic cells along few clusters of dysplastic cells are seen in all the sections of lymph nodes. The histopathological features are suggestive of Ameloblastic Carcinoma with Cervical Lymph node metastasis.
1.5 Immunohistochemical Findings:
For further confirmation of the diagnosis, immunohistochemical expression of proliferative marker Ki-67 was performed, sporadic positivity was observed in the lesion and also in atypical odontogenic cells in the lymph node.
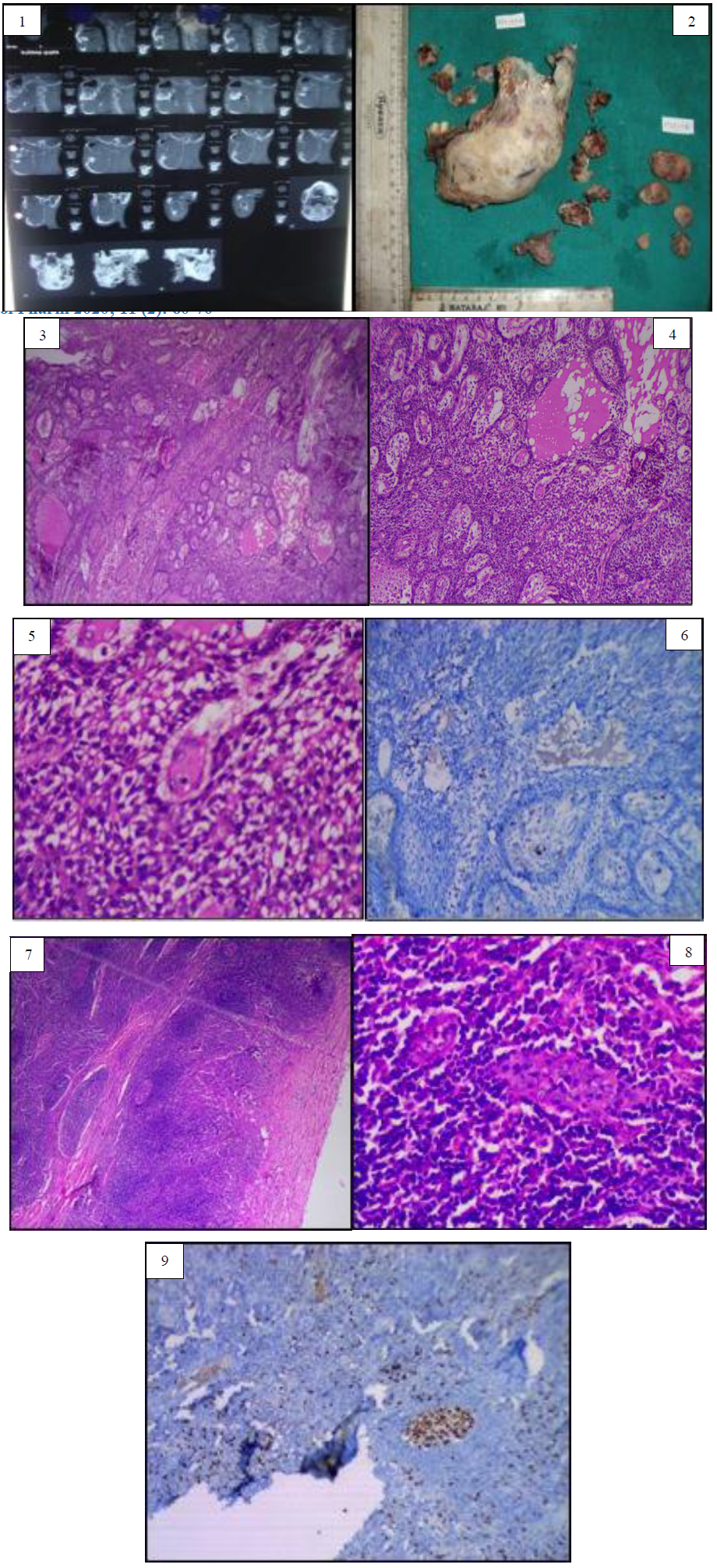
Figure 1: Computerized tomography image of case report 1 showing ill defined multilocular radiolucent lesion on left side of mandible, crossing midline anteriorly and posteriorly extends upto ramus and coronoid process. Figure 2: Gross image of the excised specimen of case report one. Figure 3: Histopathological image (4X) shows numerous odontogenic islands in the connective tissue stroma. Figure 4: Histopathological image of the specimen (10X) reveal numerous odontogenic islands in dense connective tissue stroma with peripheral ameloblast like cells with peripheral palisading and hyperchromatic nuclei and central cells resemble angular shaped stellate reticulum like cells. Figure 5: Higher magnification (40X) reveals cytological atypia in the central stellate reticulum like cells. Figure 6: Immunohistochemical analysis of first case shows sporadic positivity of Ki-67 in atypical odontogenic cells. Figure 7: Histopathological image reveal lymph node sections of first case in which dysplastic odontogenic cells with cellular atypia. Figure 8: Histopathological examination of the first case revealed odontogenic cells with cytological atypia in lymph node section under higher magnification. Figure 9: Immunohistochemical expression of lymph node section revealed Ki-67 positivity in epithelial cells. Insets show Ki67 positive epithelial cells in higher magnification.
