An Algorithmic Approach for and Special Characteristics of Veno-Venous Extracorporeal Life Support in Patients With Severe Asthma Attack: A Case Report and Review of the Literature
Article Information
Linna HUANG, Qingyuan ZHAN*
Department of Pulmonary and Critical Care Medicine, Centre for Respiratory Diseases, China-Japan Friendship Hospital, Beijing, P. R. China
*Corresponding Author: Qingyuan ZHAN, Department of Pulmonary and Critical Care Medicine, Centre for Respiratory Diseases, China-Japan Friendship Hospital, Beijing, P. R. China
Received: 31 May 2019; Accepted: 10 June 2019; Published: 14 June 2019
Citation: Linna HUANG, Qingyuan ZHAN. An Algorithmic Approach for and Special Characteristics of Veno-Venous Extracorporeal Life Support in Patients With Severe Asthma Attack: A Case Report and Review of the Literature. Archives of Clinical and Biomedical Research 3 (2019): 241-256.
View / Download Pdf Share at FacebookAbstract
Little is known about the indicators and special characteristics of veno-venous extracorporeal life support (VV-ECLS) in patients with a severe asthma attack. We included a new case with near-fatal asthma (NFA) in a medical intensive care unit (MICU). Cases with severe asthma attack requiring VV-ECLS from 1980 to 2018 from the literature were added. The general information, laboratory examinations, treatments and outcomes were summarized. There were seventeen cases with severe asthma attack supported with VV-ELCS from the literature, including our case; six of them received extracorporeal CO2 removal (ECCO2R). The average age of the patients was 37 (±18) years, and only three had an underlying disease. Among the seventeen patients, most had a history of prior exacerbations and improper therapies before the current exacerbation, for which there were eleven (64.7%) and nine (52.9%) patients, respectively. As high as fourteen patients (82.4%) had a combination of complications related to asthma pre-ECLS. The most common complication was severe and refractory acidosis. Arterial blood gas analysis values and respiratory mechanics improved significantly post-ECLS. The average blood flow and gas flow of the patients receiving VV-ECLS were 2.3 (±1.2) L/min and 4.0 (±2.6) L/min, respectively. The mean duration of ECLS was 48 (35-96) hours. Complications related to ECLS occurred in 35.3% of the patients, among which hemorrhage was the high prevalence (23.5%). An algorithmic approach to VV-ECLS was described for a patient with a history of prior exacerbations and improper therapies that should be actively supported by ECLS once he becomes unresponsive to maximal therapies or presents with a combination of severe complications. Considering the short duration and the high risk of haemorrhage, anticoagulation without heparin might be a choice.
Keywords
Severe Asthma Attack; Algorithmic Approach; VV-ECLS; No Anticoagulation
Article Details
Introduction
Severe and refractory asthma attacks often require invasive ventilation, which is attributed to 30% mortality [1,2]. Mechanical ventilation might lead to several complications attributed to the related mortality of 7-8% [3-5]. Extracorporeal life support (ECLS) is an alternative strategy, allowing time for “inflammation resolution of the airway” and avoiding complications effectively. There have been several scattered case reports on the successful treatment of a severe asthma attack by VV-ECLS in recent years. The selected patients had some common characteristics, but there was no systematic summary of these cases. Thus, we describe herein a new case from our centre and a comprehensive review of cases reported in the literature [5-17], with a particular focus on the indicators and the special characteristics of VV-ECLS in severe asthma attacks compared with other diseases.
Materials and methods
1. Study population Our case
We included a new case with near-fatal asthma (NFA) admitted to the medical intensive care unit (MICU) of the China-Japan Friendship Hospital.
Cases from the literature
In addition to our case, we searched the English-language published literature using PubMed/Medline with the search terms “NFA” or “status asthmaticus” and “ECMO” or “Extracorporeal CO2 removal (ECCO2R)” from 1980 to 2018. The cases with veno-arterial ECLS were excluded.
2. Diagnostic Criteria Definition of status asthmaticus
Status asthmaticus is an acute exacerbation of asthma that does not respond to standard treatments of bronchodilators and corticosteroids. Symptoms include chest tightness, rapidly progressive dyspnoea, dry cough, use of accessory respiratory muscles, fast and/or labored breathing, and extreme wheezing. It is a life-threatening episode of airway obstruction and is considered a medical emergency [18].
