Antibiotic use in Primary care in Northern Ireland
Article Information
Naomi Hamilton and Heather M. Coleman*
1School of Pharmacy and Pharmaceutical Sciences; Ulster University, Coleraine, UK
*Corresponding author: Heather M. Coleman, School of Pharmacy and Pharmaceutical Sciences; Ulster University, Coleraine, UK.
Received: 16 April 2024, Accepted: 23 April 2024, Published: 27 May 2024
Citation:
Naomi Hamilton and Heather M. Coleman. Antibiotic use in Primary care in Northern Ireland. Archives of Microbiology and Immunology. 8 (2024): 198-213.
View / Download Pdf Share at FacebookAbstract
Research is scarce regarding antimicrobial use and antimicrobial resistance (AMR) for specific localities in Northern Ireland (NI). Additionally, the effects of COVID-19 on antimicrobial prescribing patterns and AMR are unknown. Between the European Union (EU) and United States (US), AMR causes approximately 68,000 deaths annually. An electronic survey was developed and distributed to eighty-five community pharmacists in Belfast. Yearly and monthly prescription data was gathered from the Business Services Organisation and COVID-19 statistics from Gov.uk. Over- all response rate was 46%. Most pharmacists (82%) failed to address prescriber non-compliance with guidelines and antimicrobial dosing errors (59%). The most common indication for antibiotics was upper respiratory tract infections (URTIs). Overprescribing of antibiotics was perceived as the leading cause of AMR, therefore 69% of pharmacists indicated increased General Practitioner (GP) compliance with guidelines would reduce AMR. Pharmacists’ are in an ideal position to reduce AMR through patient education however, it is demonstrated that pharmacists failed to adequately counsel patients on antibiotic use. All GP practices demonstrated inappropriate antibiotic use, especially for URTIs which suggests antibiotic appropriateness should be reviewed. Many patients avoided contact with GP’s during COVID-19 which may have resulted in reduced antibiotic use. This re- search established amoxicillin as the most commonly prescribed antibiotic, which is contributing to increased AMR with its broad-spectrum activity and has recognised a decrease in antibiotic pre- scribing during COVID-19. Based on the results found and a critical review of the literature it is recommended antimicrobial guidelines should be reviewed and improved, enhanced training should be provided to pharmacists and the antibiotic guardian (AG) campaign should be re-energised.
Keywords
Antimicrobial use, antimicrobial resistance, COVID-19, pharmacists, primary care
Article Details
1. Introduction
Antimicrobial resistance (AMR) occurs when a microorganism evolves following exposure to an antimicrobial drug, resulting in failure of the antimicrobial medication [1]. According to the WHO, 2022 [2], the successful treatment of infections caused by bacteria, fungi, viruses and parasites are dwindling due to AMR. The overuse and misuse of antimicrobials combined with reduced manufacture of new antimicrobials are major factors influencing AMR [3]. Reports have demonstrated that primary care accounts for 80% of antibiotics prescribed and between 20-50% of these are estimated to be inappropriate [4,5]. The extensive use of broad-spectrum antibiotics is contributing to an increase in serious systemic fungal infections [6]. AMR is of increasing global concern and was described as a “ticking time bomb” by the Chief Medical Officer (CMO) of England, highlighting the seriousness of this issue [7]. New mechanisms of resistance are rapidly evolving, hindering health care professional’s (HCP) ability to treat infections [1]. Previously, AMR was mostly associated with hospital and care settings, however primary care currently has increasing numbers of resistant infections [3]. Immediate action is needed to prevent a post-antibiotic era in which common infections are a cause of death [1]. It is estimated that between the European Union (EU) and the United States (US), 68,000 people die annually as a result of an antimicrobial resistant infection [8, 9]. These infections cost the NHS approximately £180 million annually [10].
Antimicrobials play an essential role in the treatment of serious or life threating infections such as malaria and pneumonia. They are also vital in the prevention of infection in neonates, patients undergoing chemotherapy, transplants or invasive surgical procedures such as caesarean sections [3]. Research by Clancy et al, 2020 [11] predicted a rise in AMR due to the increased, unnecessary use of broad-spectrum antibiotics during COVID-19. On the other hand, Collignon and Beggs, 2020 [12] suggested that the pandemic will reduce AMR due to a reduction in the spread of resistant microorganisms through increased infection control practices in both the community and healthcare facilities. These contrasting views demonstrate there is a significant lack of knowledge and evidence relating to AMR levels during and post the first wave of the COVID-19 pandemic [13]. A five-point global action plan was designed which aimed to reduce AMR. The action plan intended to educate the community on AMR, decrease infection rates through enhanced sanitation and infection control measures, improve knowledge on AMR through research, ensure optimal antimicrobial use and reduce the cost associated with AMR through investments in vaccine programmes, manufacturing of new antimicrobial drugs and diagnostic tools [14].
There is a global misconception that antibiotics cure viral self-limiting infections such as the cold or flu [15]. Thus, antimicrobial stewardship (AMS) aims to optimise appropriate use of antimicrobial drugs through selection of the most suitable drug for the relevant infection, given by the correct route, at the right dose and for the required time [16].. In primary care, pharmacists are key in implementing AMS through evidence-based medicine optimisation and antimicrobial prescribing reviews [17]. The antibiotic guardian (AG) campaign was introduced during the 2014 European Antibiotic Awareness Day (EAAD) as a pledge for HCPs and the public. The aim of this campaign for HCP including pharmacists was to empower champions of AMS within their role and remit, including education of HCPs and the public on antibiotic use and in turn reduce AMR [18]. Previous research by Mone, 2018 [19] considered antibiotic use and AMR in Northern Ireland (NI) as a whole, however there continues to be a dearth of evidence regarding antimicrobial use in relation to AMR in specific localities in Northern Ireland (NI), there- fore this research will build upon these findings to enhance knowledge of AMR with regards to antimicrobial use in the Belfast area through exploring pharmacists’ knowledge, attitudes, opinions and behaviours. The effects of the COVID-19 pandemic are largely unknown with regards to antimicrobial use with a link to AMR. The study’s undertaken by Clancy et al, 2020 [11] and Collignon and Beggs, 2020 [12] are contradictive, therefore it is important to establish how COVID-19 has impacted antimicrobial use and in turn AMR. This study will also attempt to glean information in relation to this important aspect of AMR and make recommendations to improve the current alarming position. The overall aim of this research is to determine antibiotic prescribing patterns within primary care in the Belfast locality and determine any link with AMR.
Results
Demographics
46% of pharmacists completed the survey, with the highest response rate in East Belfast (65%) and the lowest in West Belfast (25%). Of the thirty-nine responses, twenty-five (64%) were between 24-34 years old, eleven (28%) were between 35-44 years old, two (5%) were between 45-54 years old, one (3%) was between 55-64 and no response was obtained from pharmacists over 64 years old. Most responses were seen in the age range 24-34, while the least were seen by over 64-year olds.
Misuse of Antibiotics
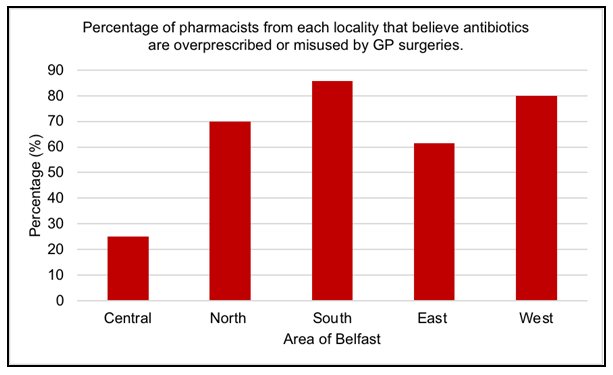
Twenty-six out of thirty-nine pharmacists (67%) were of the view that antibiotics were overprescribed or misused by GP surgeries in their area. In contrast, thirteen pharma- cists (33%) believed antibiotics were not overprescribed or misused by GP practices in their area. The majority of pharmacists from North (70%), South (85.7%), East (61.5%) and West (80%) Belfast believe that antibiotics are overprescribed or misused by GP’s, however in Central Belfast the opposite was reported in that 75% pharmacists believe antibiotics are not overprescribed or misused (Figure 1). There was no statistical signifi- cance observed between the area of Belfast and pharmacist opinion on overuse or mis- use of antibiotics (p=0.368; Fisher’s exact test).
