Assessment of Structure and Natural Regeneration Capacity of Avicennia Marina and Bruguiera Gymnorrhiza Species of Mangroves in Mida Creek Kilifi County, Kenya
Article Information
David Amakanga Erasto1*, Benards Okeyo2, Najma Dharani3
1Department of Environmental Sciences, Pwani University, Mombasa-Malindi Highway: PO Box 195 - 80108 Kilifi county, Kenya
2Department of Environmental Sciences. Pwani University, Mombasa-Malindi Highway. PO Box 195 - 80108 Kilifi county, Kenya
3Department of Plant Sciences, Kenyatta University, P.O BOX 34844-00100, Nairobi, Kenya
*Corresponding Author: David Amakanga Erasto, Department of Environmental Sciences, Pwani University, Mombasa-Malindi Highway: PO Box 195 - 80108 Kilifi county, Kenya
Received: 27 March 2021; Accepted: 12 April 2021; Published: 21 May 2021
Citation: David Amakanga Erasto, Benards Okeyo, Najma Dharani. Assessment of Structure and Natural Regeneration Capacity of Avicennia Marina and Bruguiera Gymnorrhiza Species of Mangroves in Mida Creek Kilifi County, Kenya. International Journal of Plant, Animal and Environmental Sciences 11 (2021): 262-294.
View / Download Pdf Share at FacebookAbstract
The study assessed the structure, natural regeneration capacity and Biotic agents of Avicennia marina and Bruguiera gymnorrhiza species in Mida creek, Kilifi County. The study used both cross section and descriptive research design. Avicennia marina species was more dominant with a greater complexity index (A2.7, B0.4), basal area (A588cm2, B484cm2) and mean height (A26m, B10m) compared to Bruguiera gymnorrhiza species with both having an aggregate dispersion pattern. During dry season, Height, and diameter of Avicennia marina had a correlation of 0.56 while Bruguiera gymnorrhiza species had 0.78. During rainy season both had a correlation of 0.67. B. gymnorrhiza species had more straight poles than A. marina species, 75.3% of B. gymnorrhiza trees assessed had straight pole compared to 1.7% of Avicennia marina trees. Both Avicennia marina and Bruguiera gymnorrhiza species had fair regeneration capacity where the number of seedlings was greater than saplings and number of saplings was less than mature trees. Littoraria scabra fed mostly on micro-organisms and algae, Littoraria Glabrata fed mostly on mud surface, Sesarma guttata and Sesarma leptosoma fed on mangrove leaves, Cerithidea decollata fed on deposits and organic matter, Terebralia palustris and Selatium elongatium fed more on algae and leaves, Sesasrmi ortmanni, Metopograpsus oceanicus and Neosarmatium meinerti fed on leaves and young propagules, Barnacles and Oyster bunch on the roots and stems of B. gymnorrhiza species. Biotic agents were insignificant in affecting regeneration capacity of A. marina and B. gymnorrhiza species however combinations with climatic and anthropogenic factors affected Regeneration capacity of the two species.
Keywords
Structure; Natural regeneration capacity; Biotic agents; Complex index; Dispersion pattern
Structure articles; Natural regeneration capacity articles; Biotic agents articles; Complex index articles; Dispersion pattern articles
Article Details
1.0 Introduction
The mangrove ecosystem is a unique and exceptional ecosystem found between the tropical and subtropical coastline offering a wide array of ecosystem services and goods. These include fuel, timber production, protection of the shoreline from wave erosion, breeding grounds for fish, pollution amelioration, and lime production among others (Natividad and Jimenez, 2015). A mangrove is a woody tree or shrub that has developed adaptations characteristics such as vivipary of seeds and salt excretion glands and aerial roots that thrive in between the tropical and subtropical coastlines.Natural regeneration is the process by which Mangroves are restocked by trees that develop from coppice shoots, root suckers, and seeds that fall and germinate in situ (Van Leeuwen and Nieuwenhuis, 2010).
Mangroves are found in tropical and subtropical regions of the world. The largest percentage of mangroves is found between the 5° N and 5° S latitudes. In the past decades, Mangrove forest cover has been reducing across the world, it is estimated that one-third of the forest has been lost over the years. As of 2005, the mangrove forest that existed was estimated to be 15.2 million hectares, a decrease from 18.8 million hectares in 1980 (FAO, 2005).
Giri et al, (2011) recorded that mangrove forests occupy approximately less than 14 million ha. Spalding et al, (2010) indicated 35% of the original forest cover has been degraded. According to IUCN, (2020), Half of the World's mangrove forest which covers an area of 32 million hectares has been deforested. Hamilton and Casey (2016) recorded that the rate of loss of mangrove slowdown in most of the areas since 1980 but they remained significant. However, most countries still had an annual loss rate of up to 3.1% yearly. The continent that suffered a major net loss of greater than 1.9 million hectares at the regional level was Asia. The North, Central America, and Africa significantly contributed to the decline of mangrove area at the global level with a loss of about 690 000 and 510 000 ha (FAO, 2008).
In the Africa continent, Mangrove forests cover over 3.2 million ha, accounting for about 19% of the world mangrove forest coverage area (Ajonina et al, 2008). Mangroves forest in Africa grows in countries that are found along the east and west coasts, from South Africa to Egypt on the east and from Angola to Mauritania on the west. Seventy percent of Africa's mangroves are found within five countries: Madagascar, Guinea, Cameroon, Mozambique, and Nigeria. Mangrove in the Eastern side of Africa is in Mozambique, Tanzania, Kenya, and Somalia with the majority found in northern Tanzania and southern Kenya. Tanzania had the highest tidal amplitudes of 3.2 meters, and Kenya had 3.5 meters and Mozambique had 5.6 meters (Spalding et al, 1997).
Mangroves have well developed an aerial rooting system that helps in the exchange of gases, provides support in the muddy sediments, and absorption of nutrients by the tree (Alongi, 2009). Mangrove use salt excretion glands to eliminate the absorption of salt at the root level and removal of extra salt at the leaves level by cuticular transpiration. Another mechanism is by accumulating the salt in leaf cells or by shedding the leaves (Alongi, 2009). Mangroves depend on seedling propagation for forest sustainability and maintenance of biodiversity (Feller and Sitnik, 1996).
Mangroves have two reproductive strategies: hydrochory and vivipary (Feller and Sitnik, 1996). Hydrochory is when the mangrove seeds, fruits, and propagules are dispersed by water. The water waves and tides carry away the mangrove diaspore to some distance from the point of origin. Vivipary is a process where the propagule embryo starts germinating while still attached to the parent tree (Elmqvist and Cox, 1996). Mangroves depend on propagules and seeds for natural regeneration. Predation of propagules before and after dispersion is very common (Clarke and Kerrigan, 2002). The most common predation of the mangrove is by snails, decapods, insects, monkeys, and fish (Dahdouh- Guebas et al, 1998). Seeds and seedling predation hinder the natural establishment of seedlings (Amarsinghe and Vidanage, 2007).
Mangrove forest cover in Kenya was estimated at 50,000-60,000 hectares (FAO, 2016) representing a decline of almost one-fifth since 1985. Global warming which results to rise in temperature in the past years causing an increase in sea levels threatens the growth of the mangrove. In Kenya, most of the people living along the coastline rely on mangrove forests for building poles and firewood (Thomas, 2017). According to (Bosire, 2014) the year between 1992 and 2009, Tudor lost 86.9% of the mangrove forest while Mwache lost 45.4% which marked the highest degradation rate of 5.1 and 2.7% per annum, respectively.