Definition of NFA
NFA is a serious condition of asthma attack that has the characteristics of hypoxemia, hypercapnia, and altered mental status and often requires invasive ventilation, which is attributed to 30% of mortality [1, 2].
3. Data collection
Data were retrospectively collected. Demographic data included the age, gender, underlying disease, history of smoking, history of prior exacerbations of asthma and oral corticosteroid administration, and whether the proper therapies were given before the exacerbation was also recorded. Pharmacological therapies, arterial blood gas (ABG) results, ventilator parameters, respiratory mechanics, and complications pre- and post-ECLS were collected. Whether the patients underwent “awake ECLS” and the duration of invasive positive-pressure ventilation (IPPV) and ECLS were recorded as well.
Statistical Analysis
All of the analyses were carried out using SPSS 17.0 software. Normally distributed continuous variables were expressed as the mean±SD and compared using a paired t-test. Non-normally distributed continuous variables were expressed as medians and quartiles and compared using a Wilcoxon rank-sum test. P values<0.05 were considered significant.
Results
1. The new case from our MICU
A 51-year-old woman presented to the emergency department for progressive shortness of breath and asthmatic orthopnoea. She was a farmer and allergic to cat and dog hair with no history of smoking. The patient had a known history of asthma for 20 years, with no irregular therapies because of poor compliance. There were more than 4 previous hospital admissions for exacerbation, none of which required noninvasive or invasive ventilation. On clinical examination, she was confused, cyanotic, diaphoretic, and using accessory muscles. Her initial ABG, on an inspiratory fraction of oxygen (FiO2) of 0.33, showed pH: 7.14, partial pressure of arterial carbon dioxide (PaCO2): 88 mmHg, partial pressure of arterial oxygen (PaO2): 54 mmHg, HCO3-: 29.2 mmol/L, and lactate (Lac): 1.7 mmol/L. A routine blood test showed a white blood cell count (WBC) of 14.18*10^9/L and an eosinophil count of 0. The patient was treated with inhaled salbutamol in repeated doses, nebulized ipratropium bromide and budesonide, intravenous fluids and a high dose of steroids (equivalent to 120 mg of methylprednisolone). However, the condition of the patient deteriorated despite the therapy. The repeat ABG on FiO2 0.3 showed pH: 7.08, PaCO2: 107 mmHg, PaO2: 71 mmHg, HCO3-: 31.7 mmol/L, and Lac: 2 mmol/L. She was intubated and transferred to the MICU.
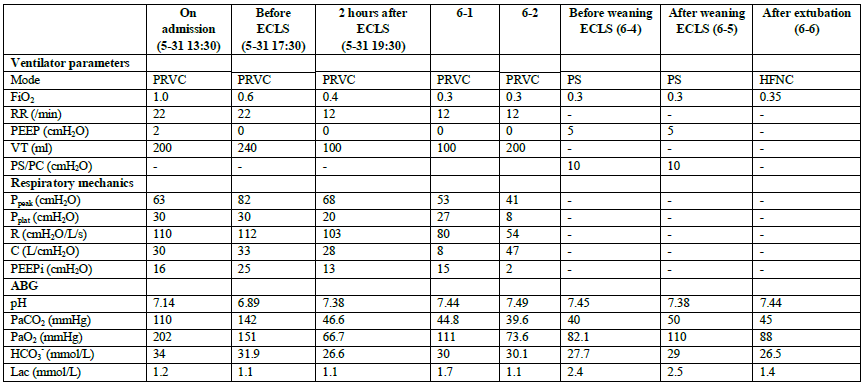
We treated her with pressure-regulated volume-controlled (PRVC) mode ventilation at a tidal volume (VT) of 200 ml, a of 22/min (then to 12/min), a positive end-expiratory pressure (PEEP) of 2 cmH2O (then to 0 cmH2O), and a FiO2 of 1.0. In addition, propofol, midazolam, morphine and rocuronium bromide were continuously infused to facilitate synchrony between the patient and ventilator. Inhaled salbutamol with a spacer, nebulized ipratropium bromide and
budesonide 4 times a day, approximately 3000 ml of intravenous fluid and 160 mg of methylprednisolone steroid per day were administered. The initial index of respiratory mechanics was a peak airway pressure (Ppeak) of 63 cmH2O, plateau pressure (Pplat) of 30 cmH2O, intrinsic levels of PEEP (PEEPi) of 16 cmH2O, airway resistance (R) of 110 cmH2O/L/s and respiratory compliance (C) of 30 L/cmH2O. However, there were no improvements in the index of respiratory mechanics and ABG values despite the regulation of the ventilator and sufficient pharmacological therapy after 4 hours (Table 1). The patient was still on persistent high Ppeak, high Pplat, and PEEPi and developed hyperkalaemia and anuria. Meanwhile, the blood pressure declined, and the patient required norepinephrine at 0.4 μg/kg/min. The decision to transition to VV-ECLS was made 8 hours after leaving the emergency department. The blood flow was 3.5-4.1 L/min, and the gas flow was 4 L/min, with heparin infusion and monitoring of the target, activated partial thromboplastin time (APTT), within 55-65 s. The ABG values improved after 4 hours of ECMO, while the resistance of the airway and the PEEPi were improved 2 days later, improving gradually as the therapy continued (Table I).
