Anticholinesterase Properties of Mareya Micrantha (Benth) Mull. Arg. (Euphorbiaceae) and its Fractions on the Cholinesterase Model of Isolated Rabbit Duodenum
Article Information
Dosso1*, T.Y. Soro2, M.A. Libra1, F. Traore2, J.D. N’guessan3
1Department of Biochemistry-Genetics, UFR Biological Sciences, Peleforo Gon Coulibaly University, B.P 1328 Korhogo, Ivory Coast
2Laboratory of Animal Physiology, UFR Biosciences, Félix Houphouët-Boigny University, 22 B.P. 582 Abidjan22, Ivory Coast
3Laboratory Pharmacodynamics Biochemistry, Department of Biosciences, Félix Houphouët-Boigny University, 22 BP 582 Abidjan 22, Ivory Coast
*Corresponding Author: Mamadou Dosso, Department of Biochemistry-Genetics, UFR Biological Sciences, Peleforo Gon Coulibaly University, B.P 1328 Korhogo, Ivory Coast
Received: 20 May 2020; Accepted: 01 June 2020; Published: 05 June 2020
Citation: Mamadou Dosso, Tianga Yaya Soro, Michel Archange Libra, Flavien Traore, Jean David N’guessan. Anticholinesterase Properties of Mareya Micrantha (Benth) Mull. Arg. (Euphorbiaceae) and its Fractions on the Cholinesterase Model of Isolated Rabbit Duodenum. International Journal of Applied Biology and Pharmaceutical Technology 11 (2020): 121-130.
View / Download Pdf Share at FacebookAbstract
Abstract
The study of the anticholinesterase properties of Mareya micrantha, a plant used in African pharmacopoeia as a laxative, was carried out on cholinesterases of the rabbit duodenum. At 0.15 mg / ml, the aqueous extract (MAR), its fraction (F5) and its sub-fraction (F52), depending on their state of purity, decrease the catalytic speed of cholinesterases during the hydrolysis of the acetylthiocholine (ASCh), without significantly influencing the Michaelis constant. The sub-fraction (F52) with the best anti-cholinesterase and myostimulant effects revealed by the bio-guided study is 25% more effective than MAR. A study of the High performance liquid chromatography (HPLC) chromatogram of this sub-fraction F52 shows that this plant is rich in phenolic molecules.
Keywords
Laxative; Mareya micranth; Fraction; Cholinesterases; HPLC
Article Details
Introduction
Constipation accompanies or announces some pathologies. It manifests in an insufficient frequency of defecation, formation of dehydrated stool, hard and difficult to evacuate. To remedy this, the laxatives available in pharmacies are used. These, like most synthetic drugs, often have side effects. As an alternative, and even more for cultural and economic reasons, the vast majority of the African population uses medicinal plants, an undeniable source of active ingredients. Mareya micrantha one of these plants is a shrub, about four meters tall with simple leaves, oblong and shapely. It occurs mainly in central and western Africa (Traoré et al., 2004). In Ivory Coast, is called in the vernacular "Oyia" and "Ochibouo-m’pi" respectively by the Dida and M’Bato. It grows on secondary formations. In traditional medicine, this plant is used in the treatment of gastrointestinal dysfunctions, leprosy, leishmaniasis, malaria, trypanosomiasis, and as an oxytocic agent during difficult deliveries (Guede-guina et al., 1995, Mac Foy et al., 1990; Thes et al., 2011). Previous studies have shown that its aqueous extract, endowed with cholinergic, histaminergic and even laxative activities (Traoré et al., 2004; Meité et al., 2010 , Dosso et al., 2013) is also cardio protector (Doumbia et al., 2014); the methanoic extract of this plant has antimicrobial effects; its essential oil is antifungal according to Thes (20114). However, these studies provide little information on the effect of Mareya micrantha on cholinesterases, enzymes that play a pivotal role in cholinergic mechanisms. To supplement this need for information, the present study, motivated by the use of this plant as a laxative, will evaluate the anticholinesterase properties of MAR and its fractions based on the cholinesterase model of the isolated rabbit duodenum.