2 Case Report 2:
2.1 Case History:
A 69-year-old female patient walks into the outpatient unit of Department of Oral Surgery, KGMU with a chief complaint of swelling and pain in left lower jaw region since 6-7 months. The onset of the swelling started about 6 months ago and was progressive in nature. No relevant family history was evident. Patient had a habit of Bidi smoking one packet in 2-3 days since 40 years and areca nut chewing once a day since 40-50 years. Patient quit the habit of Bidi smoking 2 years back but continued with areca nut chewing.
2.3 Clinical Findings:
On extra-oral examination, swelling at left angle of mandible was exhibited which was mildly tender on palpation. Lymph nodes were non-palpable. Intra oral examination revealed the swelling of the lesion extended from left angle of the mandible starting from premolar to ramus of mandible. The lesion was firm in consistency and was tender on palpation. No discharge were associated with it.
2.4 Radiographic Findings:
Radiographic examination revealed mixed radiolucent lesion over left angle region of the mandible. Computerized Tomography revealed an ill defined bony expansile lesion present on left side of mandible extending from distal to 35 to ramus area marked bony reaction. On the basis of clinical and radiological examination, provisional diagnoses of Ameloblastoma and the differential diagnosis of Osteomyelitis, Malignancy and Central Giant cell Granuloma were made. An incisional biopsy was then performed from left angle region of the mandible. Tissue was sent for histopathological examination in formalin fixed bottle.
2.5 Gross Examination:
Gross examination of the tissue revealed two soft tissue specimen were creamish-brown in color, firm in consistency, smooth surface and were oval in shape. Both the tissues were kept for routine processing.
2.6 Histopathological Findings:
The H and E stained sections showed odontogenic islands in the moderately dense connective tissue stroma. The tumor islands show peripheral palisading cells with hyperchromatism and increased mitotic figures. Increased nuclear cytoplasmic ratios were also seen. The connective tissue stroma showed moderately dense connective tissue stroma and endothelial lined blood vessels along with muscle cross-sections.