Mida creek has the highest hectares of Mangrove in Malindi, with an average area of 1600 ha (Wairungu et al, 2009). Mida creek lost 8.8 ha of mangrove forest between 1969 and 2010 (Alemayehu, 2014). The destruction of the mangrove ecosystem has always led to the loss of ecosystem services like fish habitat and coastal protection, which has serious impacts on livelihoods for local communities (Alemayehu et al, 2014). Eight species of mangroves are found in Mida Creek, and the distribution depends on the salt concentration gradients, dissolved oxygen, soil’s pH, and depth of the water table. Avicennia marina grows in sandy soils, Rhizophora mucronata in muddy soils, Ceriops tagal grows in dry areas, Bruguiera gymnorrhiza in areas that are wet with sediment deposition, and Lumnitzera racemosa and Xylocarpus granatum grows in the landward peripheral, indicating the change to brackish water (Chapman, 1977). Sonneratia alba is a pioneer species growing on open seas, with Heritiera littoralis and Bruguiera gymnorrhiza often found behind it.
Avicennia marina is known as Mchu (Swahili) and Mtswi (Giriama) is an evergreen distributed mangrove tree or shrub that grows to a height of between 3 and 5 m, A. marina has thick branches, breathing root, and round crown. The bark Avicennia marina is yellow-green, smooth in nature, and releases resin when cut. New branches have short and white hairs. Leaves are opposite, oval with lengths of between 4-11 cm. They have small and fragrant flowers that are cream orange (turning black). Fruit are grey and capsule are oval. The seeds are vivipary (Dharani, 2019).
Bruguiera gymnorrhiza is known as: (Muia in Swahili) is an average mangrove tree with a height of up to 30–35 m. The diameter of B. gymnorrhiza ranges between 15 and 35 cm. The leaves are large and usually grow as one stem tree with short buttresses that are characterized by horizontal roots that form above ground. (Allen, 2008). The bark of Bruguiera gymnorrhiza is pale brown usually darker when wet with a thickness larger than 2 cm and rough in nature. They have simple leaves, dark green in color. Leaves are between 8 to 22 cm in length and between 5 and 8 cm in width, with petioles of 2 to 4 cm. Leaf-blades are elliptic and about 15 cm long and 6cm wide (Dharani, 2019).
Bruguiera gymnorrhiza is viviparous where the propagule germinates while still attached to the parent tree. Hypocotyl arises from the calyx and is dark green in color, cylindrical, elongated in shape, and angular in shape. The root tips are pointed with dimensions of between 15 and 25 cm long and 2cm wide. The worn calyces often remain attached to the mother plant after mature propagules fall. (Allen, 2006).
Mida Creek mangrove forest is under the protection of the Forest Act (2005). Mida community rely on mangrove forest for livelihood resulting in overharvesting, this overexploitation resulted in the opening of ways in the forest for high tides that affect the mangrove ecosystem hence threatening the sustainability of Mangrove forest. Kairo, (2002) recorded exploitation of mangroves in Mida creek does not necessarily cause a change in the forest but also a change in species composition.
Alongi, (2002) and Giri et al, (2011) found that degradation of Mangrove forests in Mida creek has been instigated by anthropogenic activities. Siltation, salinization, rise in temperature, change in ocean tides and storms negatively impact on growth and establishment of mangroves hence contributing to the changes in Mangrove vegetation cover.
Alemayehu et al, (2016) found Mida creek Land use rate to be 2.5% with coastal bush having a decline coverage rate of -6.5%. Warui, (2011) recorded a loss of 105.2ha of Mangrove forest in Mida creek and an increase of bare patches and islands by 32.1 ha and 107.4ha. Mangrove forests in Mida play a key ecological function with an average of 90% of aquatic animals spends part of their lives within the mangrove forest (Benfield, 2002) hence necessitate the need to protect the mangrove ecosystem (Farnsworth and Ellison, 1997).
Numerous studies have been undertaken on the regeneration and establishment of mangrove trees (Blanchard and Prado, 1995). Factors of fruit dispersal (Middleton, 2005), predation by crab (McGuinness, 1996), and properties of soil. Fruit dispersal, Clarke and Allaway, (1993) gives a distribution range of a few 100 meters for Avicennia marina. According to Blanchard and Prado (1995), Rhizophora mangle mainly settles some meters away from the adult tree and McGuinness, (1996) reported dispersal range of Ceriops tagal to be around 3 m in mangroves around Darwin, North Australia.
Mchenga and Ali, (2014) found that at the species level, A. marina, C. Tagal and B. gymnorrhiza recorded high regeneration in Manda and Mwache. Alemayehu and Chemuku, (2017) found mangrove regeneration in Mwache, Tudor, and Kilifi creeks to be high with some mangrove species i.e., Rhizophora mucronata and Ceriops tagal dominating the sites. Regeneration for R. mucronata was high; seeding for the same species was also heavy especially in Mwache creek. The natural regeneration of Bruguiera gymnorrhiza was equally high in Manda Island. Kairo et.al (2002) found the sapling density of mangroves in Mida creek varied greatly.
2.0 Material and Method
Ecological Techniques
The study used stratified sampling in Avicennia marina species since they have distinct zonation while random sampling technique in Bruguiera gymnorrhiza species which grow mixed with other species. Line transect line was established across the single stand forest of A. marina and mixed forest of B. gymnorrhiza species using Point Centered Quarter Method to enable assess the structure, regeneration rate and biotic agents of Avicennia marina and Bruguiera gymnorrhiza species Deshmukh et al, (1994).
A total of 15 belt transects of 10m by 10m were established along the transect line of Avicennia marina single stand forest and another 15 belt transects of 10m by 10m were established on Bruguiera gymnorrhiza mixed forest. The distance between the belt transects was 50 m, tree sampling was done in 10m by 10m quadrats, sapling on 5m by 5m and seedlings on 1m-by-1m quadrats. Within each quadrat all individuals’ mature trees, saplings and seedlings were measured and counted.
2.1 Data collection techniques
Primary data was collected through ecological survey, measurement, and direct observation while secondary data was sourced from, journals, newspapers, academic research findings and reports from government and non-government organizations. A preliminary site visit was undertaken to identify and mark the plot coordinates before the primary data was collected. Primary data collected were number of trees at seedling, sapling and mature level, tree height, stem diameter at ground and breast level (DBH and DGL), quantity of stumps, fallen or standing dead mangrove, quality of pole of Avicennia marina and Bruguiera gymnorrhiza species and biotic agents.
Seedlings less than 0.5 m in height were classified as regeneration class I (RCI), sapling between 0.5 m and 1.5 m height and diameter below 2.5 cm were classified as regeneration class II (RCII) while all small trees with height greater than 1.5m height and 2.5cm butt diameter were classified as regeneration class III (RCII) (Fourqurean and Robblee, 1999). Wood quality was assessed through trees categorization into classes depending on how straight the main stem is. Stems that were straight were assigned tree form 1, intermediate poles that needed slight modification before building were assigned tree form 2 and the crooked poles that are unsuitable for building were assigned tree form 3.
Diameter (DBH, DGL) was measured using metric fabric diameter tape and height (H) of the trees was measured using suunto clinometer. GPS was used to mark coordinates and diameter tape used to establish a regular line transect. Identification of biotic agents was done in the quadrats established through observation of the shell form and colour and feeding habit.