Materials And Methods
Plant Material
The fresh leaves of Mareya micrantha were collected in the locality of Akoupé, in June 2007, identified and authenticated by Pr Ake Assi Laurent of the Botany Department of the Félix Houphouët-Boigny University (Côte d'Ivoire). After identification, one specimen was deposited under number 1804 at the herbarium of the National Floristic Center of the Félix Houphouët-Boigny University.
Animal Material
For our pharmacological and enzymatic experiments, we used rabbits of the species Oryctolagus cuniculus (Leporidae), from the commune of Abobo (Abidjan, Ivory Coast). They weighed on average 2 kg and were acclimatized under ambient conditions (28°± 3°C) for one week in the animal house of the Biosciences Training and Research Unit (UFR) of the Félix Houphouët-Boigny University of Cocody. The photoperiod is 12/24 hours.
Experimental Device
The experimental device consists of a thermostatically controlled water bath, an insulated organ tank filled with physiological solution of the Mac Ewen type, contained in flasks placed 40 cm from the apparatus. The liquids contained in the vials are placed in polyvinyl catheters and then coils that allow these solutions to warm up to a temperature of 38°C. This liquid supply is controlled by a multi-way selector valve. The insulated organ vessel can be drained through a drain at the bottom of the device.
Methods
Method for the Preparation of Aqueous Extract of Mareya micrantha
The leaves of Mareya micrantha were air-dried and then crushed. Eighty grams of the dry powder were mixed with two liters of distilled water. The mixture was shaken for 24 hours at room temperature by means of an agitator (AGIMATIC-N). The macerated mixture was filtered through cotton wool. The filtrate was evaporated using a Buchi rotary evaporator at 70°C. After total evaporation, a powder is obtained, part of which is used as an aqueous extract (MAR) and the other part is fractionated.
Method of Partitioning Aqueous Extract Fractionation
The aqueous extract of Mareya micrantha (1.5 g) is dissolved in one litre of 70% ethanol (Ethanol/water; 70/30; v/v). The mixture is stirred for 6 hours and left to stand in a separating flask for 24 hours. A hydroalcoholic supernatant (F1) and a precipitate (P) were obtained, collected separately and then evaporated using a Buchi rotary evaporator at 60°C. The resulting pellets are dried in an oven at 50°C. A portion of F1 taken up with a cyclohexane-water mixture (5:5; v/v) is subjected to the same process. A cyclohexane fraction (F2) and an aqueous fraction (F3) are obtained. The extract obtained from the aqueous phase (F3) is taken up in an ethyl acetate-water mixture (5:5; v/v). The solution undergoes the same treatment as the other mixtures. An ethyl acetate fraction (F4) and an aqueous fraction (F5) are obtained. F5 with 1371.42 mg strength revealed by the bio-guided study, presents the best myostimulant effect. It will therefore be retained for solid-liquid fractionation.
Method for Solid-Liquid Fractionation on Sephadex G25 gel from F5
800 mg of fraction F5 dissolved in 10 mL of distilled water is passed over a column (50 cm x 1.5 cm) of Sephadex G25 gel with distilled water as the mobile phase. The rate of elution was adjusted to 2 mL per minute. Volumes of 10 mL were collected and then grouped based on their ccm profile into three sub-fractions F51, F52 and F53. These fractions were evaporated at reduced pressure using a BÜCHI 461 Water Bath rotary evaporator. The powders obtained were used for physiological tests and the F52 with the best positive inotropic effect is analysed using the RP-HPLC (C18) preparative apparatus.
Method for the Preparation of Enzyme Extract
The method of preparation of the enzyme extract is based on that of Khoa and Ochillo (1987) (Khoa et al., 1987), taken up by Bahi (1998). After a 24-hour fast, the rabbit is stunned by a club blow on the neck and bled rapidly through the carotid artery. Following a midline laparotomy, four grams of intestine (duodenum) is immediately removed and immersed in 0.5 M mono and di-sodium phosphate buffer, pH 7.8, and then crushed with a T 25 micro-mill at 2500 rpm for 2 minutes. The grind obtained is then centrifuged at 2500 rpm for half an hour in an Alresa centrifuge. The supernatant obtained constitutes the enzyme preparation.