The most common method used by fifteen out of thirty-nine (39%) pharmacists to re- duce antimicrobial use was to provide patients with advice for self-limiting infections. Fourteen pharmacists (36%) counselled patients on correct antibiotic use and twelve pharmacists (31%) discouraged GP visits for patients with self-limiting infections. Thirty-two out of thirty-nine (82%) pharmacists did not address non-compliance with antimicrobial guidelines. Seven out of thirty-nine (18%) pharmacists addressed non- compliance 1-3 times per week. Non-compliance with local guidelines were never ad- dressed more than three times per week. There was no statistical significance established between the locality in Belfast and the average number of times per week pharmacists addressed non-compliance with local antimicrobial guidelines (p=0.367; Fisher’s exact test). Dosing errors were not addressed by twenty-three out of thirty-nine (59%) pharmacists. Sixteen out of thirty-nine (41%) pharmacists addressed dosing errors 1-3 times per week. Dosing errors were never addressed more than three times per week. There was no statistical difference found between the area in Belfast and the average number of times per week pharmacists contacted prescribers with regards to dosing errors for antimicrobials (p=0.215; Fisher’s exact test).
Antibiotic use
Friedman’s rank was applied to obtain the mean rank of pharmacists’ opinion on each of the above antibiotics. This was then compared to the actual rank from accessing the Business Service’s Organisation data for primary care prescribing in Northern Ireland [20]. Table 1 demonstrates amoxicillin was the most commonly dispensed antibiotic followed by doxycycline, both in actual rank and Friedman’s rank. Actual rank and pharmacists’ opinion ranked by Friedman’s test were inconsistent for the eight other antibiotics.
Table 1: Pharmacists opinion on the most commonly prescribed antibiotics compared to the actual rank order of the most commonly prescribed antibiotics
|
Antibiotic |
Mean Rank |
Rank by Friedman test |
No of prescriptions in primary care [20] |
Rank |
|
Amoxicillin |
1.05 |
1 |
510881 |
1 |
|
Doxycycline |
3.55 |
2 |
163572 |
2 |
|
Trimethoprim |
3.97 |
4 |
142739 |
3 |
|
Flucloxacillin |
4.16 |
5 |
128608 |
4 |
|
Phenoxymethyl penicillin |
3.74 |
3 |
108236 |
5 |
|
Clarithromycin |
6.55 |
7 |
97230 |
6 |
|
Co-amoxiclav |
6.47 |
6 |
70241 |
7 |
|
Cephalexin |
8.82 |
10 |
61303 |
8 |
|
Erythromycin |
8.11 |
8 |
31648 |
9 |
|
Ciprofloxacin |
8.58 |
9 |
24670 |
10 |
41% of pharmacists suggested the most common antibiotic indication in Belfast was an upper respiratory tract infection (URTI), 26% suggested chest infection, while 18% indicated urinary tract infection (UTI). Only 3% of pharmacists believed skin and soft tissue infections were the most common indication for antibiotics. 13% of pharmacists believed the most common indication for antibiotics were of other origin than listed above. The other indication suggested was dental infections with 10% of pharmacists noting this as a common indication in their pharmacy. 3% of pharmacists responded with information not known. There was no statistical difference observed between the area of Belfast and the most common indication for antibiotics (p=0.896; Fisher’s exact test). The most common age range for antibiotics prescribed in Belfast, indicated by 62% of pharmacists was between 51-70 years old, while 21% suggested 21-50 years old and 13% believed the most common age range was over 70 years old. Only 5% of pharmacists believed most antibiotics were prescribed for children between 4-10 years old with none indicating the under 3 years old or 11-20 years ranges as common for antibiotic prescriptions.
What is the problem?
Friedman’s rank was applied to obtain the mean rank of pharmacists’ opinion for each of the suggested causes of AMR shown in Table 2. The most common opinion for the main cause of AMR was overprescribing of antibiotics and the least common was poor patient hygiene and sanitation.
Table 2: Pharmacists opinion on the main cause of antimicrobial resistance
|
Cause |
Mean Rank |
Rank by Friedman test |
|
Overprescribing of antibiotics |
2.62 |
1 |
|
Antibiotic overuse or misuse |
3.13 |
2 |
|
Inappropriate use of broad spectrum antibiotics |
3.54 |
3 |
|
Patient demand on GP |
3.92 |
4 |
|
Failure to finish the course of antibiotics |
4.41 |
5 |
|
Inappropriate duration of anti microbial |
5.95 |
6 |
|
Incorrect dose of antimicrobial |
6 |
7 |
|
Sharing antibiotics with friends and family |
7.54 |
8 |
|
Poor patient hygiene and sanitation |
7.9 |
9 |
Reducing Antimicrobial Resistance
100% of pharmacists indicated that they understood the meaning of AMR, however one pharmacist (3%) failed to identify the correct definition of AMR. Most pharmacists (69%) suggested increasing GP compliance with local guidelines when prescribing antibiotics will reduce AMR, while 62% of pharmacists indicated increasing patient counselling on appropriate antibiotic use and 56% recommended increasing delayed antibiotic prescriptions (DAPs). One out of thirty-nine (3%) pharmacists selected “other” in which their suggestion was to implement prescription/GP consultation charges. Table 3 demonstrates the majority of pharmacists (44%) believed that DAPs were beneficial, whilst 15% of pharmacists believed that DAPs have the potential to be beneficial, however changes need to be implemented such as ensuring the pharmacist is aware that the prescription is delayed. In addition, pharmacists specified patients should be educated on the growing problem of resistance in which a small delay in antibiotic prescribing will not have a detrimental effect for the patient but may improve the future for antibiotics. A large percentage of pharmacists (41%) disagree with antibiotic prescribing as they feel that it pro- vides conflicting views for the patient. Furthermore, it is felt that patients will keep anti- biotics for another time they are unwell.
Table 3: Pharmacist opinion on delayed antibiotic prescriptions (DAPs)
|
Opinion |
Number of Pharmacists |
% of pharmacists |
|
Useful |
17 |
44 |
|
Useful only if changes are |
6 |
15 |
|
made to the current system |
||
|
Unhelpful |
16 |
41 |
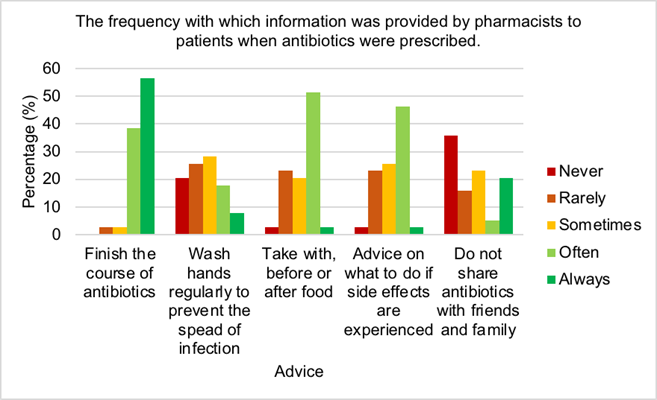
Over half of pharmacists (56.4%) always advised patients to finish the course of antibiotics, while only 3% never addressed this issue. The majority of pharmacists (74%) sometimes, rarely or never provided advice to patients on hand washing to prevent the spread of infection. Just 3% of pharmacists always guided patients on when to take their antibiotic for best effect, however over half of pharmacists (51%) often counselled patients on this. Only 3% of pharmacists always advised patients on what to do if side effects were experienced, while the majority of pharmacists (46%) often advise patients of this. 36% of pharmacists never provided information to patients regarding the sharing antibiotics, while 21% of pharmacists always stipulated this to patients. Figure 2 illustrates just over half of pharmacists (56.4%) always advised patients to finish the course of antibiotics, while only 3% never addressed this issue. The majority of pharmacists (74%) sometimes, rarely or never provided advice to patients on hand washing to prevent the spread of infection. Just 3% of pharmacists always guided patients on when to take their antibiotic for best effect, however over half of pharmacists (51%) often counselled patients on this. Only 3% of pharmacists always advised patients on what to do if side effects were experienced, while the majority of pharmacists (46%) often advise patients of this. 36% of pharmacists never provided information to patients regarding the sharing antibiotics, while 21% of pharmacists always stipulated this to patients.
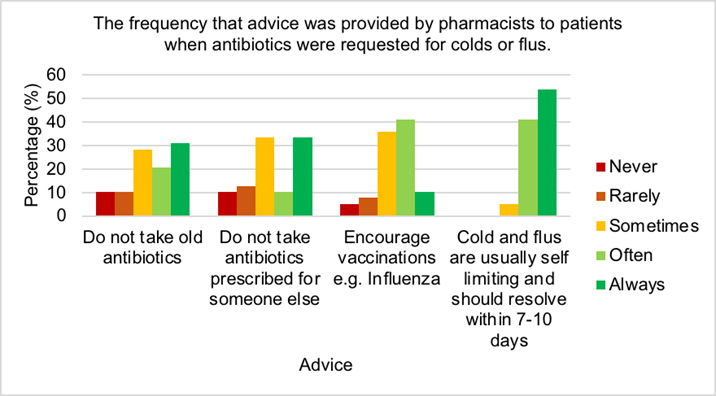
Figure 3 shows the highest percentage of pharmacists (54%) always advised patients with self-limiting cold and flus that they should resolve within 7-10 days, while no pharmacists never or rarely informed patients of this. Few pharmacists (10%) always urged patients to get vaccinations while most often (41%) or sometimes (36%) recommended this. One third always or sometimes encouraged patients to refrain from taking antibiotics prescribed for someone else and just 10% of pharmacists never counselled on this. Most pharmacists (31%) always suggested to patients to abstain from taking old antibiotics, while only 10% never advised patients on this.