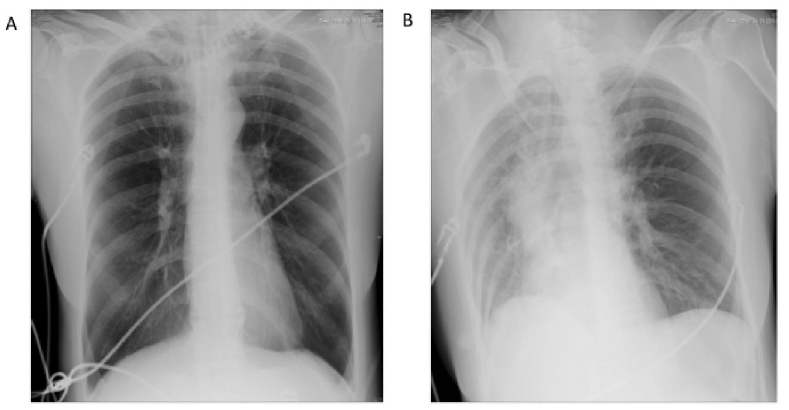
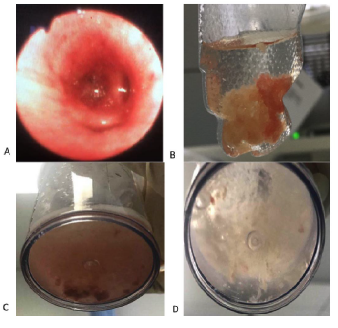
The chest X-ray (CXR) on the first day of admission showed bilateral hyperventilation and a narrow cardiac shadow without obvious infiltration, while pulmonary atelectasis appeared the day after ECLS support (Figure 1). A fibro-optic bronchoscopy was subsequently performed, which revealed a large amount of thick bronchial secretions occluding the middle lobe of the right lung and the upper and lingual lobe of the left lung. Mucous plugs could be observed in bronchoalveolar lavage fluid (BALF) (Figure 2). Cytological classification of the BALF revealed that the majority were neutrophil granulocytes, which accounted for 57.5% of the cells, while the eosinophil granulocytes accounted for 5%.
VV-ECLS was continued for 120 hours (5 days), and the intubation for IPPV was removed after 156 hours. There was no ECLS-related complication. The patient transferred to the ward on the 13th day and was discharged on the 23rd day after admission.