Method of Preparing Ellman's Reagent for Enzyme Assays
The study of total cholinesterase activity is performed with Ellman's substrate reagent, acetylthiocholine (ASCh) at 0.014 M concentration prepared with 0.4 g acetylthiocholine iodide dissolved in 100 mL phosphate buffer. Ellman's reagent is prepared extemporaneously with the mixture of 100 µL of 0.014 M acetylthiocholine iodide, 100 µL of 0.01M DTNB (Dithiobisnitrobenzoate) (obtained by dissolving 0.4 g of DTNB in 100 mL of 0.5 M phosphate buffer, pH 7.8) and 800 µL of 0.5 M phosphate buffer, pH 7.8.
Method for Studying the Activity of Rabbit Duodenum Cholinesterases in the Presence and Absence of the Effector
For the study of cholinesterase activity, 100 µL of Ellman's reagent with increasing concentrations of acetylthiocholine (10-5 M to 10-2 M) is added to the reaction medium. This medium consists of 1 mL of phosphate buffer (0.5 M, pH 7.8) and 50 µL of enzyme solution pre-incubated at 38°C for 5 min. During 10 min incubation, cholinesterases in the presence or absence of 30µL of effector at 0.15 mg/mL, hydrolyze acetylthiocholine (of varying concentrations) from the Ellman reagent. The hydrolysis products are acetate and thiocholine, which reacts with DTNB to give a yellow coloration, the intensity of which is measured by spectrophotometer at 412 nm against a control containing no enzyme solution. The change in optical density (OD) relative to the control, measured and plotted against the incubation time (dA/min), represents the initial rate. The LINERWEAVER-BURK representation (1/V as a function of 1/S) is constructed from these values and used to determine Vmax and KM.
Method of Removal and Placement of the Isolated Organ
The rabbit who fasted for 24 hours is knocked out. Following a midline laparotomy, 3 cm long segments of duodenum are immediately removed and kept alive in a glucose- and oxygenated Mac Ewen solution. One of the fragments is mounted on the recording apparatus in a well containing 250 ml of Mac Ewen in (mM) Nacl 130; Kcl 2.5; Cacl2 2.40; NaH2PO3 1.18; NaHCO3 11.90; MgCl2 0.24; glucose 2.2 of pH 7.4 continuously oxygenated and temperature 38°C.
With the aid of a wire passed through the wall of the duodenum fragment, a knot is made at one end of the duodenum fragment enabling it to be hooked into the interior of the insulated organ vessel. The other end is connected by another wire to the stylus, the nib of which is in contact with a paper coated with black smoke, wound on a cylinder subjected to a rotation of constant speed.
Experimental Protocol
Using a graduated syringe, a volume of the dilutions made from the test substances are introduced into the single-organ vessel, a well containing 250 ml of Mac Ewen. To move from one test to the next, the preparation is washed thoroughly with Mac Ewen (750 ml) to avoid the cumulative effect of the test substances.
Method for the Analysis of the Phenolic Constituents of the F52 Sub-Fraction
For the analysis of the phenolic constituents of the F52 fraction, we used the varian RP- HPLC (C-18) apparatus, equipped with a PDA (photo diode array) detector. The injection volume is 20µl, the analysis temperature is 25°C and the pump flow rate is 1mL /mn. The mobile phases used during this analysis are: water at 0.1% TFA for eluent A and acetonitrile for eluent B. The different gradients used for the analysis are: mobile phase [T=0: 95% (A), 5% (B)]; [T=10: 85% (A), 15% (B)]; [T=45 70% (A), 30% (B)]; [T=50: 0% (A), 100% (B)]; [T=55: 0% (A), 100% (B)].
Statistical Analysis
Statistical data expressed as mean ± standard error (M± SEM) were obtained from (n=4) separate experiments. The calculated means were compared from the Student (t) test. When p ≤ 0.05, the difference is said to be significant. The curves and statistical analysis were performed using Graph Pad Prism 5.01, San Diego, CA, USA.