2.7 Immunohistochemical Findings:
Immunohistochemical analysis of Ki-67 was performed and sporadic positivity was seen in the stellate reticulum like central cells of the odontogenic islands confirming the diagnosis of Ameloblastic Carcinoma.
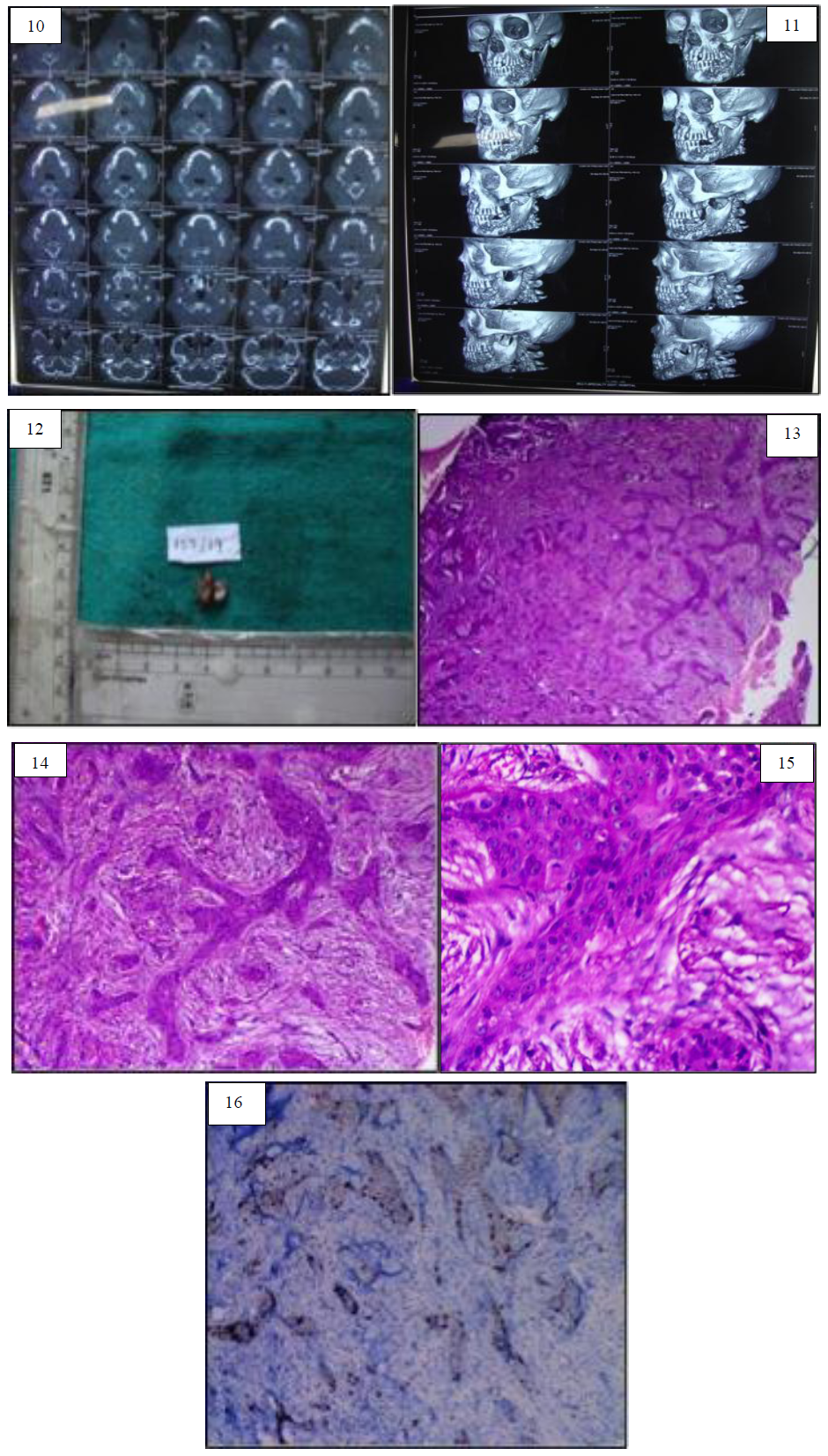
Figure 10: Computerized Tomography Image for Case report 2 showing ill defined bony expansile lesion present on left side of mandible extending from distal to 35 to ramus area marked bony reaction. Figure 11: Three-dimensional reconstructed computed tomography image of second case report. Figure 12: Gross image of case report 2. Figure 13: Histopathological image (4X) for second case reveals strands and chords of odontogenic islands in dense connective tissue stroma. Figure 14: Histopathological image (10X) of second case shows strands of odontogenic islands showing peripheral palisading tall columnar and hyperchromatic nuclei with central stellate reticulum like cells. Figure 15: Histopathological image under higher magnification (40X) of second case cytological atypia seen in the odontogenic islands. Figure 16: Immunohistochemical analysis of second case shows sporadic positivity of ki-67 seen in central cells of the odontogenic islands.
Discussion:
Ameloblastic carcinoma is extremely rare, aggressive malignant epithelial odontogenic tumor with a poor prognosis [5]. Deng et al conducted a systematic review in the year 2018 and reviewed a total 125 cases of Ameloblastic Carcinoma [9]. Kallianpur et al reported total incidence of 1.6% cases of AC of all the odontogenic lesions was reported in the literature [10]. Since Ameloblastic Carcinoma presents as varied clinical presentation, accurate histopathogical diagnosis has immense value in concluding a diagnosis. It can arise de novo or in a preexisting benign ameloblastoma [5]. Even though, it is believed that AC is derived from the dental embryonic remnants or entrapped salivary gland epithelium, the origin cells are unknown [11, 12].