Only 10% of the pharmacists in the study were Antibiotic Guardians. However, 67% of the pharmacists had heard of Antimicrobial Stewardship.
The effect of covid-19
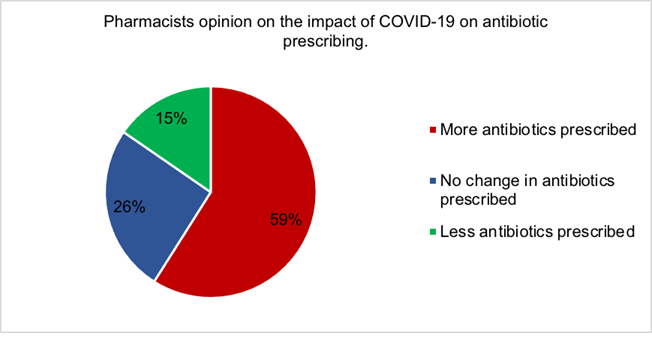
Figure 4 demonstrates the majority of pharmacists (59%) believed COVID-19 had increased the number of antibiotics prescribed. Only 15% of pharmacists thought antibiotic prescribing had decreased, while 26% believed there was no change in antibiotic prescribing during the COVID-19 pandemic.
Table 4 shows most pharmacists believed COVID-19 had no effect on patients’ perceptions for receiving antibiotics. Many pharmacists were concerned that patients were able to receive antibiotics without being appropriately examined by a doctor. It was suggested that patients were relying more on the pharmacy/pharmacists and avoided contact with the GP unless very unwell.
Table 4: Pharmacist opinion on how COVID-19 impacted patients’ perception on receiving antibiotics.
|
Opinion |
Number of Pharmacists |
|
No effect |
14 |
|
Patients are blindly prescribed antibiotics without being examined, which is dangerously altering patients’ expectations on antibiotic use |
11 |
|
Patients expect antibiotics |
9 |
|
Patients rely more on pharmacy |
4 |
|
Unsure |
3 |
|
Patients are less likely to contact the GP unless really unwell. |
3 |
|
Doxycycline was prescribed to elderly patients awaiting a COVID test |
1 |
|
Patients are less likely to demand antibiotics |
1 |
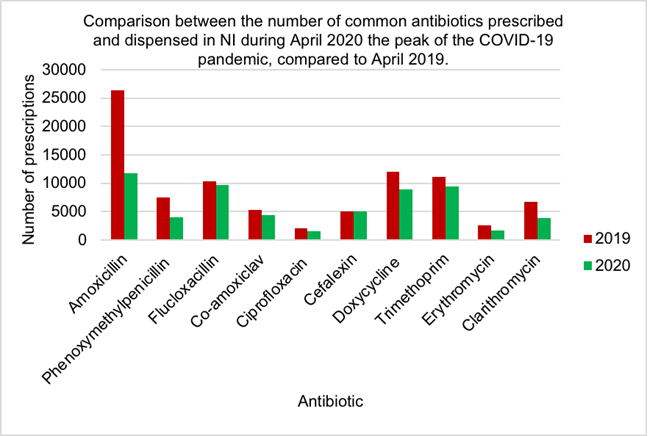
Figure 5 demonstrates prescriptions for nine out of ten antibiotics have decreased during the peak of the COVID-19 pandemic compared to April 2019. Amoxicillin showed the largest decrease in antibiotic use in April 2020 (11801) compared to 2019 (26364) while cefalexin had a slight increase in 2020 (5005) compared to 2019 (4999).
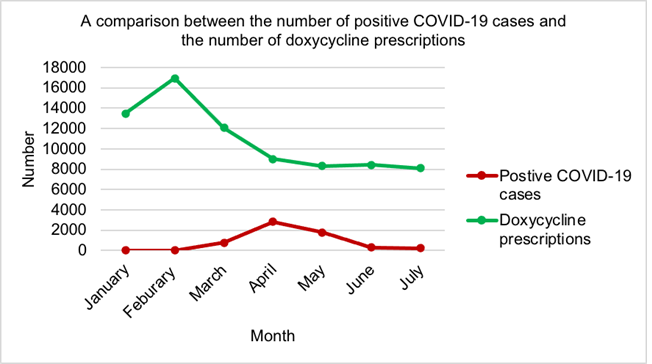
Figure 6 illustrates the first wave of the COVID-19 pandemic reached its peak in April 2020. The most prescriptions for doxycycline were seen in February 2020 (16928) with the lowest observed in July 2020 (8105). Since March 2020 (12069), doxycycline prescriptions had been decreasing monthly until May 2020 (8310), where there was a slight increase in June (8409).
Prescribing data
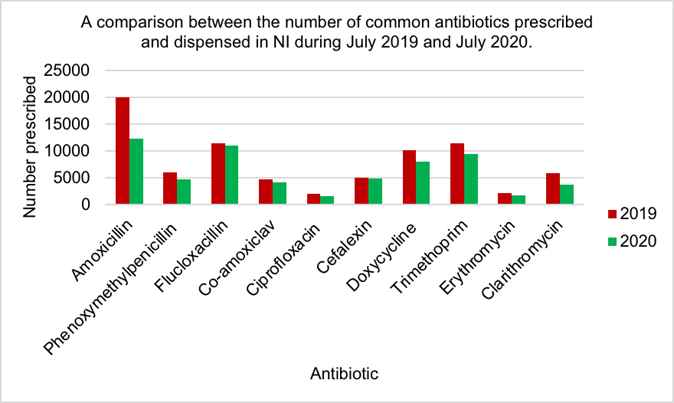
Figure 7 shows amoxicillin was the most commonly prescribed and dispensed anti- biotic in both July 2019 (19932) and 2020 (12344). Ciprofloxacin was the least pre- scribed antibiotic in 2019 (2088) and 2020 (1679). The biggest reduction in antibiotic use was seen by amoxicillin while the least reduction was seen by cefalexin. All anti- biotic use has decreased in 2020 compared to 2019.
Antibiotic use in the EU
Table 4 demonstrates the highest antibacterial consumption was observed in primary care. The Netherlands had the lowest consumption of antibacterial agents both in primary (8.9 Defined Daily Dose (DDD)) and secondary care (0.84 DDD). The highest levels were seen in Greece for primary care (32.4 DDD) and the UK for secondary care (2.47 DDD). The UK consumption of systemic antibacterial agents in primary care (16.3 DDD) was lower than the EU average (17.1 DDD), however for secondary care was higher (2.47 DDD) than the EU average (1.64 DDD). Ireland (20.9 DDD and 1.79 DDD) and Greece (32.4 DDD and 1.66 DDD) were both above the EU average for primary and secondary care respectively. The UK had the highest consumption. of systemic antibacterial agents in secondary care out of twenty-four countries in the EU and was ranked the twelfth lowest overall in primary care out of twenty-six EU countries, however Ireland was the fifth highest in primary care and the tenth highest in secondary care [24].
Table 5: Consumption of systemic antibacterial agents used in primary and secondary care in selected EU countries during 2018 [24].
|
Country |
Consumption of systemic antibacterial agents in primary care (DDD per 1000 inhabitants) |
Consumption of systemic antibacterial agents in secondary care (DDD per 1000 inhabitants) |
|
EU Average |
17.2 |
1.64 |
|
UK |
16.3 |
2.47 |
|
Ireland |
20.9 |
1.79 |
|
Greece |
32.4 |
1.66 |
|
Netherlands |
8.9 |
0.84 |
Discussion
Demographics
Research by Ebert et al, 2018 [25] and Manikkath et al, 2020 [26] discovered response rates for electronic surveys were between 36% and 37%. Electronic questionnaires for this study yielded an overall response rate of 46%. According to Wu et al, 2022, the average online survey response rate is 44%, indicating that this study is above average response rate [27]. The increased response rate may have been due to the two follow-up emails sent to pharmacists as sixteen new responses were obtained following the emails. Manikkath et al, 2020 [26] found the majority of survey participants were between 26-30 years old which is similar to this research as most participants were between 24-34 years old. Young pharmacists may be more inclined to complete student questionnaires as in the last 10 years they have completed a university research project, thus may under- stand the difficulty in obtaining survey responses, therefore can relate to the researcher [28]. Smith et al, 2019 [29] established older participants were hard to recruit for studies, whereas younger participants were more intrigued. In this study, as participant age increases, response decreases. Additionally, older pharmacists may have been owners or managers in the pharmacy, therefore did not have time to complete the questionnaire, due to flu vaccination clinics, nursing home dispensing or checking prescriptions. Younger pharmacists may have been locums, therefore had a reduced workload.