Results
Influence of MAR on Total Cholinesterase Activity of Rabbit Duodenum
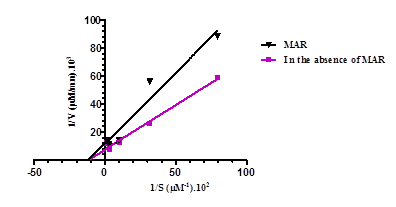
Figure 1. shows the effect of MAR at 0.15 mg/mL on the catalytic activity of total cholinesterases in the rabbit duodenum. In the presence of MAR, the maximum rate and affinity constancy values are 83.3 µM/min (P < 0.001) and 8.69 ± 1.7 µM, respectively, while in the absence of an effector, Vmax and KM are 125 ± 1.3 µM/min and 8.69 ± 0.9 µM, respectively. In the presence of MAR, KM does not change while Vmax decreases significantly (P < 0.001), indicating that MAR is a cholinesterase inhibitor.
MAR decreases Vmax without modifying the KM of cholinesterases.
The values express a variation of KM and Vmax of cholinesterases in the presence and absence of MAR and its F5 fraction (SEM ± mean, ***P< 0.001; n=4).
Influence of F5 on Total Cholinesterase Activity of Rabbit Duodenum
Table 1. represents the effect of F5 at 0.15 mg/mL on the catalytic activity of total rabbit duodenal cholinesterases. The maximum rate (Vmax) and affinity constancy (KM) values are 66.6 µM/min and 8.69 ± 0.9 µM in the presence of F5 versus 125 ± 1.3 µM/min and 8.69 ± 0.9 µM in the absence of an effector, respectively. The Vmax value decreases significantly (P < 0.001) in the presence of F5 while the KM values do not change. The hydrolysis activity of ASCh by cholinesterases decreases. Thus, F5 is an inhibitor.
Table1 : Kinetic parameters of rabbit duodenum cholinesterase in the presence and absence of F5
|
Kinetic parameters of cholinesterases in the presence and absence of the effector |
KM (µM) |
Vmax (101µM/mn) |
|
Cholinesterase |
8,69±1,5 |
12,50±1,3 |
|
Cholinesterase+ MAR |
8,69±1,7 |
8,33±1,2*** |
|
Cholinesterase+ F5 |
8,69±0,9 |
6,66±0,97*** |
Like MAR, F5 decreases Vmax without modifying the KM of cholinesterases.
The values express a variation of KM and Vmax of cholinesterases in the presence and
absence of MAR and its F5 fraction (SEM mean, ***P < 0.001; n=4).
Effect of F5 Fractions on the Contractile Activity of the Rabbit Duodenum
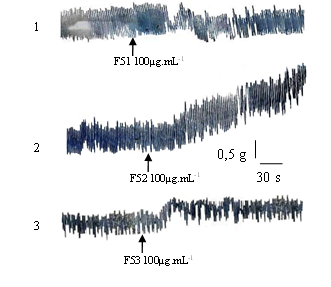
Chromatographic fractionation of F5 on sephadex G25 gel yielded fractions F51, F52 and F53. Figure 2 shows the effect of these fractions on the contractile activity of the rabbit duodenum. F52 and F53 at 100 µg.mL-1 show myostimulant effects, whereas F51 at the same concentration has myorelaxing properties. F52 and F53 increase contraction by 2428.57 ± 5 mg strength (P < 0.001) and 534.74 ± 5 mg strength (P < 0.05), respectively.
1-Effect of F51 100 μg.mL-1 2-Effect of F52 100 μg.mL-1 3-Effect of F53 100 μg.mL-1;
F5 has a dose-increasing myostimulant effect
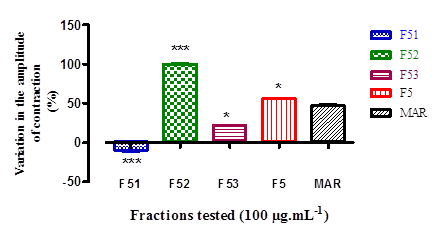
Figure 3 shows the percentage change in rhythmic contraction of the rabbit duodenum as a function of the F51, F52 and F53 fractions tested at 100 µg.mL-1. F52 induces the greatest myostimulant effect (P < 0.001).
These values express a variation in contraction as a function of the fraction of F5 tested (SEM mean,*P< 0.05; ***P <0.001, n= 4).