ABG, arterial blood gas; ECLS, extracorporeal life support; FiO2, inspiratory fraction of oxygen; RR, respiratory rate; PEEP, positive end-expiratory pressure; VT, tidal
volume; PS/PC, pressure support/ pressure control; Ppeak, peak airway pressure; Pplat, plateau pressure; R, airway resistance; C, respiratory compliance; PEEPi, intrinsic
level of positive end-expiratory pressure; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen; Lac, lactate
2. Pooled analysis of our case and the literature
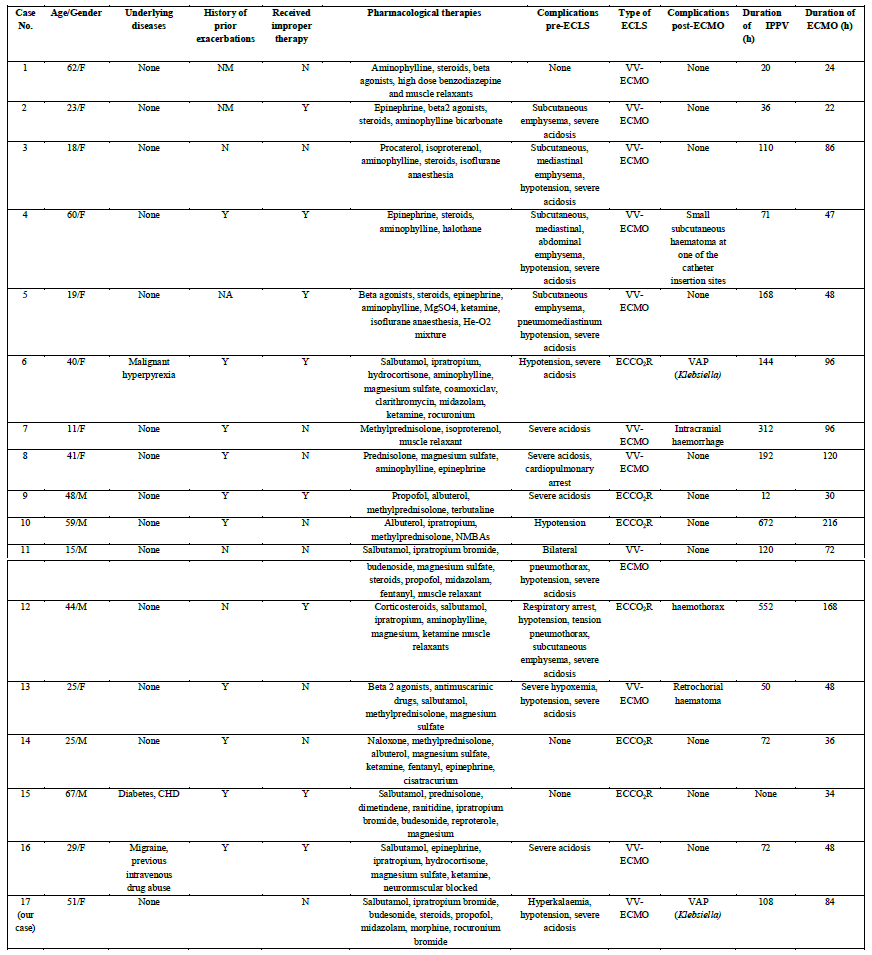
In addition to our case, there were sixteen cases of severe asthma attacks supported with VV-ECLS from literature review [5-17]. Six of them received extracorporeal CO2 removal (ECCO2R). The details are presented in Table II.
General information and complications before ECLS
The average age of the patients was 37 (±18) years, and 41.2% (7/17) of them were male. Among the seventeen patients, only three (17.6%) had an underlying disease, one of them had malignant hyperpyrexia, another complained of diabetes and coronary heart disease, and the other patient suffered from migraine and previous intravenous drug abuse. The number of patients with a history of prior exacerbations and improper therapies before the current exacerbation was eleven (64.7%) and nine (52.9%), respectively, while the number of patients with a history of smoking and oral corticosteroids was four
(23.5%) and three (13.6%), respectively. As high as fourteen patients (82.4%) presented with a combination of complications related to asthma pre-ECLS. The most common complication was severe and refractory acidosis, accounting for 70.6% (12/17) the complications, which was defined as a pH below 7.1 despite maximal pharmacological therapies and full strength of ventilator support. The second most common was hypotension or shock because of severe acidosis or cardiac dysfunction related to dynamic lung inflation, which accounted for 47.1% (8/17) (Table III).