Misuse of antibiotics
Most pharmacists (67%) felt antibiotics were overprescribed or misused by GP’s in their locality (Figure 1). Potential reasons for antibiotic misuse or overprescribing is the difficulty for GP’s to differentiate between bacterial and viral infections [30]. Furthermore, one pharmacist suggested regular patients presented multiple antibiotic prescriptions each month with no clinical predisposing factors as to the reason. This suggestion was supported in research by Palin et al, 2019 [31] which concluded the chance of receiving an antibiotic was greater if a patient was previously prescribed an antibiotic. Most pharmacists in North, South, East and West Belfast believed overprescribing or misuse of antibiotics was a problem in their area, however the opposite is true for Belfast City Centre (Figure 1). A reason for this may be the small population of three thousand people in Belfast city centre [32]. Macrotrends, 2020 [33] recorded a larger population of six-hundred and thirty-one thousand for North, South, East and West Belfast, therefore indicating increased demands on GP’s for antibiotics.
Fleming et al, 2011 [34] recommended that community pharmacists should counsel patients on correct use of prescribed antibiotics, educate patients on the dangers and ad- verse effects of AMR and select over the counter (OTC) treatments for patients with self- limiting infections. This agrees with the most common methods used by pharmacists to prevent antimicrobial misuse in this study. Antimicrobial guidelines are used to ensure correct and consistent antimicrobial use, while providing evidence-based treatment [35]. Shockingly, 82% of pharmacists in this study never addressed prescriber non-compliance with local antimicrobial guidelines. Interestingly, one participant highlighted contacting clinicians regarding inappropriate antibiotic use or misuse led to friction between the prescriber and pharmacist, therefore these issues are no longer addressed by this pharmacist. This may account for the high percentage of pharmacists who failed to address prescriber non-compliance and dosing errors (59%). Additionally, pharmacists may not have time to address these issues due to the increased patient demands on pharmacy during COVID-19. Treatment failure and AMR are probable when subtherapeutic doses of antimicrobials are prescribed [36]. Conversely, excessive doses of antimicrobials are known to exacerbate adverse effects and can result in toxicity [36]. A study by Iftikhar et al, 2019 established that the major contributors to antimicrobial prescribing errors in children were sub- therapeutic doses and increased dosing frequency [37]. In contrast, since the increase in children’s dose for amoxicillin in 2014, the probability of children receiving a therapeutic dose has increased considerably to 94% [36]. The majority of pharmacists failed to address dosing errors for antimicrobials, which could be a major driver for AMR in Belfast pharmacies. Furthermore, only 5% of pharmacists addressed dosing errors of antimicrobials as a way to combat antimicrobial misuse.
Antibiotic Use
Amoxicillin is the most commonly used antibiotic in NI primary care (Table 1). It is a beta-lactam antibiotic and a penicillin derivative which exhibits broad-spectrum activity [38]. Amoxicillin is first line therapy for a range of infections including acute otitis media, pericoronitis, dental abscess, lower respiratory tract infection (LRTI) and helicobacter pylori infection [39]. Doxycycline is a broad spectrum, second-generation tetracycline which has antimicrobial, anti-inflammatory and antiviral properties [40, 41]. It is the second most commonly prescribed antibiotic in primary care (Table 1). Pharmacists correctly identified the two most common antibiotics prescribed in primary care, however there was a discrepancy in their knowledge for the remaining eight compared to the actual figures published by the BSO [20]. This may be consequent of changed antimicrobial prescribing pat- terns during the COVID-19 pandemic, or perhaps pharmacists were taking into account private prescriptions which the BSO data does not include. Furthermore, this research only considered Belfast pharmacies, however prescription data included all of NI. The most common infections in primary care were respiratory tract infections (RTIs) and UTIs for which significant numbers were prescribed antibiotics [42, 43]. This agrees with findings from this study. Pouwels et al. established a substantial number of antibiotics prescribed for RTIs in primary care England were inappropriate, which was indicative by the percentage of antibiotics prescribed for self-limiting conditions such as sore throats (59%), rhinosinusitis (88%) and coughs (41%) [44]. It is suggested a large number of anti- biotics prescribed for URTI and chest infections in the Belfast area were inappropriate, however in order to prove this further research is required. It is recommended appropriateness of antibiotic use should be investigated in primary care by undertaking a study focusing on GPs choices for antibiotics and comparing this to the published local guide- lines. Dolk et al, 2018 demonstrated that the average age range for antibiotic prescriptions was over 65 years old [42]. This study demonstrates a similar trend as pharmacists suggested most prescriptions were seen in patients between 51-70 years old. Antibiotics for viral infections were more common among this age group [45]. This may be due to the early initiation of antibiotics in the elderly due to fear of their condition deteriorating [46].
What is the problem?
Smieszek et al, 2018 documented inappropriate antibiotic prescribing in all English practices investigated, with the highest being 52.9% [47]. Overprescribing of antibiotics was suggested as the main cause of AMR (Table 4). Despite access to antimicrobial guide- lines, doctors commonly prescribed broad-spectrum antimicrobials as opposed to the first line treatment [48]. Antibiotic overuse or misuse was selected as the second most common cause of AMR (Table 4). This portrays an urgent need for increased education and support for prescribers, both in Belfast and the wider community to enhance prescribing decisions and reduce AMR. NPS was used to measure pharmacist opinion on the AMR problem in NI. The score was -7.69 which indicated pharmacists do not think that AMR is a problem, however this may not reflect true opinions as Lewis and Mehmet, 2020 suggested the boundaries of the NPS were flawed as passive participants were shown to have a similar attitude as promoters [49].
Reducing Antimicrobial Resistance
Community pharmacists are in an ideal position to assist in reducing levels of AMR by patient education and minor ailments consultations [50]. To do this, pharmacists must have adequate knowledge of AMR. All pharmacists assumed they knew the correct definition of AMR and when asked to select the correct definition, 97% identified the correct answer which was excellent. Hayhoe et al, 2019 established over half of the UK general public believed antibiotics could be used to cure viral infections which indicates that public understanding of antibiotic use was unexceptional [51]. Mason et al, 2018 demonstrated patients counselled by community pharmacists showed significantly better knowledge on appropriate antibiotic use [50], therefore indicating the importance of this method. However in this study, just over 61% of pharmacists believed counselling would reduce AMR. It is demonstrated that community pharmacists lacked insight with regards to public perception of antibiotic use, therefore enhanced education should be provided to pharmacists on this area to improve counselling and in turn reduce antibiotic use and AMR. Research by Aljayyousi et al, 2019 found 27% of patients admitted to taking old antibiotics, while 37% used antibiotics prescribed for someone else, which may be a result of the lack of counselling provided by community pharmacists (Figures 2 and 3) [52]. Count- less patients received antibiotics for infections that could have been prevented by hand- washing, yet shockingly 46% of pharmacists never or rarely counselled patients regarding handwashing (Figure 2) [53]. This demonstrates an urgent need for counselling improvement by community pharmacists to combat AMR. Furthermore, patients confessed they discontinued antibiotic use when symptoms improved, however most pharmacists al- ways (56%) or often (39%) instructed patients to finish the course (Figure 2) [50]. More information should be provided to patients on the reasons and benefits for finishing anti- biotic course. It is recommended that some antibiotics are taken on an empty stomach, for example flucloxacillin, however research by Gardiner et al, 2018 established taking flu- cloxacillin with food had no effect on the efficacy but reduced side effects such as nausea [54]. This suggests flucloxacillin would be beneficial to be taken with food. Most pharmacists often provided advice to patients regarding management of side effects (46%) and recommended to take either before, with of after food (51%) (Figure 2). Vaccinations help to prevent infections, hence avoiding antimicrobial use and reducing AMR [55]. The majority of pharmacists often or sometimes encouraged patients to receive vaccines (Figure 6.11). These figures could be significantly increased by pharma- cist education on the benefits of vaccination programmes for patient health. It is crucial pharmacists reassure patients that colds and flus are self-limiting, provide symptomatic relief and educate patients on the reasons why antibiotics are inappropriate [56]. It is positive that all pharmacists sometimes (5%), often (41%) or always (54%) provided advice to patients regarding self-limiting infections (Figure 3).