The mean age of occurrence in various literatures is 5-7 decade [9,10, 13,14]. However; the first case of ours which presented in 2nd decade defied the age range, the second case reported in the 7th decade was in conjunction with other studies. Although rare, but the ameloblastic carcinoma cannot be excluded if presented in younger population. Ameloblastic Carcinoma predominantly occurs in male populations [9, 13, 14,15]. Braimah et al reported almost two-third cases reported in male population [15]. The second case reported in our study was seen in a female patient. The most common site for occurrence of Ameloblastic Carcinoma is mandible. Both the cases were seen in Mandible. Soyele et al in their literature reported that Ameloblastic Carcinoma in Mandible is seen in 76.9% cases and 69.2% cases were reported in posterior mandible [14]. The most common clinical presentation of ameloblastic carcinoma is a facial swelling and pain. Other clinical presentation includes cystic lesion with benign clinical features or a large tissue mass with ulceration, bone resorption, and tooth mobility [16]. Both the cases were presented as swelling and pain. Radiological finding in Ameloblastic Carcinoma is deceptive; ill defined radiolucency was seen in 69.23% in a study [17]. Both the cases were presented as ill-defined radiolucency.
Histological presentation of Ameloblastic Carcinoma shows the architectural features of Ameloblastoma with features of cellular dysplasia, such as: pleomorphism, hyperchromatism, and abnormal mitotic figures with altered nuclear/cytoplasmic ratio. Squamous metaplasia and keratin pearl formation, clear cells and ghost cell can also be seen in Ameloblastic Carcinoma [18] Our case presented as Ameloblastoma with all dysplastic features. However, rare features such as clear cells, ghost cells were not seen in our cases.
Metastasis of Ameloblastic Carcinoma is infrequent. Deng et al conducted a systematic review in the year 2018 and included 125 cases, lymph nodes were seen in only 9 cases till date [9]. Lymph node metastasis was seen in our first case. A thorough evaluation is needed in case of Ameloblastic Carcinoma.
Many proliferation markers had been used to diagnose AC, especially, Ki-67, PCNA, and MCM proteins. Bello et al., in 2009 found that Ki-67 labeling index in Ameloblastic Carcinoma is three times that of Ameloblastoma [19]. Bologna-Molina et al. in 2013 studied PCNA and Ki-67 expression in AC and found that Ki-67 was a more specific marker for the proliferation of ameloblastic tumor cells [20].
Table: Key Findings In Ameloblastic Carcinoma
|
Features |
Ameloblastic Carcinoma |
|
Incidence |
1.6% of Odontogenic Lesions |
|
Age |
5th to 7th decade |
|
Gender |
Male preponderence |
|
Site |
Mandible> Maxilla |
|
Clinical Findings |
Swelling, pain, ulceration, tooth mobility, bone resorption |
|
Radiographic Findings |
Ill-defined radiolucency |
|
Histopathological Features |
Architectural features of Ameloblastoma along with cellular dysplasia |
|
Metastasis |
May be present (Local or Distant) |
|
Immunohistochemical Marker |
Proliferative markers such as Ki 67, PCNA and MCM |
|
Differential Diagnosis |
Ameloblastoma, Basaloid Squamous Cell Carcinoma, Primary Intraosseous Squamous Cell Carcinoma, Calcifying Epithelial Odontogenic Tumors, Keratoameloblastoma and Acanthomatous Ameloblastoma |
Differential Diagnosis includes Ameloblastoma, Basaloid Squamous Cell Carcinoma, Primary Intra-osseous Squamous Cell Carcinoma, Squamous Odontogenic Tumor, Calcifying Epithelial Odontogenic Tumors, Keratoameloblastoma and Acanthomatous Ameloblastoma, High grade Mucoepidermoid Carcinoma and Odontogenic Sarcoma and Carcinosarcoma [21]. Ameloblastoma can be differentiated from Ameloblastic Carcinoma as no cellular atypia will be seen the former. Also, Ki-67 positivity ruled out Ameloblastoma. Micro-cystic spaces will show PAS positivity of Basaloid Squamous Cell Carcinoma. Primary Intra-osseous Carcinoma can be ruled out as no ameloblastomatous component and no peripheral palisading and stellate reticulum like cells will be seen in them. Similarly, no ameloblastomatous differentiation and cytological atypia will be seen in Squamous Odontogenic Tumor and instead benign squamous epithelium with micro-cystic spaces and keratinization will be noted. In, CEOT, amyloid deposition will be seen. Keratoameloblastoma and Acanthomatous Ameloblastoma exhibit extensive squamous metaplasia, keratinizing cysts but no cellular atypia will be noted. Rare differential diagnosis includes Metastatic Carcinoma of Jaws from lungs, breasts, Gastro-intestinal tract and salivary gland tumors.