CHD, coronary heart disease; NM, not mentioned; N, no; Y, yes; VV-ECMO, veno-venous extracorporeal membrane oxygenation; ECCO2R, extracorporeal CO2 removal
|
Variables |
No. of patients (%) |
|
|
|
|
|
|
Age, year (mean±SD) |
37±18 |
|
|
|
|
|
|
Sex, male |
7 |
(41.2) |
|
|
|
|
|
Underlying disease |
3 |
(17.6) |
|
|
|
|
|
History of prior exacerbations |
11 (64.7) |
|
|
|
|
|
|
History of oral corticosteroid |
4 |
(23.5) |
|
|
|
|
|
History of smoking |
3 |
(17.6) |
|
|
|
|
|
Received improper therapy |
9 |
(52.9) |
|
|
|
|
|
Complications pre-ECLS |
14 (82.4) |
|
|
|
|
|
|
Barotrauma |
6 |
(35.3) |
|
|
|
|
|
Hypotension or shock |
8 |
(47.1) |
|
|
|
|
|
Cardiopulmonary arrest |
1 |
(5.9) |
|
|
|
|
|
Severe acidosis (pH<7.1) |
12 (70.6) |
|
|
|
|
|
|
Severe hypoxemia (PaO2/FiO2 <100 mmHg) |
2 |
(11.8) |
ECLS, extracorporeal life support
Table III: General information, ABG and respiratory mechanics before ECMO in patients with severe asthma attack
Changes in ABG values and respiratory mechanics in patients on ECLS
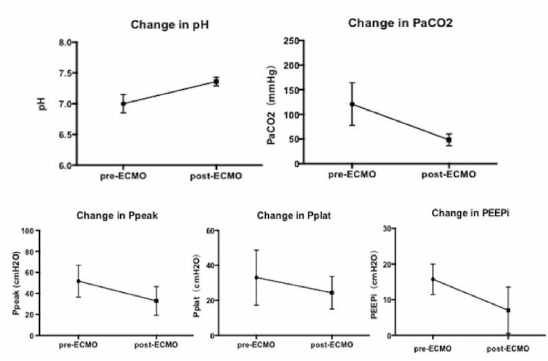
The ABG analysis, which included pH and PaCO2, and the respiratory mechanics, which contained Ppeak and PEEPi (since airway resistance and the compliance were not contained in most of the studies, we did not present them in the results) were all improved after ECLS support. The pH value changed from 7.01 (±0.15) to 7.36 (±0.07) (p<0.01), while PaCO2 decreased from 117 (±47) mmHg to 48 (±12) mmHg (p<0.01). Ppeak and PEEPi decreased from 53 (±17) cmH2O to 33 (±14) cmH2O (p<0.01) and from 17 (±6) cmH2O to 7 (±6) cmH2O (p=0.024), respectively. Pplat and PaO2 pre- and post-ECLS support showed no significant differences (Figure 3).
Parameters of ECLS, ECLS-related complications and duration of mechanical support
The average blood flow and gas flow of the VV-ECLS therapies were 2.3 (±1.2) L/min and 4.0 (±2.6) L/min, respectively, which were relatively lower than those of the patients with hypoxemic respiratory failure supported by ECLS. Among those seventeen patients, only two (11.8%) underwent “awake ECLS”. Complications post-ECMO occurred in 35.3% (6/17) of the patients, among which haemorrhage was the highest prevalence at 23.5% (4/17). The complication of haemorrhage included subcutaneous haematoma at one of the catheter insertion sites, intracranial haemorrhage, haemothorax and retro-chorial haematoma. Two (11.8%) patients suffered from ventilator-associated pneumonia, with Klebsiella isolated from the lower respiratory tract. The mean duration of ECLS and IPPV were 48 (35-96) hours and 108 (43-180) hours, respectively (Table IV).
Figure 3: Changes in ABG values and respiratory mechanics in patients on ECMO. The arterial blood gas (ABG) analysis, which included pH and PaCO2, and the respiratory mechanics, which contained Ppeak and PEEPi, were all improved after ECMO support. The pH value changed from 7.01 (±0.15) to 7.36 (±0.07) (p<0.01), while PaCO2 decreased from 117 (±47) mmHg to 48 (±12) mmHg (p<0.01). Ppeak and PEEPi decreased from 53 (±17) cmH2O to 33 (±14) cmH2O (p<0.01) and from 17 (±6) cmH2O to 7 (±6) cmH2O (p=0.024), respectively.