Based on a study conducted in primary care in England, there were significantly more antibiotics prescribed than anticipated, based on antimicrobial guidelines [44]. This is indicative of prescriber non-adherence to guidelines. 69% of pharmacists believed the best way to combat AMR was increased GP compliance with local antimicrobial guide- lines. Reasons for prescriber non-adherence were lack of clarity and applicability of the guidelines [57]. It is suggested antimicrobial guidelines should be reviewed to improve clarity for prescribers and additional guidelines provided to assist prescribers to select the correct treatment plan for patients that do not fall into the ideal patient category, such as patients with co-morbidities and pregnant women [57]. Clinicians were not comfortable issuing DAPs as they felt mixed messages were pro- vided to patients regarding antibiotic use [58,59]. In contrast, over half of pharmacists felt increased use of delayed antibiotic prescribing would contribute to reduced AMR in Bel- fast. Reduced antibiotic use has been proven with DAPs, furthermore they provide an opportunity for clinicians to educate patients on antibiotic use and AMR [59]. Introducing GP/prescription charges in Northern Ireland was suggested by one pharmacist as a method to reduce AMR. In the Republic of Ireland, where charges exist, GP’s felt obligated to provide antibiotics to paying patients [60]. Ireland showed higher levels of antibiotic use compared to the UK (Table 5), therefore this method does not seem useful in achieving the aim desired. Conversely, by implementing prescription charges, the number of patients requesting antibiotics unnecessarily may reduce, therefore decreasing AMR levels and achieving the desired aim.
Francis et al, 2012 established that 67% of patients who received DAPs commenced the antibiotic course the day of the consultation [61]. Lowest antibiotic use (14%) was seen when clinicians advised patients to return if symptoms persisted, however patients were not satisfied. DAPs were a compromise which decreased antibiotic use to 31% compared to immediate use at 93%, whilst still achieving patient satisfaction [62]. Pharmacists had mixed opinions on DAPs (Table 3). Many pharmacists expressed opinion that patients either need an antibiotic or do not, therefore failed see the benefits of DAPs. Others felt this strategy was useful if counselling was provided by both clinicians and pharmacists, whilst most felt this was a good strategy which should be implemented more often. From personal experiences, many DAPs were not collected from the pharmacy, which anecdotally suggests that DAPs are a useful measure to reduce AMR. Sadly a large number of pharmacists are not AGs suggesting the objectives of the AG campaign were not met [63]. Perhaps it would be timely and advantageous to re-energise this campaign. The intention of AMS in primary care is to reduce antibiotic prescribing through patient education and adaption of clinicians’ behaviours. For AMS to be successful, HCPs must be committed to change [64]. Worryingly, only 67% of pharmacists have heard of AMS, however, recently this was implemented as part of the core learning in the undergraduate MPharm degrees in NI. Pharmacists should be integral to AMS programmes in primary care as they are in an ideal position to educate patients and HCPs [4].
The effect of COVID-19
There is little research into the effect of COVID-19 on antibiotic prescribing which was demonstrated by the conflicting views regarding antibiotic prescriptions by Clancy et al, 2020 and Collignon and Beggs, 2020 [11,12]. Most pharmacists suggested that anti- biotic use had increased during the COVID-19 pandemic (Figure 4), however all antibiotic use decreased in 2020 compared to the same point in 2019 (Figure 7), with a similar observation noted during the peak of surge one (Figure 5). At the beginning of the pandemic GPs were advised to replace face to face consultations with phone and video consultations [65]. Many pharmacists iterated concern that antibiotics were prescribed without patient examinations. Brookes-Howell et al, 2012 dis- covered the most common method used by clinicians to determine patient need for anti- biotics was chest auscultations [66]. This method is of utmost importance when assessing patients’ need for antibiotics for URTI or chest infections, the most common indications for antibiotics. Chest auscultations are impossible to do via digital consultations, therefore there is questionable evidence for appropriate antibiotic use during COVID-19. Interestingly, one pharmacist suggested that the next pandemic will be resistance caused without immediate changes however, MacIntyre and Chau, 2017 confirmed AMR may complicate a pandemic, however could not be the main trigger [67].
When the pandemic started in Wuhan, it was reported that 90% of positive, hospitalised patients received antibiotics despite no evidence of a bacterial infection [68]. Research is scare regarding antibiotic use in EU primary care settings during COVID-19, but Abelenda-Alonso et al, 2020 concluded that during January and February 2020, just before the pandemic struck, antibiotic consumption in a Spanish hospital was relatively consistent with 2019 figures [69]. However, during the peak of the first surge in April 2020, antibiotic consumption dramatically increased, compared to April 2019. This study dis- covered in NI most antibiotics examined in primary care had decreased during the peak of surge one, with only a minor increase seen in cefalexin (Figure 5). A potential reason for this is the significant reduction of face-to-face consultations by GPs which have moved to phone and video consultations [70]. Furthermore, some patients failed to contact the GP as they felt they were a burden during this crisis [71]. Moreover, from personal experiences more patients sought advice from community pharmacists for infections due to the difficulty in accessing GP services. Even when pharmacists advised patients to contact the GP for antibiotics, they were reluctant. Antibiotic use in Spanish hospitals may have increased as a result of microbiology results being inaccessible, delay in antibiotic reviews and the fact the majority of critically ill patients receive antibiotics [68]. The main symptoms of COVID-19 include hyperpyrexia, anosmia, hypogeusia and a new continuous cough [72]. Bonzano et al, 2020 established once daily administration of doxycycline 200mg rapidly improved all symptoms of COVID-19 [73]. Guidelines for the treatment of COVID-19 pneumonia suggest doxycycline should be offered if the patient is at high risk of complications especially in the elderly or patients with existing co-morbidities [74, 75, 76]. Figure 6 displays no correlation between COVID-19 numbers and doxycycline prescriptions in NI primary care which is potentially due to use in hospitalised patients with a severe infection.
Antibiotic Use in the EU
An overall decrease is seen for EU antibacterial consumption of systemic agents in 2018 (Table 5), compared to findings by Mone, 2018 considering 2017 figures [19]. A re- duction can also be seen in the UK and Ireland figures in both primary and secondary care. Greece had the highest levels of systemic antibacterial consumption in primary care (Table 5). This may be because systemic antibiotics such as co-amoxiclav, can be bought from community pharmacies without a prescription. Secondly, Greek doctors overprescribe antibiotics for self-limiting infections due to patient expectations or incentives from pharmaceutical companies. Often the wrong antibiotic is selected by Greek clinicians, for example for otitis media first line treatment is amoxicillin, however frequently co-amoxiclav is prescribed [77]. The Netherlands had the lowest level of antibacterial consumption both in primary and secondary care (Table 5). Potentially this may be due to the fact anti- biotics must be prescribed by physicians, however they proactively avoid overprescribing of antibiotics, therefore patients’ expectations of antibacterial use are altered. Further- more, Dutch GPs follow strict antimicrobial prescribing guidelines produced by the College of General Practitioners (NHG), additionally GP’s believe self-limiting infections such as otitis media should be treated with paracetamol as opposed to antibiotics [78]. Ireland had the 5th highest level of antimicrobial consumption in primary care which was higher than the EU average (Table 5). Factors which could have contributed to 20.9 DDD were Irish GP’s struggled to interpret antimicrobial guidelines due to variability in patients’ complaints, meaning diagnoses were often not straightforward. GP’s also felt obligated to provide paying patients with an antibiotic prescription to meet patients’ expectations of receiving an antibiotic [60]. The UK was 12th lowest for consumption of antibacterial agents and was just under the EU average (Table 5). Although levels of antibacterial consumption in primary care were decreasing, levels were still high, which may have been a result of inappropriate use of broad-spectrum antibiotics such as amoxicillin. Nowakowska et al, 2019 discovered that only 62% of prescriptions for URTI were appropriate [79]. Additionally, clinician’s poor adherence to antimicrobial guidelines potentially is contributing to high antibacterial consumption. A possible reason for the UK having the highest antibacterial consumption in secondary care (Table 5), is the uncertainty of appropriate antibacterial use due to variation in decisions based on factors such as clinicians’ experiences, training or the worry of a patient deteriorating [80]. This goes hand in hand with difficulties in interpreting guidelines based on patients varying conditions [60].
Materials and Methods
Questionnaire
Development
An electronic survey was developed on Microsoft forms. Pharmacies where phoned to obtain their e-mail address, then the surveys were distributed via e-mail. This method was chosen given it required no face-to-face contact it was the safest way to distribute questionnaires during the COVID-19 pandemic. Electronic surveys are advantageous as they are inexpensive, anonymous and have the ability to achieve an 100% completion rate for questions within the survey. According to Hardigan et al, 2016 this method is proven to have the quickest response time compared to postal mail [81]. It is recommended questionnaires should not take respondents longer than 15 minutes to complete, as long questionnaires detrimentally effect response rate [82]. Therefore, the aim of questionnaire development was to make a short questionnaire, whilst asking appropriate questions in order to obtain as much relevant information as possible. Twenty-three questions were developed strategically using the project aims and literature pertaining to questionnaire design [82-87]. Demographics were required to establish if antimicrobial use and AMR differs based on locality in Belfast. Research by Blair et al, 2014 demonstrated that commonly background information such as demographics are required in questionnaires to potentially explain differences in findings [82].