2.2 Data analysis and presentation
NCSS12 Data, XTLSTAT statistical software and Ms. excel were used to analyze data for vegetation structure, Regeneration capacity and biotic agents. Analysis of variance, correlation, regression analysis and univariate analysis was used to analyze the different data parameters.
Watson’s inundation class method was used to determine the regeneration capacity of mangrove species. RCI represented good regeneration capacity where the numbers of seedlings > saplings > adults, RCII represented fair regeneration where seedlings > or ≤ saplings ≤ adults, RCIII represented poor regeneration where species survived only in sapling stage where sapling> or ≥ adults and if the species present was only mature it was considered not regenerating and it was represented by RCIV. Recruitment rate data from class I to class III was used to make prediction for future mangrove layer.
The qualitative data was analyzed through categorization and content analysis and then presented in figures and tables. The Morisita index equation was used to determine the dispersion pattern of Mangrove species. The spatial pattern arrangement of the tree species was classified using Silveira Neto et al, (1976) where it was classified as uniform or regular when it was less than one (Id<1), aggregate when the index is greater than one (Id>1) and random when the Morisita index was equal to one (Id=1).
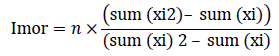
Density, dominance, frequency, abundance and relative density, relative frequency, relative dominance, and importance value was calculated using the formulae below:
|
Density |
= |
Total number of individuals of species in all quadrats/Total number of quadrats studied |
|
Dominance |
= |
Total basal area of a species/Total area sampled |
|
Frequency (%) |
= |
Number of quadrats in which the species occurred/Total number of Quadrats studied×100 (%) |
|
Abundance |
= |
Total number of individuals of a species in all quadrats/Total number of quadrats in which the species occurred |
|
Relative density |
= |
Number of individuals of the species/Number of individuals of all the species×100 (%) |
|
Relative Frequency |
= |
Number occurrence of the species/ Number of occurrences of all the species×100 (%) |
|
Relative dominance |
= |
Total basal area of the species/Total basal area of all the species ×100 (%) |
|
Importance value |
= |
Relative frequency + Relative density + Relative dominance |
3.0 Results
3.1 Structural Composition of Avicennia marina and Bruguiera gymnorrhiza mangrove species.
Avicennia marina dominated the highest land ward zone while Bruguiera gymnorrhiza mangrove species was recorded growing as a mixed forest with other species in Mida creek. During dry season Avicennia marina species had a dominance of 4.2±1, density of 7.1±4, frequency of 93.3±40 and abundance of 7.6±4. Bruguiera gymnorrhiza species had a dominance of 4.3±3, density of 4.3±2, frequency of 93.3±41, and abundance of 4.6±1 as shown in Table 4.1 below. During rainy season Avicennia marina species had a dominance of 8.2±5, density of 7.3±4, frequency of 93.3±42 and abundance of 7.6±3. Bruguiera gymnorrhiza species had a dominance of 3.8±3, density of 4.3±2, frequency 93.3±31and abundance of 3.4±1.
3.2. Occurrence density distribution
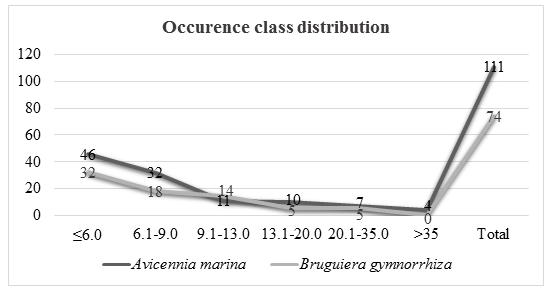
There were 111 Avicennia marina trees species in 0.15 hectare assessed in the eulittoral zone and 74 of Bruguiera gymnorrhiza trees in 0.15hectare in supra littoral zone. A total of 46 trees had diameter less than 6.0 cm, 32 trees had diameter between 6.1 and 9.0 cm, 11 trees had a diameter between 9.1 and 13.0 cm, 10 trees had diameter between 13.1 and 20.0 cm, 7 trees had a diameter between 20.1 and 35 cm and 4 trees had diameter above 35 cm.
Bruguiera gymnorrhiza occupied supra littoral zone. There was a total of 74 trees in 0.15 hectares assessed. A total of 32 trees had a diameter less than 6cm, 18 had a diameter between 6.1 and 9.0 cm, 14 had a diameter between 9.1 and 13.0 cm, 5 trees had diameter between 13.1 and 20.0 cm, 5 trees had adiameter between 20.1 and 35.0 with 0 trees having a diameter above 35cm.
3.3. Structural characteristics of Avicennia marina and Bruguiera gymnorrhiza species of Mida Creek
There were variations in structural characteristics of Avicennia marina and Bruguiera gymnorrhiza species of Mida Creek. Avicennia marina tree species of diameter class less than five had a stem density of 28, mean height of 3.0 m, average basal area of 11.2 cm2 and a complexity index of 9.4×103; trees with class diameter of between 5and 10 had a stem density of 45, mean height of 4.0 m, average basal area of 38.5 cm2 and complexity index of 6.9×102.
Trees with diameter class between 10 and 15 had a stem density of 13, mean height 5.3m, average base area 115.5 cm2 and complexity index 7.9×102; trees with diameter class greater than fifteen had a stem density of 18, mean height of 25.7 m, average basal area of 587.9 cm2 and complexity index of 2.7. Bruguiera gymnorrhiza tree species of class diameter less than five had a stem density of 74, mean height of 5.5 m, average basal area of 6.8 cm2 and complexity index of 2.7×102; tree with diameter class between 5 and 10 had stem density of 32, mean height 5.7 m, average basal area 43.3 cm2 and complexity index of 7.8×102; trees with diameter class between 10 and 15 had a stem density of 8, mean height of 7.6 m, average basal area of 122.6 cm2 and complexity index of 7.4×102; trees with diameter class above 15 had a stem density of 8, mean height of 10.4m, average basal area of 43.8 cm2 and complexity index of 0.4.