|
Variables |
No. of patients (%) |
|
Blood flow, L/min (mean±SD) |
2.3±1.2 |
|
Gas flow, L/min (mean±SD) |
4.0±2.6 |
|
“Awake ECLS” |
2 (11.8) |
|
Complications post-ECLS |
6 (35.3) |
|
Haemorrhage |
4 (23.5) |
|
Nosocomial infection |
2 (11.8) |
|
Duration of ECLS, hours, median (IQR) |
48 (35-96) |
|
Duration of IPPV, hours, median (IQR) |
108 (43-180) |
ECLS, extracorporeal life support; IPPV, invasive positive-pressure ventilation
Table IV: Parameters, complications and duration of ECLS in patients with severe asthma attack
3. An algorithmic approach to VV-ECLS in patients with severe asthma attack
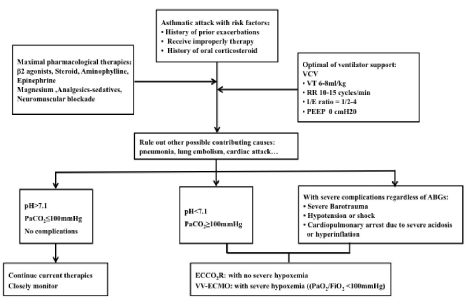
From a comprehensive review and summary of the scattered case reports with VV-ECLS, we found that most patients requiring ECLS support had a history of prior exacerbations (64.7%) and improper therapy before the current attack (52.9%). Those patients often represented severe respiratory acidosis [pH 7.01 (±0.15) or PaCO2 117 (±47) mmHg] even maximal pharmacological therapies and the full strength of ventilator supports was administered. In addition, a large number of patients requiring ECLS often presented with a combination of severe complications (82.4%) due to hyperinflation or severe acidosis. Thus, we concluded an algorithmic approach for VV-ECLS support in patients with a severe asthma attack that is presented in Figure 4.
Figure 4: An algorithmic approach to VV-ECMO in patients with severe status asthmaticus or NFA. A patient with some high-risk factors should be actively supported by ECMO once he becomes unresponsive to maximal pharmacological therapy and optimal ventilator support or presents with a combination of severe complication.
Discussion
The biggest strength of our study was that we first made a comprehensive review and summary of the scattered case reports to explore an algorithmic approach for and several special characteristics of VV-ECLS in patients with severe status asthmaticus or NFA.
An algorithmic approach to VV-ECLS in patients with severe asthma attack
Mechanical ventilation as well as the high dose of sedation, analgesics and muscle relaxant required for good tolerance in patients with severe asthma might increase the risk of dynamic air trapping and hyperinflation, which increased the work of breathing, caused barotrauma and cardiac dysfunction [19-21] and ultimately contributed to death [3-5]. ECMO allows reduction of both the tidal volume and minute ventilation, which subsequently reduces ventilator-related complications, thus decreasing the mortality [5].
From the first case successfully rescued by ECLS in the year 1981 [22], several case reports [5-17] and two relatively large retrospective studies [2, 23] showed the efficacy and safety of ECMO as a rescue therapy in patients with a severe asthma attack. However, no studies have specifically explicated the algorithmic approach to VV-ECLS support.
From a comprehensive review and summary of the scattered case reports with VV-ECLS, we concluded an algorithmic approach for VV-ECLS support in patients with a severe asthma attack that is presented in Figure 4. Since severe asthma attack is a reversible pulmonary disease, a patient with some high-risk factors should be actively supported by ECLS once he becomes unresponsive to maximal pharmacological therapy and optimal ventilator support or presents a with a combination of severe complications.
Several special characteristics of VV-ECLS in patients with severe asthma attack Whether there is the necessity for anticoagulation?
The complications post-ECLS in our study occurred in 35.3% (6/17) of the patients, among which haemorrhage was the highest at 23.5% (4/17). Another study revealed that the total complication rate was 65.1%, with haemorrhagic complications being the most common (28.3%) [23]. Since the pathophysiology of asthma mainly involves airway smooth muscle constriction, airway oedema, airway plugging by mucus or cellular debris rather than lung injury [15], the mean duration of ECLS was relatively lower than for those with hypoxemic respiratory failure, which was 48 (35-96) hours in our study. Therefore, we speculated that the lower target for anticoagulation or anticoagulation without heparin might be an effective way to avoid haemorrhage as well as maintain the normal function of the oxygenator considering the high risk of haemorrhage and the short duration of ECLS support.
Whether there is the necessity for “awake ECLS”?
“Awake ECLS” is defined as a patient supported by ECLS without intubation, which results in a direct reduction of the patients’ respiratory effort, alleviation of hyperinflation while the airways are left untouched and spontaneous breathing
The advantages of keeping the patient “awake” are avoidance of VAP, reduction of delirium, reduction of feeding problems, and allowing social contacts with friends and family, as well as allowing sufficient physiotherapy to reduce myopathy and critical care illness [25, 26].
In our opinion, since the duration of ECLS was relatively short, the advantages above were weakened. Meanwhile, the patient discomfort, pain, and anxiety in the “awake approach” might be to such an extent that starting deep sedation and mechanical ventilation is inevitable, and all advantages are lost [16]. Thus, we speculated that “awake ECLS” might not be necessary for patients with a severe asthma attack.