Piloting
According to Hassan et al, 2006, piloting questionnaires is considered an invaluable part of research [88]. The Chief Investigator piloted the questionnaire with three pharma- cists that were not directly involved in the study. During piloting, the contributors were sent a link to the questionnaire via email and asked to complete. This pilot aimed to identify questions that were misunderstood or led the contributor to become confused, to enable questions to be rephrased; it also provided a guide for completion time [89]. Contributors were asked to comment on their understanding of the questionnaire and suggest any changes that would improve questionnaire design. Piloting established that the average completion time for the survey was 5 minutes 16 seconds which was ideal. No further issues were identified by the contributors and responses were easily accessed by the investigator on Microsoft forms.
Target Population
The target population for questionnaire distribution were community pharmacists working in the Belfast area. According to the Business Services Organisation, there are one hundred and thirty registered pharmacies in the Belfast area [90]. Ninety community pharmacies were randomly selected from yell.com for questionnaire distribution. On yell.com “pharmacy” was entered as the search term and the location entered was the first part of each Belfast area postcode. All ten pharmacies in Belfast City Centre were selected from the postcode areas BT1 and BT2. It was decided that twenty questionnaires would be sent to the other Belfast localities. In North Belfast the postcode regions used were BT15 and BT14, in South Belfast BT6, BT7 and BT8 were used, in East Belfast the postcode regions used were BT4, BT5 and BT16 and in West Belfast BT10, BT11, BT12, BT13 and BT17 were used. The number of pharmacies in each locality were counted and allocated a number. The number of pharmacies was entered into a random number generator and the number selected was not included in questionnaire distribution. This was repeated until twenty pharmacies remained in each locality.
Ethical approval
The School of Biomedical Sciences Ethics Filter Committee granted ethical approval for this research.
Distribution
Following questionnaire development and ethical approval permission, the ninety selected pharmacies were contacted by phone. The nature of the project was explained, and the pharmacists were asked to participate by completing a short electronic survey. If they agreed to participate, they were asked for an email address so that the questionnaire link could be sent. When contacted, thirteen pharmacies did not wish to participate, therefore the selection process was carried out a second time to replace non-participants. Unfortunately, Belfast City Centre and South Belfast pharmacies had all been contacted previously, therefore the numbers could not be redeemed. The result of this was Belfast City Centre pharmacies received nine questionnaires, South Belfast pharmacies received sixteen questionnaires and North, East and West Belfast pharmacies received twenty questionnaires. The total number of distributed questionnaires was eighty-five. Two follow up reminder emails were sent to the pharmacists who had agreed to participate in the questionnaire.
Analysis
Data was entered onto Microsoft Office Excel® enabling pie charts, tables and graphs to be constructed based on the results. Relevant data was transferred to IBM® SPSS® Statistics 25 were descriptive statistics were generated from statistical tests such as Friedman’s test and Fisher’s Exact test. The Friedman’s test was used to analyse related samples of pharmacist opinion for the rank order of the most commonly prescribed antibiotics in primary care in addition to pharmacist opinion on the main cause of AMR, which enabled calculation of the mean rank [91]. The Fisher’s exact test was used to determine if there was a significant difference between locality in Belfast and pharmacist opinion on antibiotic overuse or misuse. Secondly, the Fisher’s exact test was used to establish if there was a significant difference between the area of Belfast and the number of times per week pharmacists addressed prescriber non-compliance with local antimicrobial guidelines. In addition, this test was used to determine if there was a statistical difference between the area and the average number of times per week pharmacists contacted prescribers with regards to dosing errors. Finally, the Fishers exact test was used to ascertain if there was a statistical difference between locality and pharmacist opinion on the most commonly used indication for antibiotics. This method was selected as sample sizes were small and 80%, 70%, 70% and 97% of the data respectively had an expected count of less than five, therefore approximation methods were insufficient [92]. No significant difference was observed if the p-value was greater than 0.05 [93]. Pharmacist opinion on how big the problem of AMR is in NI was analysed by counting the number of promoters (9-10), passives 7-8) and detractors (0-6) and percentage was calculated for each. Net promoter score (NPS) was then calculated by subtracting the percentage of detractors from the percentage of promoters [94].
Antimicrobial Use in NI
Data Collection
The BSO publishes monthly statistics on all National Health Service (NHS) prescriptions submitted for payment from community pharmacy, following the dispensing of the medication which was prescribed by GPs or non-medical prescribers within GP practices. These statistics only include prescriptions that have been dispensed from an NHS pre- scription, therefore it does not take into consideration private prescriptions [22]. For the purpose of this study yearly and monthly prescription statistics were gathered from the BSO [20-22]. In addition, the number of COVID-19 positive cases for each month were gathered from Gov.uk, 2020 where daily statistics were available from 5th March 2020 [23].
Sample Size
Firstly, data on ten commonly prescribed antibiotics amoxicillin, phenoxymethylpenicillin, flucloxacillin, co-amoxiclav, ciprofloxacin, cefalexin, doxycycline, trimethoprim, erythromycin and clarithromycin were collected for the year 2019. This enabled a rank order to be produced so comparison could be made against pharmacists’ opinion on the most commonly used antibiotics in primary care. Then, data for the same ten antibiotics was gathered for July 2019 and July 2020 to provide a comparison to establish if antibiotic use has changed this year compared to the same time period last year. July was selected as it was the most up to date figures available from the BSO. Data was also gathered for the same ten antibiotics during April 2019 and April 2020 to compare how antibiotic use had changed during the peak of the COVID-19 pandemic. Doxycycline prescription data and positive COVID-19 statistics were gathered for the months: January, February, March, April, May, June and July. Doxycycline and COVID-19 statistics were compared as one pharmacist suggested a rise in doxycycline prescriptions for elderly patients awaiting COVID-19 test results.
Data Analysis
GP prescribing data for the ten antibiotics during 2019 and the chosen months April and July 2019 and April and July 2020, was extracted from the BSO, the available forms and strengths for each preparation were added together and were entered into Microsoft Office Excel® separately. This enabled tables and graphs to be produced to show usage during this time. The extracted data for doxycycline prescriptions and positive COVID-19 cases were transcribed onto Microsoft Office Excel® where a graph was produced to allow visual determination to ascertain if there was a correlation between the two sets of data.
Antimicrobial Use in the EU
Data Collection
Surveillance data from ECDC, 2020 was collated to enable a comparison of antimi- crobial consumption between the UK and other EU countries in primary and secondary care [24]. Previous studies conducted in the EU on antibacterial consumption were located using USearch.
Sample Size
Four countries were selected: The UK, Ireland, Greece and the Netherlands. Ireland was selected for comparison with the UK as it borders NI, therefore is closely related to this study. The Netherlands was selected to represent countries with low levels of anti- bacterial consumption and Greece was chosen to represent countries with high levels of antibacterial consumption.
Data Analysis
The EU average was calculated for both primary and secondary care by adding all the values together and dividing by the number of countries that submitted data. Data from the EU was entered onto Microsoft Office Excel® were a table was produced to visually display the data. Previous studies were used to explain the statistical data found from ECDC, 2020 [24].
Conclusion
This research established no variation in antibiotic prescribing patterns with regards to locality in Belfast primary care. Amoxicillin was the most commonly prescribed antibiotic, which is contributing to increasing AMR due to its broad-spectrum activity. Furthermore, COVID-19 has reduced antimicrobial use in NI, which has clarified previous contradictory literature. Pharmacists believed the most prevalent cause of AMR was overprescribing of antibiotics by GPs and suggested that the best way to reduce AMR was to improve GP compliance with local antimicrobial guidelines. It was established that the majority of pharmacists failed to provide appropriate advice to patients when antibiotics were prescribed and when patients requested antibiotics from the pharmacist for self-limiting infections, despite the ideal position they are in to aid the reduction of AMR through patient education. This study has established that the first wave of COVID-19 contributed to decreased antibiotic use in NI, however it is unclear whether antibiotics prescribed in primary care were appropriate during this time as GP’s were unable to conduct face-to- face clinical examinations.
Author Contributions:
Conceptualization, H.C.; methodology, H.C.; software, N.H.; validation, H.C and N.H..; formal analysis, N.H and H.C.; investigation, H.C. and N.H. resources, H.C. and N.H.; data curation, H.C. and N.H.; writing—original draft preparation, H.C. and N.H.; writing— review and editing, H.C. and N.H.; visualization, H.C.; supervision, H.C.; project administration, H.C..; funding acquisition, H.C. All authors have read and agreed to the published version of the manuscript.
Funding:
This research received no external funding.