3.4. Height diameter distribution of Avicennia marina and Bruguiera gymnorrhiza species during dry season of February and March 2019
3.4.1: Height diameter distribution of Avicennia marina species
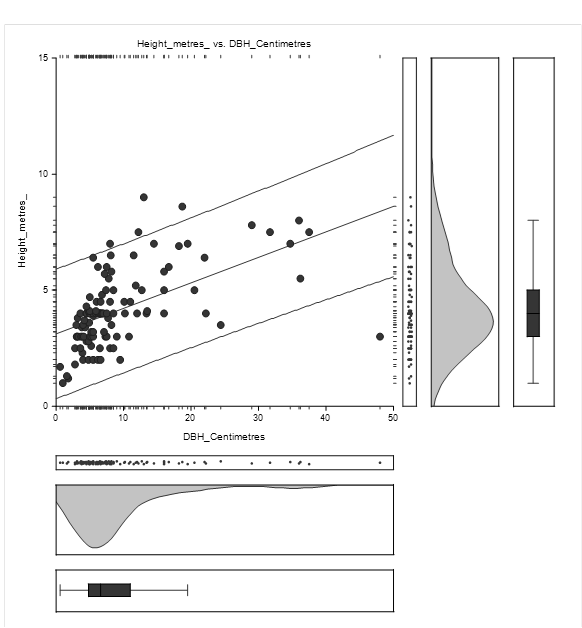
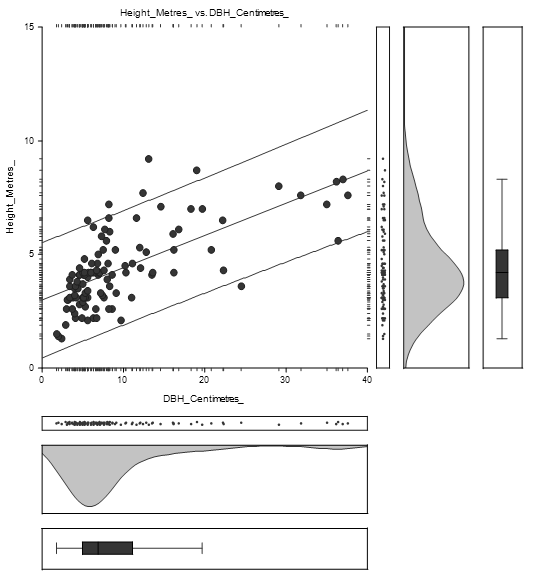
The equation of the straight-line relating Height and DBH of Avicennia marina was estimated as: Height = (3.1) + (0.1) DBH as shown in Figure 4.2. The diameter concentration was between 0 and 15 centimetres while height concentration was between 1 and 7 metres.
3.4.2: Height diameter distribution of Bruguiera gymnorrhiza mangrove species
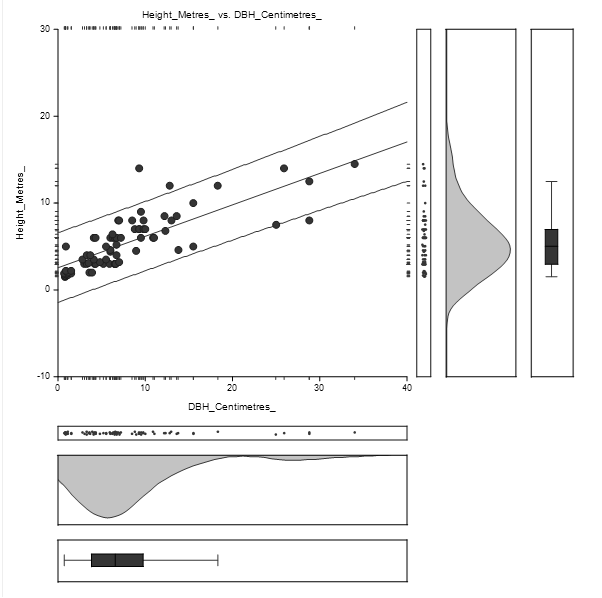
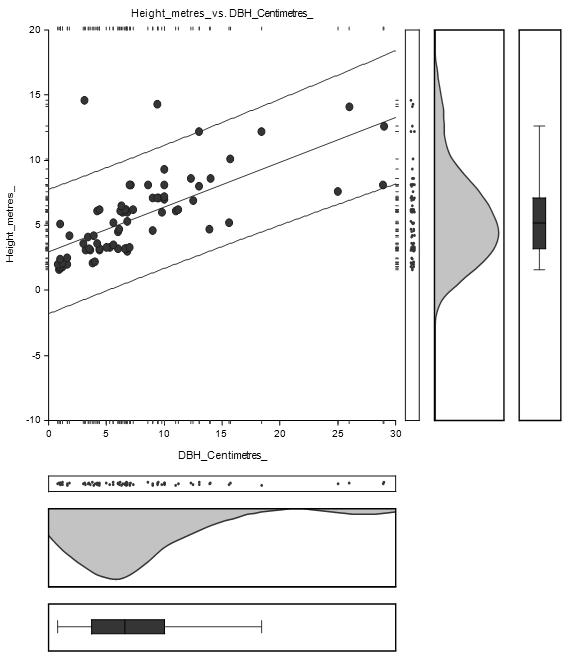
The straight-line equation relating Height and DBH of Bruguiera gymnorrhiza species was estimated as: Height = (2.6) + (0.4) DBH as shown in Figure 4.3. The high concentration of DBH lies between 1 and 15 cm while Height concentration lies between 1 and 10 m.
3.5. Height Diameter Distribution of Avicennia marina and Bruguiera gymnorrhiza species for Rainy season of November 2019
There were trivial variations in stem diameter and tree height distribution between Avicennia marina and Bruguiera gymnorrhiza species as shown in Figure 4.4 and Figure 4.5.
3.5.1. Height Diameter Distribution of Avicennia marina species.
The straight-line equation relating Height and DBH of Avicennia marina was estimated as: Height = (3.0) + (0.1) as shown in Figure 4.4. The high concentration of height lies between 1 and 8 metres while DBH concentration was between 1 and 15 centimetres.
3.5.2. Height Diameter Distribution of Bruguiera gymnorrhiza species
The equation of the straight-line relating Height and DBH was estimated as: Height = (3.0) + (0.3) DBH as shown in Figure 4.5. The concentration of DBH lies between 1 and 15 centimetres while the concentration of height lies between 1 and 10 metres.
3.6. Dispersion pattern of Mangrove trees species
Avicennia marina and Bruguiera gymnorrhiza were aggregately dispersed with Avicennia marina having dispersion index of 1.3 and Bruguiera gymnorrhiza having dispersion index of
3.7. Quality of Avicennia marina and Bruguiera gymnorrhiza poles
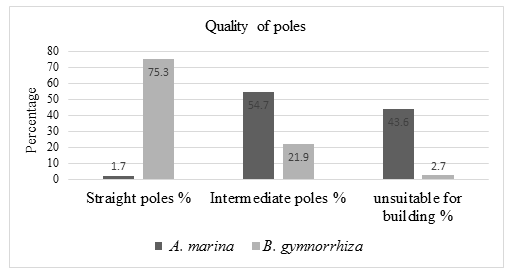
The quality of poles of Avicennia marina species varied with Bruguiera gymnorrhiza species, B. gymnorrhiza was more suitable for building more than A. marina species. 75.3% of the Bruguiera gymnorrhiza tree species assessed had a straight pole, 21.9% of the tree had intermediate poles that need slight modification for construction and 2.7% of trees were unsuitable for building. Avicennia marina tree species, 1.7% of total trees assessed had a straight pole that can be used for building, 54.7% trees had intermediate poles that can be modified and 43.6% trees unsuitable for buildings.
3.8. Regeneration capacity of Avicennia marina and Bruguiera gymnorrhiza species
3.8.1. Regeneration capacity of Avicennia marina and Bruguiera gymnorrhiza species during dry season of between February and March 2019
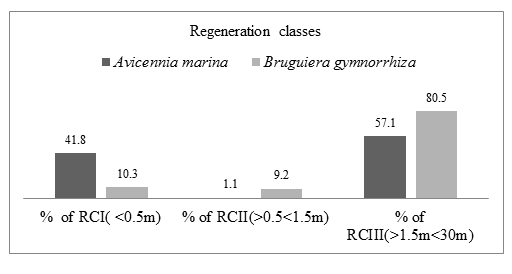
During dry period in Mida creek, Avicennia marina and Bruguiera gymnorrhiza species had a fair Regeneration capacity. The number of seedlings (RCI) was higher than number of saplings (RCII) while the number of saplings was less than the number of mature trees (RCIII). Avicennia marina species RCI had 79 individuals (41.8%), RCII had 2 individuals (1.1%) and RCIII had 108 individuals (57.1%). Bruguiera gymnorrhiza species RCI had 9 individuals (10.3%), RCII had 8 individuals (9.2%) and RCIII had 70 individuals (80.5%). Table 4.5 summarizes the number of A. marina and B. gymnorrhiza species recorded in the three regeneration classes.