Is ECCO2R better than VV-ECMO?
ECCO2R could effectively remove CO2 with low blood flow; however, it was ineffective in improving oxygenation [27]. As our study revealed, severe and refractory hypercapnia was a more common complication (70.6%) than severe hypoxemia (11.8%) in patients with a severe asthma attack; meanwhile, the average blood flow of the patients receiving VV-ECLS was 2.3 (±1.2) L/min, which was relatively lower than that of patients with hypoxemic respiratory failure, making ECCO2R possible. In addition, ECCO2R could be achieved by placing just one double-lumen cannula conveniently [12, 16]. We speculated that patients without severe hypoxemia could receive ECCO2R as the first choice when requiring ECLS.
Whether bronchoscopy is necessary and safe?
Bronchoscopy is a useful tool for phenotyping asthma with objective data obtained from BAL, endobronchial biopsy, and brushings for the direction of therapeutic decisions [28-30]. Moreover, thick bronchial secretions and mucous plugs were commonly observed in critically ill asthma patients, which obstructed the airway, causing persistent high airway resistance and aggravating hyperinflation. In our case, aggressive pulmonary hygiene, including frequent bronchoscopies, was employed to improve the patient’s respiratory status. It also enables pulmonary hygiene that could be lifesaving in the case of an asthma-associated plastic bronchitis [10]. ECLS made it possible and safe for aggressive pulmonary hygiene in patients with a severe asthma attack.
The limitations of our study
The limitation of this study is that the number of selected cases is relatively small and may not represent all patients with a severe asthma attack. There is still an urgent need to expand the sample size of the study and perform a case-controlled study comparing patients with VV-ECLS and conventional mechanical ventilation in the future.
Acknowledgements
Not applicable.
Funding
This study is supported by the “National Key Research and Development Programme” Major Chronic Non-communicable Diseases’ Prevention and Control (QML 2016YFC1304300).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
All authors made substantial contributions to the conception and design of the study or to the data acquisition, analysis or interpretation; reviewed and approved the final manuscript; and significantly contributed to this study. Dr. Zhan took full responsibility for the integrity of the submission and publication and was involved in the study design. Drs. Huang and Zhan were involved in data collection, had full access to all of the data in the study, took responsibility for the integrity of the data and the accuracy of the data analysis and were responsible for data verification and analysis, as well as the drafting of the manuscript.
Ethics approval and consent to participate
This study was approved by the ethics committee of the China-Japan Friendship Hospital, Beijing, P.R. China, and written informed consent was obtained from the patients or their next of kin.
Consent for publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
References
- Restrepo RD, Peters J: Near-fatal asthma: recognition and management. Current opinion in pulmonary medicine 14 (2008): 13-23.
- Di Lascio G, Prifti E, Messai E, et al: Extracorporeal membrane oxygenation support for life-threatening acute severe status asthmaticus. Perfusion 32 (2017): 157-163.
- Krishnan V, Diette GB, Rand CS, et al: Mortality in patients hospitalized for asthma exacerbations in the United States. American journal of respiratory and critical care medicine 174 (2006): 633-638.
- Khawaja A, Shahzad H, Kazmi M, et al: Clinical course and outcome of acute severe asthma (status asthmaticus) in adults. JPMA The Journal of the Pakistan Medical Association 64 (2014): 1292-1296.
- Alzeer AH, Al Otair HA, Khurshid SM, et al: A case of near fatal asthma: The role of ECMO as rescue therapy. Annals of thoracic medicine 10 (2015): 143-145.
- Tajimi K, Kasai T, Nakatani T, et al: Extracorporeal lung assist for patient with hypercapnia due to status asthmaticus. Intensive care medicine 14 (1988): 588-589.
- Shapiro MB, Kleaveland AC, Bartlett RH: Extracorporeal life support for status asthmaticus. Chest 103 (1993): 1651-1654.
- Kukita I, Okamoto K, Sato T, et al: Emergency extracorporeal life support for patients with near-fatal status asthmaticus. The American journal of emergency medicine 15 (1997): 566-569.
- Leiba A, Bar-Yosef S, Bar-Dayan Y, et al: Early administration of extracorporeal life support for near fatal asthma. The Israel Medical Association journal : IMAJ 5 (2003): 600-602.