Institutional Review Board Statement: The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Ulster University Biomedical Ethics Committee (FCBMS-20-082-B, 25th September 2020) for studies involving humans.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest:
The authors declare no conflict of interest.
References
- World Health Organisation (2021) Antimicrobial Resistance (2021).
- World Health Organisation. Global antimicrobial resistance and use surveillance system (GLASS) report 2022. Swit- zerland: World Health Organisation Press (2022).
- World Health Organisation. ‘The WHO AWaRe (Access, Watch, Reserve) antibiotic book’ (2022).
- Liaskou M, Duggan C, Joynes R and Rosado H. Pharmacy’s role in antimicrobial resistance and stewardship. Clinical Pharmacist, 10 (2018).
- Brinkmann I and Kibuule D. Effectiveness of antibiotic stewardship programmes in primary health care settings in developing countries. Research in Social & Administrative Pharmacy 16 (2020): 1309-1313.
- World Health Organisation. ‘Antimicrobial Resistance’ (2023).
- Walsh F. Antibiotics resistance 'as big a risk as terrorism' - medical chief. BBC News (2013).
- European Centre for Disease Prevention and Control. Antimicrobial consumption in the EU/EEA (ESAC-Net) Annual Epidemiological Report for 2021 (2022).
- Centres for Disease Control and Prevention, (CDC). Biggest Threats and Data - 2019 Antibiotic Resistance Threats Report (2019).
- Poudel NA, Zhu S, Cooper N, Little P, Tarrant C, Hickman M, et al. The economic burden of antibiotic resistance: A systematic review and meta-analysis’ PLoS ONE 18 (2023).
- Clancy C, Buehrle D and Nguyen H. PRO: The COVID-19 pandemic will result in increased antimicrobial resistance rates. JAC - Antimicrobial Resistance 2 (2020).
- Collignon P and Beggs J. CON: COVID-19 will not result in increased antimicrobial resistance prevalence. JAC - Antimicrobial Resistance 2 (2020).
- Hsu J. How covid-19 is accelerating the threat of antimicrobial resistance. British Medical Journal 369 (2020): m1983.
- World Health Organisation. Global action plan on antimicrobial resistance. Switzerland: World Health Organisation (2015).
- Dadgostar P. Antimicrobial Resistance: Implications and Costs. Infect Drug Resist 12 (2019).
- Dyar O, Huttner B, Schouten J and Pulcini C. What is antimicrobial stewardship? Clinical Microbiology and Infection 23 (2017): 793-798.
- Liaskou M, Duggan C, Joynes R and Rosado H. Pharmacy’s role in antimicrobial resistance and stewardship. Clinical Pharmacist 10 (2018).
- Kesten J, Bhattacharya A, Ashiru Oredope D, Gobin M and Audrey S. The Antibiotic Guardian campaign: a qualitative evaluation of an online pledge-based system focused on making better use of antibiotics. BMC Public Health 18 (2017): 1-13.
- Mone P. Antibiotic Use and Antimicrobial Resistance in Northern Ireland – What is the problem? Ulster University (2018).
- Business Services Organisation. Prescription Cost Analysis (2019a).
- Business Service Organisation, (BSO) GP Prescribing Data (2019b).
- Business Services Organisation, (BSO). Pharmaceutical list (2020b).
- Gov uk. Coronavirus (COVID-19) in the UK - Cases in Northern Ireland (2020).
- European Centre for Disease Prevention and Control (ECDC). (2020) Antimicrobial consumption database Rates by country.
- Ebert J, Huibers L, Christensen B and Christensen M. Paper- or Web-Based Questionnaire Invitations as a Method for Data Collection: Cross-Sectional Comparative Study of Differences in Response Rate, Completeness of Data, and Financial Cost. Journal of Medical Internet Research 20 (2018): 21.
- Manikkath J, Matuluko A, Leong A, Ching D, Dewart C, Lim R, et al. Exploring young pharmacists' and pharmaceutical scientists' needs and expectations within an international pharmacy organization: Find- ings from FIP's needs assessment survey. Research in Social and Administrative Pharmacy (2020).
- Wu MJ, Zhao K and Filis-Aime F. Response rates of online surveys in published research: A meta-analysis. Computers in Human Behaviour Reports 7 (2022): 100206.
- Saleh A and Bista K. Examining Factors Impacting Online Survey Response Rates in Educational Research: Per- ceptions of Graduate Students. Journal of MultiDisciplinary Evaluation 13 (2017): 63-74.
- Smith M, Witte M, Rocha S and Basner M. Effectiveness of incentives and follow-up on increasing survey re- sponse rates and participation in field studies. BMC Medical Research Methodology 19 (2019): 1-13.
- Saliba-Gustafsson E, Roing M, Borg M, Rosales-Klintz S and Lundborg C. General practitioners' perceptions of delayed antibiotic prescription for respiratory tract infections: A phenomenographic study. Public Library of Science 14 (2019b).
- Palin V, Mölter A, Belmonte M, Ashcroft D, van Staa T, White A, et al. Antibiotic prescribing for common infections in UK general practice: variability and drivers. The Journal of Antimicrobial Chemotherapy, 74 (2019): 2440- 2450.
- Rice C. Belfast: Is plan to boost city centre living realistic? Northern Ireland: BBC News (2019).
- Belfast, UK Metro Area Population 1950-2020 (2020).
- Fleming N, Barber S and Ashiru-Oredope, D. Pharmacists have a critical role in the conservation of effective anti- biotics. The Pharmaceutical Journal 287 (2011): 465.
- Allison R, Lecky D, Beech E, Ashiru-Oredope D, Costelloe C and Owens R. Local implementation of national guidance on management of common infections in primary care in England: findings of a mixed-methods national ques- tionnaire. The Pharmaceutical Journal 304 (2020).
- Rann O, Sharland M, Long P, Wong I, Laverty A, Bottle A, et al. Did the accuracy of oral amoxicillin dosing of children improve after British National Formulary dose revisions in 2014? National cross-sectional survey in England. BMJ Open 7 (2017): e016363.
- Iftikhar S, Sarwar M, Saqib A, Sarfraz M and Shoaib Q. Antibiotic prescribing practices and errors among hos- pitalized pediatric patients suffering from acute respiratory tract infections: A multicenter, cross-sectional study in Paki- stan. Medicina (Lithuania) 55 (2019).
- Evans J, Hannoodee M and Wittler M. Amoxicillin Clavulanate. In: Anon. Treasure Island (FL). StatPearls Publishing (2020).
- National Institute for Health and Care Excellence. Summary of antimicrobial prescribing guidance – managing common infections. (2020a).
- Li Y, Wu Z, Liu K, Qi P, Xu J, Wei J, et al. Doxycycline enhances adsorp- tion and inhibits early-stage replication of porcine reproductive and respiratory syndrome virus in vitro. FEMS Microbiology Letters, 364 (2017).
- Kohli K. Doxycycline Treatment In High-Risk COVID Patients: Recent Evidence (2020).
- Dolk F, Pouwels K, Smith D, Robotham J and Smieszek T. Antibiotics in primary care in England: Which anti- biotics are prescribed and for which conditions? Journal of Antimicrobial Chemotherapy 73 (2018).
- Stuart B, Brotherwood H, Van'T Hoff C, Brown A, Moore M, Little P, et al. Exploring the appropriateness of antibiotic prescribing for common respiratory tract infections in UK primary care. Journal of Anti- microbial Chemotherapy 75 (2020): 236-242.
- Pouwels K, Dolk F, Smith D, Robotham J and Smieszek T. Actual versus 'ideal' antibiotic prescribing for com- mon conditions in English primary care. Journal of Antimicrobial Chemotherapy 73 (2018): ii19-ii26.
- Singer A, Fanella S, Kosowan L, Falk J, Dufault B, Hamilton K and Walus A. Informing antimicrobial stew- ardship: factors associated with inappropriate antimicrobial prescribing in primary care. Family Practice 35 (2018): 455-460.
- Dylis A, Boureau A, Coutant A, Batard E, François J, Berrut G, et al. Antibiotics pre- scription and guidelines adherence in elderly: impact of the comorbidities. BMC Geriatrics 19 (2019): 1-6.
- Smieszek T, Pouwels K, Dolk F, Smith D, Robotham J, Hopkins S, et al. Potential for reducing inappropriate antibiotic prescribing in English primary care. Journal of Antimicrobial Chemotherapy 73 (2018): ii36-ii43.
- Krishnakumar J and Tsopra R. What rationale do GPs use to choose a particular antibiotic for a specific clinical situation? BMC Family Practice 20 (2019): 1-9.
- Lewis C and Mehmet M. Does the NPS® reflect consumer sentiment? A qualitative examination of the NPS using a sentiment analysis approach. International Journal of Market Research 62 (2020): 9-17.