Influence of F52 on total Cholinesterase Activity of Rabbit Duodenum
In the presence of F52 at 0.15 mg/mL the affinity constant and maximum rate Vmax of rabbit duodenal cholinesterases are 8.69 ± 0.9 µM and 62.5 µM/min (P < 0.001) respectively, compared to 8.69 ± 0.9 µM and 125 ± 1.3 µM/min in the absence of an effector. Vmax decreases in the presence of F52 (P < 0.001) while KM does not vary in the presence of the effector. The hydrolysis activity of acetylthiocholine (ASCh) by cholinesterases decreases, indicating that F52 is an inhibitor. It is 25% more effective than MAR.
High Performance Liquid Chromatography (HPLC) Chromatograms of F52
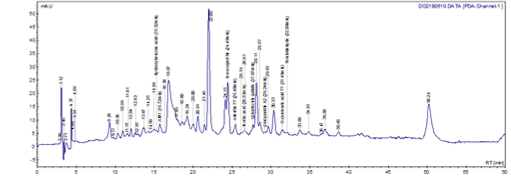
The RP-HPLC (C-18) chromatogram of sub-fraction F52 indicates that the aqueous extract of Mareya micrantha, is rich in phenolic molecules in view of the peaks recorded. Some of these molecules have been identified, they are coumarins (isoscopoletin), phenolic acids (hydroxy-benzoic acid, ferulic acid, o-coumaric acid) and flavonoids (epicatechin gallate, oriental) (Figure 4).
Discussion
The study of the anti-cholinesterase properties of Mareya micrantha was carried out on rabbit duodenum cholinesterases. It showed that in the presence of the aqueous extract of Mareya micrantha, the maximum rate (Vmax) of hydrolysis of acetylthiocholine (ASCh) from cholinesterases decreased significantly, without changing the Michaels constant (KM). These data reveal that the aqueous extract Mareya micrantha has anticholinesterase properties. It is believed to be a non-competitive inhibitor of cholinesterases. This effect of Mareya micrantha could be explained by the binding of the anticholinesterase effector molecules of this natural essence at sites different from those of the substrate (ASCh) at the enzyme level. The resulting ternary complex of enzyme, effector and substrate would therefore prevent conformational adjustment, a prerequisite for catalysis at the active site of the enzyme (Wilson et al., 1950; Froede et al., 1986; Diopoh, 2016). Similar mechanisms of competitive inhibition of cholinesterases have been demonstrated by Gnahoue (2009), using the aqueous extract of Trema guineensis.
To identify the phenolic molecules responsible for the anticholinesterase and even laxative properties of this natural essence, its aqueous extract was partitioned into different solvents. The aqueous fraction F5, more spasmogenic than MAR (Dosso et al., 2017), was retained and subjected to a new solid-liquid fractionation on sephadex G25 gel. F52 is the sub-fraction with the best positive inotropic effect. Like MAR, the F5 fraction and this F52 sub-fraction, without affecting the Michaelis constant, reduce the catalytic rate of cholinesterases in a purity-dependent manner. It is deduced that they have become progressively enriched in anti-cholinesterase substances and could act according to the same mechanism as MAR. Inhibit cholinesterases, increase intestinal contraction (Traoré et al., 2004, Dosso et al., 2013). Facilitate intestinal fluidity with electrolytes (Méité et al., 2010), probably via the activation of calcium-dependent channels and CFTR, a glycoprotein located at the apical membrane of the intestine that governs ionic movements (chlorine, sodium, water) (Hirota et McKay, 2006; Frizzell et Hanrahan, 2012). The RP-HPLC (C-18) chromatogram of the F52 fraction confirms the presence of phenolic molecules in this fraction. They are epigatechin galate, oriental, scopoletin, ferulic acid which could be endowed with anticholinesterase or even laxative effect. However, further purification studies could make it possible to clearly identify the molecules responsible for the plant's so-called effects. Plants containing phenolic molecules with anticholinesterase effects have been studied: Cynanchum atratum and Origanum majorana (Somboro et al., 2013).