Conclusion:
Ameloblastic Carcinoma is a rare aggressive odontogenic neoplasm with high propensity of metastasis. Since it has wide variety of clinical and radiological presentation, histopathological confirmation of the lesion becomes mandatory. Since, due to rare incidence of the lesion, fewer lesions have been published. Our first case was reported in young male patient. The superficial lesion showed benign Plexiform pattern of Ameloblastoma but deeper lesion exhibit Ameloblastomic Carcinoma with lymph node metastasis. The second case was reported in older female patient and it presents as Ameloblastic Carcinoma. Since the lesion exhibit ameloblastoma with features of cytological atypia.
References:
- Hall JM, Weathers DR, Unni KK. Ameloblastic carcinoma: an analysis of 14 cases. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 103 (2007): 799-807.
- Reichart PA, Philipsen HP. Odontogenic tumors and allied lesions. Quintessence Pub (2004).
- Pindborg Histological typing of odontogenic tumors, jaw cysts, and allied lesions. WHO (1971): 18-27.
- Elzay RP. Primary intraosseous carcinoma of the jaws: review and update of odontogenic carcinomas. Oral Surgery, Oral Medicine, Oral Pathology 54 (1982): 299-303.
- Slootweg PJ, Müller H. Malignant ameloblastoma or ameloblastic carcinoma. Oral Surgery, Oral Medicine, Oral Pathology 57 (1984): 168-176.
- Barnes L, Eveson JW, Sidransky D, et al, editors. Pathology and genetics of head and neck tumours. IARC (2005).
- Speight PM, Takata T. New tumour entities in the 4th edition of the World Health Organization Classification of Head and Neck tumours: odontogenic and maxillofacial bone tumours. Virchows Archiv 472 (2018): 331-339.
- Kruse AL, Zwahlen RA, Grätz KW. New classification of maxillary ameloblastic carcinoma based on an evidence-based literature review over the last 60 years. Head & Neck Oncology 1 (2009): 31.
- Deng L, Wang R, Yang M, et al. Ameloblastic carcinoma: Clinicopathological analysis of 18 cases and a systematic review. Head & Neck 41 (2019): 4191-4198.
- Kallianpur S, Jadwani S, Misra B, et al. Ameloblastic carcinoma of the mandible: Report of a case and review. Journal of Oral and Maxillofacial Pathology: JOMFP 18 (2014): S96.
- Sandoval-Basilio J, González-González R, Bologna-Molina R, et al. Epigenetic mechanisms in odontogenic tumors: A literature review. Archives of Oral Biology 87 (2018): 211-217.
- Inoue N, Shimojyo M, Iwai H, et al. Malignant ameloblastoma with pulmonary metastasis and hypercalcemia: Report of an autopsy case and review of the literature. American Journal of Clinical Pathology 90 (1988): 474-481.
- Dhir K, Sciubba J, Tufano RP. Ameloblastic carcinoma of the maxilla. Oral Oncology 39 (2003): 736-741.
- Soyele OO, Adebiyi KE, Adesina OM, et al. Ameloblastic carcinoma: a clinicopathologic analysis of cases seen in a Nigerian Teaching Hospital and review of literature. The Pan African Medical Journal 31 (2018).
- Abir B, Abouchadi A, Tourabi K, et al. Ameloblastic carcinoma of the mandible: A case report and review of the literature. Médecine Buccale Chirurgie Buccale 23 (2017): 95-98.
- Braimah RO, Uguru C, Ndukwe KC. Ameloblastic carcinoma of the jaws: Review of the literature. Journal of Dental and Allied Sciences 6 (2017): 70.
- Selvam S, Damodaran S, Ramesh V, et al. Ameloblastic carcinoma masquerading as dentigerous cyst of the mandible. Journal of Oral Research and Review 11 (2019): 81.
- Mahmoud SA, Amer HW, Mohamed SI. Primary ameloblastic carcinoma: literature review with case series. Polish Journal of Pathology 69 (2018): 243-253.
- Bello IO, Alanen K, Slootweg PJ, et al. Alpha-smooth muscle actin within epithelial islands is predictive of ameloblastic carcinoma. Oral Oncology 45 (2009): 760-765.
- Bologna-Molina R, Mosqueda-Taylor A, Molina-Frechero N, et al. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumor. Medicina Oral, Patologia Oral y Cirugia Bucal 18 (2013): e174.
- Sancheti S, Somal PK, Sarkar S. Ameloblastic carcinoma: A diagnostic dilemma. Indian Journal of Pathology and Microbiology 62 (2019): 501.


 Impact Factor: * 3.0
Impact Factor: * 3.0 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