- Tonan M, Hashimoto S, Kimura A, et al: Successful treatment of severe asthma-associated plastic bronchitis with extracorporeal membrane oxygenation. Journal of anesthesia 26 (2012): 265-268.
- Iwamoto T, Ikeda K, Nakajima H, et al: Extracorporeal membrane oxygenation is indicated for status asthmaticus refractory to maximal conventional therapy. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 110 (2013): 300-301.
- Brenner K, Abrams DC, Agerstrand CL, et al: Extracorporeal carbon dioxide removal for refractory status asthmaticus: experience in distinct exacerbation phenotypes. Perfusion 29 (2014): 26-28.
- Tiruvoipati R, Haji K, Gupta S, et al: Low-flow veno-venous extracorporeal carbon dioxide removal in the management of severe status asthmatics: a case report. The clinical respiratory journal 10 (2016): 653-656.
- Steinack C, Lenherr R, Hendra H, et al: The use of life-saving extracorporeal membrane oxygenation (ECMO) for pregnant woman with status asthmaticus. The Journal of asthma : official journal of the Association for the Care of Asthma 54 (2017): 84-88.
- Jiang C, Galaydick J, Fernandez H, et al: Adjunctive extracorporeal carbon dioxide removal in refractory status asthmaticus. BMJ case reports 2017: 2017.
- Schneider TM, Bence T, Brettner F: "Awake" ECCO2R superseded intubation in a near-fatal asthma attack. Journal of intensive care 5 (2017): 53.
- Maqsood U, Patel N: Extracorporeal membrane oxygenation (ECMO) for near-fatal asthma refractory to conventional ventilation. BMJ case reports 2018: 2018.
- Shah R, Saltoun CA: Chapter 14: Acute severe asthma (status asthmaticus). Allergy and asthma proceedings 33 (2012): Suppl: 47-50.
- Anzueto A, Frutos-Vivar F, Esteban A, et al: Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive care medicine 30 (2004): 612-619.
- Hodder R, Lougheed MD, FitzGerald JM, et al: Management of acute asthma in adults in the emergency department: assisted ventilation. CMAJ : Canadian Medical Association journal 182 (2010): 265-272.
- Schatz M, Kazzi AA, Brenner B, et al: Joint task force report: supplemental recommendations for the management and follow-up of asthma exacerbations. Introduction. The Journal of allergy and clinical immunology 124 (2009): S1-S4.
- MacDonnell KF, Moon HS, Sekar TS, et al: Extracorporeal membrane oxygenator support in a case of severe status asthmaticus. The Annals of thoracic surgery 31 (1981): 171-175.
- Yeo HJ, Kim D, Jeon D, et al: Extracorporeal membrane oxygenation for life-threatening asthma refractory to mechanical ventilation: analysis of the Extracorporeal Life Support Organization registry. Critical care (London, England) 21 (2017): 297.
- Langer T, Santini A, Bottino N, et al: "Awake" extracorporeal membrane oxygenation (ECMO): pathophysiology, technical considerations, and clinical pioneering. Critical care (London, England) 20 (2016): 150.
- Yeo HJ, Cho WH, Kim D: Awake extracorporeal membrane oxygenation in patients with severe postoperative acute respiratory distress syndrome. Journal of thoracic disease 8 (2016): 37-42.
- Kikukawa T, Ogura T, Harasawa T, et al: H1N1 influenza-associated pneumonia with severe obesity: successful management with awake veno-venous extracorporeal membrane oxygenation and early respiratory physical therapy. Acute medicine & surgery 3 (2016): 186-189.
- Burki NK, Mani RK, Herth FJF, et al: A novel extracorporeal CO2 removal system: results of a pilot study of hypercapnic respiratory failure in patients with COPD. Chest 143 (2013): 678-686.
- Moore WC, Evans MD, Bleecker ER, et al: Safety of investigative bronchoscopy in the Severe Asthma Research Program. The Journal of allergy and clinical immunology 128 (2011): 328-336 e323.
- Good JT, Jr., Kolakowski CA, Groshong SD, et al: Refractory asthma: importance of bronchoscopy to identify phenotypes and direct therapy. Chest 141 (2012): 599-606.
- Lommatzsch SE, Martin RJ, Good JT, Jr.: Importance of fiberoptic bronchoscopy in identifying asthma phenotypes to direct personalized therapy. Current opinion in pulmonary medicine 19 (2013): 42-48.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 