It can be concluded from this study that the aqueous extract of Mareya micrantha, as well as its fractions (F5 and F52) show anticholinesterase activities, they are non-competitive inhibitors; indeed, they decrease the catalytic speed of cholinesterases and have no significant influence on the consistency of Michaelis. These anticholinesterase properties of Mareya micrantha and its fractions, probably due to the phenolic content of this natural essence, could partly justify the use of this plant as a laxative in traditional medicine.
References
- Traoré F, Dosso M, Guede-Guina F, Aka KJ. Effet myostimulant d'un extrait aqueux de Mareya micrantha (Euphorbiaceae) sur l'activité contractile intestinale de lapin. Ethnopharmacologie 33 (2004): 45-59.
- Guede-Guina F, Tsai CS, Smith MO, et al. The use of isolated functional heart to pharmacologically characterize active ingredients in the aqueous extracts of Mareya micrantha. Journal of Ethnopharmacology 45 (1995): 19-26.
- Macfoy CA, Cline EI. In vitro antibacterial activities of three plants used in traditional medicine in Sierra Leone. Journal of ethnopharmacology 28 (1990): 323-327.
- Thes PM, Soumahoro JA, Ackah JA, et al. A pharmaceutical dermatological soap combining plant oils (MISCA) for the treatment of ringworm. Phytotherapi 9 (2011): 354-358.
- Méité S, Bahi C, Yéo D, et al. Laxative activities of Mareya micrantha (Benth.) Müll. Arg. (Euphorbiaceae) leaf aqueous extract in rats. BMC Complementary and Alternative Medicine 10 (2010): 7.
- Dosso M, Meite S, Yeo D, et al. Cholinergic and Histaminergic Activities of the Aqueous Extract of Mareya micrantha (Benth). Müll Arg (Euphorbiaceae). Asian Journal of Biochemistry 8 (2013): 24-32.
- Doumbia I, Ouattara K, Coulibaly AF, et al. Évaluation de l’effet cardioprotecteur de l’extrait aqueux de Mareya micrantha (Euphorbiaceae) chez le lapin. Phytothérapie 12 (2014): 128-134.
- Bui K, Ochillo RF. Characterization of cholinesterase of muscularis muscle of Bufo marinus. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 87 (1987): 107-111.
- Bahi. Mécanismes biochimiques d’une action cholinolytique de BGG, un concentré de source végétale. Thèse de doctorat 3ème cycle. Université de Cocody (1998): 103.
- Wilson IB, Bergmann F. Acetylcholinesterase VIII. Dissociation constants of the active groups. Journal of Biological Chemistry 186 (1950): 683-692.
- Froede HC, Wilson IB, Kaufman H. Acetylcholinesterase: theory of non competitive inhibition. Archives of Biochemistry and Biophysics 247 (1986): 420-423.
- K. Diopoh. Biochimie métabolique, Enzymologie générale-métabolisme, Nouvelle Edition Balafons (2016): 235.
- Gnahoué G, N’guessan JD, Koffi E, et al. In vitro anticholinestarase and cholinergic effect of the aqueous extract of Trema guineensis on rabbit duodenum. Tropical Journal of Pharmaceutical Research 8 (2009): 11-17.
- Dosso M, Soro D, Koffi AE, et al. Isolement par partition bio guidé du principe actif myostimulant de l’extrait aqueux de Mareya micrantha (Benth) Mull. Arg.(Euphorbiaceae). Journal of Applied Biosciences 114 (2017): 11336-11344.
- Hirota CL, McKay DM. Cholinergic regulation of epithelial ion transport in the mammalian intestine. British Journal of Pharmacology 149 (2006): 463-479.
- Frizzell RA, Hanrahan JW. Physiology of epithelial chloride and fluid secretion. Cold Spring Harbor Perspectives in Medicine 2 (2012): a009563.
- Somboro AA, Diallo D, Sidibe L, et al. Activités anticholinestérasiques des alcaloïdes totaux extraits des feuilles, fruits, écorces de racines et écorces de tronc de Guiera senegalensis, une plante médicinale Malienne. International Journal of Biological and Chemical Sciences 7 (2013): 1723-1728.


 Impact Factor: * 3.0
Impact Factor: * 3.0 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