The Figure 4.7. below show the relationships of the three regeneration classes of Avicennia marina and Bruguiera gymnorrhiza mangrove species for Dry season. It shows a fair regeneration capacity of Avicennia marina and Bruguiera gymnorrhiza mangrove species as the number of seedlings are greater than the number of saplings and the number of saplings is less than the number of mature trees (RCI>RCII<RCII).
3.8.2. Regeneration capacity of Avicennia marina and Bruguiera gymnorrhiza species during rainy season of November 2019
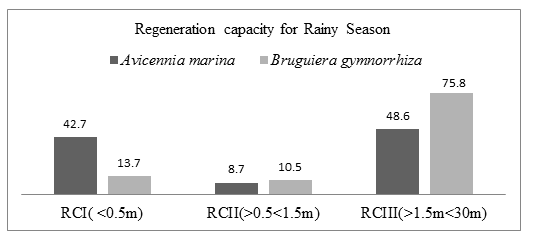
During rainy period in Mida creek, A. marina and B. gymnorrhiza species had a fair Regeneration capacity. The number of seedlings (RCI) was higher than number of saplings (RCII) while the number of saplings was fewer than the number of mature trees (RCIII). Avicennia marina species RCI had 108 individuals (42.7%), RCII had 22 individuals (8.7%) and RCIII had 123 individuals (48.6%). Bruguiera gymnorrhiza species RCI had 13 individuals (13.7%), RCII had 10 individuals (10.5%) and RCIII had 72 individuals (75.8%). figure 4.8 summarizes the number of individuals species recorded in the three regeneration classes.
3.9. Biotic agents and their impacts on Avicennia marina species
3.9.1. Biotic agents on Avicennia marina mangrove
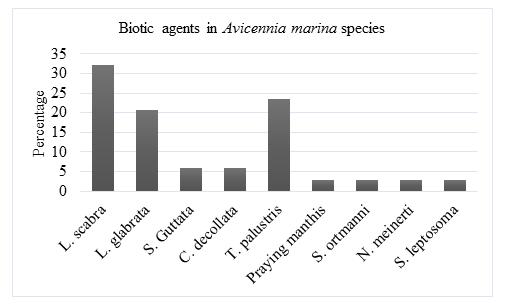
The biotic agents that were observed in Avicennia marina mangrove species includes: Littoraria scabra, Littoraria glabrata, Sesarma guttata Cerithidia decollata, Terebralia palustris, Praying manthis, Sesarma ortmanni, Neosarmatium meinerti and Sesarma leptosoma. 32.1% of the biotic agents were Littoraria scabra, 20.6% Littoraria glabrata, 5.9% Sesarma guttata, 5.9% Cerithidia decollata, 23.5% Terebralia palustris, 2.9% Praying manthis, 2.9% Sesarma ortmanni, 2.9% Neosarmatium meinerti and 2.9% Sesarma leptosome.
3.9.2. Effects of biotic agents on growth of Avicennia marina mangrove species
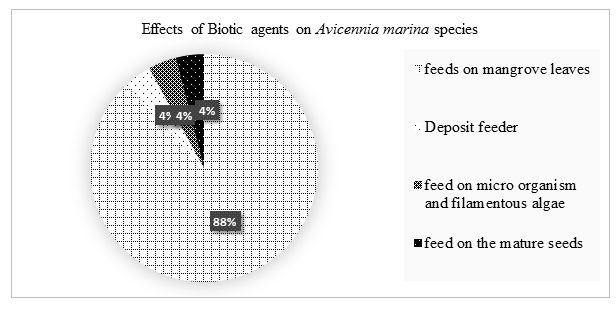
Biotic agents had moderate (reasonable limit) and negligible (small, unimportant, or so little consequence as to warrant attention) effects on the growth of Avicennia marina species. 88% of the biotic agents fed on mangroves leave reducing the surface area for transpiration hence accumulation of salts, 4% were deposit feeder, 4% feed on microorganism and filamentous algae and 4% feed on mature seeds.
3.10. Biotic agents and their impacts on Bruguiera gymnorrhiza mangrove species
3.10.1. Biotic agents on Bruguiera gymnorrhiza mangrove species
The biotic agents that were observed in Bruguiera gymnorrhiza mangrove species includes: Littoraria scabra, Oyster, barnacle (Cirripedia), Mangrove ants, Lichens, Littoraria glabrata, Selatium elongatum, Sesarma Guttata, Moth Caterpillar, Loranthus (parasitic) plant and Metopograpsus oceanicus.
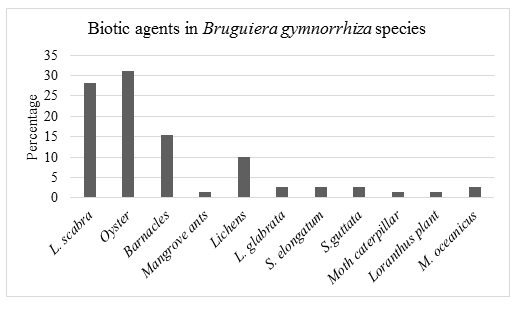
28.2% of the biotic agents recorded on Bruguiera gymnorrhiza mangrove species were Littoraria scabra, 31.0% Oyster, 15.5% barnacle (Cirripedia), 1.4% Mangrove ants, 10.0% Lichens, 2.8% Littoraria glabrata, 2.8% Selatium elongatum, 2.8% Sesarma guttata, 1.4% Moth Caterpillar, 1.4% Loranthus (parasitic) plant and 2.8% Metopograpsus oceanicus.
3.10.2. Effects of biotic agents on growth of Bruguiera gymnorrhiza mangrove species
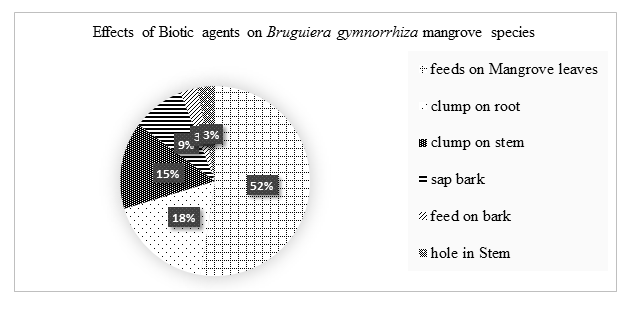
Biotic agents had insignificant effects on the growth of Bruguiera gymnorrhiza species. 52% feds on mangrove leaves reducing the surface area or transpiration hence high accumulation of salts, 18% clump on the roots, 15% clump on the stem, 9% sap nutrients and water, 3% fed on microorganisms and algae and 3% make holes on the tree stem.