- Mason T, Trochez C, Thomas R, Babar M, Hesso I and Kayyali R. Knowledge and awareness of the general public and perception of pharmacists about antibiotic resistance. BMC Public Health 18 (2018): 1-10.
- Hayhoe B, Greenfield G and Majeed A. Is it getting easier to obtain antibiotics in the UK? The British Journal of General Practice: The Journal of the Royal College of General Practitioners 69 (2019): 54-55.
- Aljayyousi G, Abdel-Rahman M, Kurdi R, El- Heneidy A and Faisal E. Public practices on antibiotic use: A cross-sectional study among Qatar University students and their family members. Public Library of Science 14 (2019).
- Burns C. It’s in our hands: RPS campaign on antimicrobial stewardship encourages good handwashing. The Phar- maceutical Journal 299 (2017).
- Gardiner S, Drennan P, Begg R, Zhang M, Green J, Isenman I, et al. In healthy volunteers, taking flucloxacillin with food does not compromise effective plasma concentrations in most circumstances. Public Library of Science 13 (2018).
- Chevalier-Cottin E, Ashbaugh H, Brooke N, Gavazzi G, Santillana M, Burlet N, et al. Communicating Benefits from Vaccines Beyond Preventing Infectious Diseases. Infectious Diseases & Therapy 9 (2020): 467-480.
- Ashiru-Oredope D. Providing self-care advice for cold and flu keeps antibiotics working. The Pharmaceutical Journal (2019).
- Catho G, Centemero N, Catho H, Ranzani A, Balmelli C, Landelle C, et al. Factors determining the adherence to antimicrobial guidelines and the adoption of computerised decision support systems by physicians: A qualitative study in three European hospitals. International Journal of Medical Informatics (2020).
- Peters S, Rowbotham S, Chisholm A, Wearden A, Moschogianis S, Cordingley L, et al. Managing self-limiting respiratory tract infections: a qualitative study of the usefulness of the delayed prescribing strategy. The British Journal of General Practice: The Journal of the Royal College of General Practitioners, 61 (2011): e579-e589.
- Saliba-Gustafsson E, Roing M, Borg M, Rosales-Klintz S and Lundborg C. General practitioners' perceptions of delayed antibiotic prescription for respiratory tract infections: A phenomenographic study. Public Library of Science 14 (2019).
- O’Doherty J, Leader L, O’Regan A, Dunne C, Puthoopparambil S and O’Connor R. Over prescribing of anti- biotics for acute respiratory tract infections; a qualitative study to explore Irish general practitioners’ perspectives. BMC Family Practice 20 (2019): 1-9.
- Francis N, Gillespie D, Nuttall J, Hood K, Little P, Verheij T, et al. Delayed antibiotic prescribing and associated antibiotic consumption in adults with acute cough. British Journal of General Practice 62 (2012): 639-646.
- Spurling G, Del Mar C, Dooley L, Foxlee R and Farley R. Delayed antibiotic prescriptions for respiratory infec- tions. The Cochrane Database of Systematic Reviews 9 (2017): CD004417.
- Kesten J, Bhattacharya A, Ashiru-Oredope D, Gobin M and Audrey S. The Antibiotic Guardian campaign: a qualitative evaluation of an online pledge-based system focused on making better use of antibiotics. BMC Public Health 18 (2017): 1-13.
- Avent M, Cosgrove S, Price-Haywood E and van Driel M. Antimicrobial stewardship in the primary care setting: from dream to reality? BMC Family Practice 21 (2020): 1-9.
- Thornton J. Covid-19: how coronavirus will change the face of general practice forever. BMJ - British Medical Jour- nal 368 (2020).
- Brookes-Howell L, Hood K, Cooper L, Coenen S, Goossens H, Little P, et al. Clinical influences on antibiotic prescribing decisions for lower respiratory tract infection: A nine country qualitative study of variation in care. BMJ Open 2 (2012).
- MacIntyre C and Chau M. Pandemics, public health emergencies and antimicrobial resistance - putting the threat in an epidemiologic and risk analysis context. Archives of Public Health 75 (2017): 1-6.
- Seaton R, Gibbons C, Cooper L, Malcolm W, McKinney R, Dundas S, et al. Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID-19 in Scottish hospitals. Journal of Infection (2020).
- Abelenda-Alonso G, Padullés A, Rombauts A, Gudiol C, Pujol M, Alvarez-Pouso C, et al. Antibiotic prescription during the COVID-19 pandemic: A biphasic pattern. Infection Control and Hospital Epidemiology (2020): 1-2.
- Corr S. GP practices in Northern Ireland still open to patients who need treatment despite perceptions, say chiefs. Belfast Live (2020).
- Bostock N. Millions of patients 'avoiding calls to GP' during COVID-19 pandemic. GP Online (2020).
- National Health Service, (NHS). Check if you or your child has coronavirus symptoms (2020).
- Bonzano C, Borroni D, Lancia A and Bonzano E. Doxycycline: From Ocular Rosacea to COVID-19 Anosmia. New insight into the coronavirus outbreak. Frontiers in Medicine 7 (2020).
- National Institute for Health and Care Excellence, (NICE). COVID-19 rapid guideline: managing suspected or con- firmed pneumonia in adults in the community (2020b).
- Malek AE, Granwehr BP and Kontoyiannis DP. Doxycycline as a potential partner of COVID-19 therapies. IDCases 21 (2020): e00864.
- Brouqui P Million M, Parola P, McCullough PA and Raoult D. Outcomes after early treatment with hy- droxychloroquine and azithromycin: An analysis of a database of 30,423 COVID-19 patients, New Microbes and New Infections 55 (2023): 101-188.
- Karakonstantis S and Kalemaki D. Antimicrobial overuse and misuse in the community in Greece and link to antimicrobial resistance using methicillin-resistant S. aureus as an example. Journal of Infection and Public Health 12 (2019): 460-464.
- Sheldon T. Saving antibiotics for when they are really needed: The Dutch example. BMJ 354 (2016).
- Nowakowska M, Van Staa T, Mölter A, Ashcroft D, Tsang J, Palin V, et al. Antibiotic choice in UK general practice: Rates and drivers of potentially inappropriate antibiotic prescribing. Journal of Antimicrobial Chemotherapy 74 (2019): 3371-3378.
- Tarrant C, Krockow E, Nakkawita D, Bolscher M, Colman A, Chattoe-Brown E, et al. Moral and Contextual Dimensions of “Inappropriate” Antibiotic Prescribing in Secondary Care: A Three-Country Interview Study. Frontiers in Sociology 5 (2020).
- Hardigan P, Popovici I and Carvajal M. Response rate, response time, and economic costs of survey research: A randomized trial of practicing pharmacists. Research in Social and Administrative Pharmacy, 12 (2016): 141-148.
- Blair J, Czaja R and Blair E. Designing Surveys. A Guide to Decisions and Procedures. 3rd ed. United States of America: SAGE (2014).
- Riiskjaer E, Ammentorp J and Kofoed P. The value of open-ended questions in surveys on patient experience: Number of comments and perceived usefulness from a hospital perspective. International Journal for Quality in Health Care 24 (2012): 509-516.
- Rowley J. Designing and using research questionnaires: MRN. Management Research Review 37 (2014): 308-330.
- Hoag J and Kuo C. Ranking question designs and analysis methods. Journal of Medical Statistics and Informatics 4 (2016).
- Smyth J, Olson K and Burke A. Comparing survey ranking question formats in mail surveys. International Journal of Market Research 60 (2018): 502-516.
- Taherdoost H. What Is the Best Response Scale for Survey and Questionnaire Design; Review of Different Lengths of Rating Scale / Attitude Scale / Likert Scale. Authors International Journal of Academic Research in Management 8 (2019): 1-10.
- Hassan Z, Schattner P and Mazza D. Doing A Pilot Study: Why Is It Essential? Malaysian Family Physician: The Official Journal of the Academy of Family Physicians of Malaysia 1 (2006): 70-73.
- Boynton P. Hands-on guide to questionnaire research: Administering, analysing, and reporting your questionnaire. BMJ: British Medical Journal 328 (2004): 1372.
- Business Services Organisation, (BSO). GP Prescribing Data (2020a).
- Eisinga R, Pelzer B, Te Grotenhuis M and Heskes T. Exact p-values for pairwise comparison of Friedman rank sums, with application to comparing classifiers. BMC Bioinformatics 18 (2017).
- Kim H. Statistical notes for clinical researchers: Chi-squared test and Fisher's exact test. Restorative Dentistry & Endodontics 42 (2017): 152-155.
- Dahiru T. P - value, a true test of statistical significance? A cautionary note. Annals of Ibadan Postgraduate Medicine 6 (2008): 21-26.
- Lewis C and Mehmet M. Does the NPS® reflect consumer sentiment? A qualitative examination of the NPS using a sentiment analysis approach. International Journal of Market Research 62 (2020): 9-17.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