Table 4.1: Structural composition of Avicennia marina and Bruguiera gymnorrhiza species during Dry season between February and March of 2019
Table 4.2: Structural composition of Avicennia marina and Bruguiera gymnorrhiza species during Rainy Season of November 2019
Key: Seedlings measured less than 0.5 m in height, Saplings measured between 0.5 m and 1.5 m height and diameter below 2.5 cm and Mature trees measured height greater than 1.5m and 2.5cm butt diameter
Table 4.3: Structural characteristics of Avicennia marina and Bruguiera gymnorrhiza species
|
Zone |
Avicennia marina zone |
Bruguiera gymnorrhiza zone |
||||||
|
Diameter Class |
<5 |
5-10 |
10-15 |
>15 |
<5 |
5-10 |
10-15 |
>15 |
|
No of species (a) |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Stem density (b) |
28 |
45 |
13 |
18 |
74 |
32 |
8 |
8 |
|
Mean height(m) (c) |
3.0 |
4.0 |
5.3 |
25.7 |
5.5 |
5.7 |
7.6 |
10.4 |
|
Average Basal area(cm2) (d) |
11.2 |
38.5 |
115.5 |
587.9 |
6.8 |
43.3 |
122.6 |
483.8 |
|
Complexity index |
9.4×103 |
6.9×102 |
7.9×102 |
2.7 |
2.7×102 |
7.8×102 |
7.4×102 |
0.4 |
Complexity index equals the product of (a), (b), (c) and (d) divided by 10^5
Table 4.4: The dispersion pattern of Avicennia marina and Bruguiera gymnorrhiza mangrove species in study area of Mida creek.
|
Single stand forest |
mixed forest |
||||
|
Species |
Dispersion Index |
Dispersion pattern |
Species |
Dispersion Index |
Dispersion pattern |
|
A. marina |
1.3 |
aggregate |
B. gymnorrhiza |
1.6 |
Aggregate |
Table 4.5 summarizes the biotic agents, their effects and impacts on Avicennia marina and Bruguiera gymnorrhiza species and level of significance in affecting the growth of mangrove tree.
Table 4.5: Effects and impact of Biotic agents on Avicennia marina and Bruguiera gymnorrhiza species
Key: Moderate - observing reasonable limit
Negligible - so small or unimportant or of so little consequence as to warrant attention.
4. Discussion
4.1 Structure and composition of Avicennia marina and Bruguiera gymnorrhiza mangrove species
Structural composition of Avicennia marina and Bruguiera gymnorrhiza mangrove species in Mida creek forest were determined based on the important value (300.0±55, 88.8±33), Avicennia marina was more dominant than Bruguiera gymnorrhiza species in Mida creek. A similar finding was recorded by (Kairo et al, 2002) where Avicennia marina species was recorded to be more dominant than Bruguiera gymnorrhiza species. Complexity index (A. marina 2.7, B. gymnorrhiza0.4) gives the quantifiable picture of the structural complexity of vegetation (Pool et al. 2007) Avicennia marina was more dominant than Bruguiera gymnorrhiza species.
In Mida creek, there was a variation in structural composition between dry season and rainy season. During rainy season, densities of seedlings, saplings, and mature trees of Avicennia marina species increased while the density of saplings and mature tree of Bruguiera gymnorrhiza species remained constant with increase in density of seedlings. This indicates the period between dry, onset of rainfall towards the end of the rainfall season there was a fair recruit from seedling, sapling and mature. A similar finding was recorded by Delgado Sanchez, (2001) the frequency and period of flooding within upper intertidal zone are vital factors influencing propagation of mangrove.
4.2. Occurrence density distribution
Avicennia marina trees were more dominant than Bruguiera gymnorrhiza species in 0.3 hectares of land assessed in Mida creek. Avicennia marina species dominated the highest land ward zone and grow as a single stand forest. Bilquees, (2019) recorded Avicennia marina association was present on the land ward edge border followed by ephemeral halophytic community in northwestern Qatar. Another study by (Pereira, 2016) record Avicennia marina species were in abundance along the 3.5km length of Kala Oya estuary that is characterized by high salinity areas ranging from 13.25 to 23mg/l near the estuary mouth which decreases along the salinity gradient.
At the edges of Mida creek where sea water meets with fresh water from mainland Avicennia marina grows to a big tree of high DBH, whereas in areas with higher salinity on seaward side the species grows as thicket of low productivity.
Bruguiera gymnorrhiza mangrove species grows mixed with other species in supra littoral zone. According to (UNEP, 1998) Bruguiera gymnorrhiza is normally found scattered within stands of R. mucronata species.
The distribution of occurrence class of diameter indicate Avicennia marina grow wider in diameter at breast height compared to Bruguiera gymnorrhiza species, these is due to growth factors. Avicennia marina grows in land ward side that is less affected by climatic and competition factors while Bruguiera gymnorrhiza grows in the sea ward side that is affected by climatic, non-climatic, genetic and competition factors.
4.3. Height diameter distribution of Avicennia marina and Bruguiera gymnorrhiza species
There were trivial differences in stem diameter and height correlation of the two species studied. A. marina had a correlation of 0.56 and B. gymnorrhiza species 0.78 in dry season while during rainy season they both had a correlation of 0.67. The growth of a tree is influenced by the structural characters rather than the age. The factors that influence growth rate includes climatic, non-climatic, genetic and competition (Alongi, 2015). Few studies have researched on formation of growth layers of Avicennia marina and Bruguiera gymnorrhiza species. Schmitz et al, (2008) presented the no-annual nature of growth layers in Avicennia marina at Gazi Bay.
4.4. Structural characteristics of Avicennia marina and Bruguiera gymnorrhiza Mangrove species of Mida Creek
The high complexity index recorded in Avicennia marina tree species of 2.7 indicated that the Avicennia marina species had greater basal area and mean height compared with Bruguiera gymnorrhiza species with complexity index of 0.4.
The structural complexity difference between the two species was attributed by the fact that Avicennia marina species grows on the land ward side without competition from other species, they grow in high saline environment because of their adaptation mechanism of controlling salt accumulation through secretion gland mechanism while Bruguiera gymnorrhiza grows along the seashores with other species hence competition of nutrients and direct impacts from waves and tides from the sea.
4.5. Spatial distribution of Avicennia marina and Bruguiera gymnorrhiza mangrove species
Avicennia marina and Bruguiera gymnorrhiza species distribution in Mida creek highly depended on the soil salinity and H+ ion concentration gradients. Avicennia marina was found growing in saline areas but not totally exempted in areas with low salinity showing their wide ecological suitability. Avicennia marina has an ecological optimum salinity of between 18.4 and 20.9 ppt (GHOSE, 2003). Bruguiera gymnorrhiza species ecological optimum salinity of between 10.0 and 15.0 ppt (GHOSE, 2003).
4.6. Quality of poles of Avicennia marina and Bruguiera gymnorrhiza mangrove species
Bruguiera gymnorrhiza species had straight poles compared to Avicennia marina species. A total of 75.3 % of Bruguiera gymnorrhiza trees assessed had straight pole while Avicennia marina species, only 1.7% trees had straight pole. A total of 21.9% of Bruguiera gymnorrhiza tree species had intermediate pole that need slight modification to be used for building while 54.7% of Avicennia marina trees had intermediate pole. A total of 43.6% of Avicennia marina trees were unsuitable for building while 2.7% of Bruguiera gymnorrhiza tree poles were unsuitable for pole use.
Kokwaro ( 1985) recorded Bruguiera gymnorrhiza trees to be straight and used as a building poles, construction poles and for telephone poles. Mainoya, (1986) found B. gymnorrhiza species growing to a height of 20 m and the wood is used as poles for buildings and Avicennia marina because of its shape was used for building canoe fittings, masts, carts, and furniture and for fittings such as handles.
4.7. Regeneration capacity of Avicennia marina and Bruguiera gymnorrhiza species
Avicennia marina and Bruguiera gymnorrhiza species had a fair regeneration capacity. The study found the number of seedlings was greater than saplings and number of saplings less than number of mature trees (RCI>RCII<RCII). The mature tree had a high density followed by seedlings and then saplings. A total of 3 % of Avicennia marina species seedlings successively recruited to sapling stage and 89% of Bruguiera gymnorrhiza seedlings successive recruited to sapling stage.
The recruitment variation was attributed to changes in physiological condition due to climatic, biotic, and anthropogenic factors. A study by Clarke and Allaway (1993) recorded that the reason why propagules establishes was because of precociously developed embryos, but the recruitment of seedlings to higher class i.e., saplings and mature tree depends on seasonal availability of regeneration niche. According to (Clarke and Myerscough, 1993) when propagules establish, seedlings survival is independent of light, salinity, and nutrients conditions.
Clarke and Kerrigan, (2002) presented that seedlings growth and survival in mangrove forest are affected under closed canopies due to reduced light. Clarke and Allaway, (1993) recorded 25% of the established seedlings recruited to sapling stage if regeneration area is wide i.e., after gross canopy and sediment disturbance, whereas after small gap disturbances average 10% recruit to next stage.
Avicennia marina species occupied the supra littoral zone, the outermost zone that boarder the mainland and the Ocean hence highly affected by edge effects. During low tide season, the water level decreases leaving a high saline environment. Seedlings of mangrove require low saline concentration (Hwang and Chen, 2001) but as they grow, they become more tolerant to increase in salinity (Kathiresan and Bingham, 2001). In Mida creek human interference was evident by the presence of animal and human along the Avicennia marina mangrove species zone.
Anthropogenic and physiological factors hinder successive recruitment of seedlings to sapling, From the observation the land area covered by Avicennia marina forest had decreased due to poor regeneration capacity leaving behind a bare land that was initially covered by the mangrove forest.
Fair Regeneration capacity of Bruguiera gymnorrhiza and Avicennia marina species threatens future sustainability of the two species in Mida Creek as they are not attaining the sustainable regeneration equilibrium of 100%.
4.8. Biotic agents of Avicennia marina and Bruguiera gymnorrhiza mangrove species
The study found the Biotic agents to be insignificant in affecting Regeneration capacity of Avicennia marina and Bruguiera gymnorrhiza species however combination with climatic and anthropogenic factors affected regeneration capacity.
Littoraria scabra belonging to snail family was observed in Avicennia marina trees. During high tides Littoraria scabra was observed on top of the trees and during outgoing tide the snails move downward slowly as they feed on the newly replenished algae, micro-organisms, and other organic tissues on the mangrove plants surface. This goes with the finding of Gallagher and Reid (1979) who found Littoraria scabra feds on microorganisms and organic tissues.
Littoraria Glabrata of genus Cerithidea feed on mud surface with slight reliance on mangrove plants, they only climb mangrove trees at various heights depending on the tide condition. Sesarma gutatta of crab’s family fed on mangrove leaves for nutrition, feeding of leaves by Sesarma gutatta reduces the surface area for excreting salts resulting to accumulation of salts which was insignificant in affecting regeneration capacity of the mangrove tree species. Sesarma leptosoma species was observed in the mangrove canopy feeding on fresh leaves reducing the salt excretion surface.
Cerithidea decollate of snail family was observed hanging on Avicennia marina mangrove trees at a very high density during low tide and feds on the deposits and organic matters. (Machiwa and Hallberg, 1995) found Cerithidea decollata common in the A. marina species on landward zone and they fed on organic matter.
Terebralia palustrisa gastropod inhabited the muddy surface of mangrove forests where they destabilize the sediments and the juvenile fed on microalgae with minor effect on mangrove growth. Neosarmatium meinerti of crab family was observed on the landward side of Avicennia marina zone and feds on the leaves and freshly gathered propagules facilitating degradation of leaves.
Steinke et al, (1993) recorded Neosarmatium meinerti to be herbivorous that plays a vital role in the process of leaf degradation in biogeochemical cycles. Dahdouh-Guebas et al, (1998) recorded Neosarmatium meinerti has unselective food preference of propagules and juveniles thus hinder efficient (re)establishment of mangrove tree species.
Barnacles (Cirripedia) bunch on the roots and stems of Bruguiera gymnorrhiza species. The poor seedling performance of the Bruguiera gymnorrhiza mangrove species in Mida creek has to some extent, been contributed by the barnacles attached to roots and stems. The barnacles clustering on the pneumatophore roots results to its smothering and bending hence reduce the capacity of gaseous exchange of mangrove plants.
The presence of Cirripedia on seedlings of Avicennia marina and Bruguiera gymnorrhiza had negligible effect on their growth. Satumanatpan and Keough, (1999) found that Elminlus covertus did had high negative impact on survival and growth of seedlings of A. marina species at Rhyll inlet. They found barnacles have effects on seedling growth for first year, but subsequent year barnacle had very little effects. Mangrove Oyster (Crassostrea gasar) attached itself on Bruguiera gymnorrhiza roots to gain a sturdy spot with little harmful effects on the growth of mangroves.
Sesasrmi ortmanni of sesarmid crab’s family fed on mangrove propagules and leaf litters of Avicennia marina, Cannicci et al, (2008) recorded Avicennia marina leaves to have a high nitrogen and low tannin content, factors considered to favors herbivory. Paulay, (2007) Metopograpsus oceanicusfed on fresh leaves off the tree. Selatium elongatium was observed on trunk feeding on algae and leaves. Morth caterpillar feed the bottom layers of mangrove leaves, leaving brown spots resulting to transparent looking leaves.
Mangrove ants depends indirectly on mangrove trees, they build a nest on the mangrove branches and feed on the honey dew on the surface of mangrove leaves and stems. Praying mantis which is carnivorous was observed feeding on insects on mangrove leaves and stems.
Manglicolous lichens were observed on both Avicennia marina and Bruguiera gymnorrhiza mangrove species. Lichens and mangrove have symbiotic relationship where mangrove plants only host lichens without lichens extracting nutrients or water from the tree tissue as they can photosynthesize (Duke and Schmitt, 2015).
Mistletoes plant which belonging to family Loranthaceae was observed in Avicennia marina species. They are parasitic though capable of photosynthesis; they tap on vascular system of Avicennia marina and sap water and nutrients depriving the plants desalinated water and nutrients. Their impact on Avicennia marina growth was insignificant and rarely kills the plant. They only cause growth modification of Avicennia marina and death of highly affected branches, (Hutchings and Saenger, 1987) find Mistletoes parasitic plants sap water and nutrients from plants causing plant modification and falling of affected branches.
Conclusion
The study recorded Avicennia marina species to be more dominant with a greater complexity index (A2.7, B0.4), basal area (A587.9, B483.8) and mean height (A25.7, B10.4) compared to Bruguiera gymnorrhiza species with both having an aggregate dispersion pattern. During dry season, the correlation between Height (m) and DBH (cm) of Avicennia marina was 0.56 while that of Bruguiera gymnorrhiza species was 0.78. During rainy season both species had a correlation of 0.67. Bruguiera gymnorrhiza trees are straighter than Avicennia marina species. A total of 75.3% of B. gymnorrhiza assessed were straight while 1.7% of total trees of Avicennia marina species were straight.
Avicennia marina and Bruguiera gymnorrhiza mangrove species have a fair regeneration capacity under natural condition, the number of seedlings (79,9) was greater than saplings (2,8) and saplings was less than mature trees (108,70) predicting unsecure future sustainability of Avicennia marina and Bruguiera gymnorrhiza (RCI>RCII<RCII). The two species are not regenerating to sustainable equilibrium in Mida creek.
Biotic agents were found to be insignificant in affecting Regeneration capacity of Avicennia marina and Bruguiera gymnorrhiza mangrove species however combinations with anthropogenic and climatic factors affected the regeneration capacity of the mangroves in Mida creek. Littoraria scabra fed mostly on micro-organisms and algae, Littoraria Glabrata fed mostly on mud surface.
The Sesarma guttata and Sesarma leptosoma fed on mangrove leaves, Cerithidea decollata fed mainly on deposits and organic matters, Terebralia palustrisand Selatium elongatium fed more on algae and leaves, Sesasrmi ortmanni, Metopograpsus oceanicus and Neosarmatium meinerti fed a lot on leaves and young propagules, Barnacles and Oyster bunch on the roots and stems of Bruguiera gymnorrhiza species.
Acknowledgement
I would like to express my deep and sincere gratitude to my research supervisors; Dr. Benard Okeyo and Dr. Najma Dharani for providing invaluable guidance throughout my research. Their dynamism, vision and motivation have deeply inspired me. They taught me the methodology to carry out the research and to present the research works as clearly as possible. It was a great privilege and honor to study under their guidance. I am grateful for what they offered me.
References
- Ajonina, G, Diamé, A, & Kairo, J. (2008). status and conservation of mangroves in Africa: An overview. World Rainforest Movement Bulletin, 133(2008), 1–6.
- Alemayehu, F, & Chemuku, W. (2017). Structure and Natural Regeneration of Mangroves in Areas Exposed to Different Agents of Degradation in Kenya. Journal of Global Biosciences, 6(1), 4695–4707.
- Alemayehu, F, Onwonga, R, Kinyanjui, M, & Wasonga, O. (2014). Assessment of Mangrove Cover Change and Biomass in Mida Creek, Kenya. Open Journal of Forestry, 4, 398–413.
- Allen, P. S, Benech, R. L, Batlla, D, & Bradford, K. J. (2008). Modeling of Seed Dormancy. In K. Bradford & H. Nonogaki (Eds.), Seed Development, Dormancy and Germination. John Wiley & Sons.
- Almahasheer, H, Duarte, C. M, & Irigoien, X. (2016). Nutrient Limitation in Central Red Sea Mangroves. Frontiers in Marine Science, 3. https://doi.org/10.3389/fmars.2016.00271
- Alongi, D. (2009). The Energetics of Mangrove Forests. Springer Science + Business Media B.V.
- Alongi, D. M. (2002). Present state and future of the world’s mangrove forests. Environmental Conservation, 29(3), 331–349.
- Amarasinghe, M. D, & Vidanage, S. (2007). Best Practice Guidelines for Restoration of Mangroves. World Conservation Union (IUCN).
- Baba, S. (2011). Close-group planting of mangroves on atolls and coral islands of the Pacific. ISME/GLOMIS Electronic Journal, 9(4), 11–12.
- Benfield, S. L, Guzman, H. M, & Mair, J. M. (2005). Temporal mangrove dynamics in relation to coastal development in Pacific Panama. Journal of Environmental Management, 76(3), 263–276.
- Blanchard, J, & Prado, G. (1995). Natural regeneration of Rhizophora mangle in strip clearcuts in northwest Ecuador. Biotropica, 160–167.
- Bosire, J. O, Kaino, J. J, Olagoke, A. O, Mwihaki, L. M, Ogendi, G. M, Kairo, J. G, Berger, U, & Macharia, D. (2014). Mangroves in peril: Unprecedented degradation rates of peri-urban mangroves in Kenya. Biogeosciences, 11(10), 2623–2634.
- Bowman, (1917). Mangrove regeneration and management. In A. K. F. Hoque 1995 (Ed.), Mimeograph.
- Brook, B. M. (2001). The effect of Coccotrypes fallax (Coleoptera; Scolytidae) on the recruitment of Rhizophora stylosa (Family Rhizophoraceae) in North Queensland mangroves. [Ph.D Thesis]. James Cook University.
- Cannicci, S, Burrows, D, Fratini, S, Smith, T. J, Offenberg, J, & Dahdouh-Guebas, F. (2008). Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquatic Botany, 89(2), 186–200.
- Cavalcanti, V. F, Soares, M. L. G, Estrada, G. C. D, & Chaves, F. O. (2009). Evaluating Mangrove Conservation through the Analysis of Forest Structure Data. Journal of Coastal Research, 390–394. JSTOR.
- Chapman, V. J. (1977). Africa B. The remainder of Africa. Ecosystems of the World.
- Clarke, P. J, & Allaway, W. G. (1993). The regeneration niche of the grey mangrove (Avicennia marina): Effects of salinity, light and sediment factors on establishment, growth, and survival in the field. Oecologia, 93(4), 548–556.
- Clarke, P. J, & Kerrigan, R. A. (2000). Do Forest Gaps Influence the Population Structure and Species Composition of Mangrove Stands in Northern Australia?1. Biotropica, 32(4a), 642–652.
- Clarke, P. J, & Kerrigan, R. A. (2002). The effects of seed predators on the recruitment of mangroves. Journal of Ecology, 90(4), 728–736. https://doi.org/10.1046/j.1365-2745.2002.00705.x
- Clarke, P. J, & Myerscough, P. J. (1993). The intertidal distribution of the grey mangrove (Avicennia marina) in southeastern Australia: The effects of physical conditions, interspecific competition, and predation on propagule establishment and survival. Australian Journal of Ecology, 18(3), 307–315.
- Cohen, R, Kaino, J, Okello, J. A, Bosire, J. O, Kairo, J. G, Huxham, M, & Mencuccini, M. (2013). Propagating uncertainty to estimates of above-ground biomass for Kenyan mangroves: A scaling procedure from tree to landscape level. Forest Ecology and Management, 310, 968–982.
- Dahdouh-Guebas, F, Koedam, N, Satyanarayana, B, & Cannicci, S. (2011). Human hydrographical changes interact with propagule predation behaviour in Sri Lankan mangrove forests. Journal of Experimental Marine Biology and Ecology, 399(2), 188–200.
- Dahdouh-Guebas, F, Verneirt, M, Cannicci, S, Kairo, J. G, Tack, J. F, & Koedam, N. (2002). An exploratory study on grapsid crab zonation in Kenyan mangroves. Wetlands Ecology and Management, 10, 179–187.
- Dahdouh-Guebas, Farid, Verneirt, M, Tack, J. F, Van Speybroeck, D, & Koedam, N. (1998). Propagule predators in Kenyan mangroves and their possible effect on regeneration. Marine and Freshwater Research, 49(4), 345–350.
- Davis, J. H. (1940). The ecology and geologic role of mangroves in Florida. Publications of the Carnegie Institution of Washington, 517, 303–412.
- Delgado-Sanchez, P. (2001). Factors Affecting Community Structure of Mangroves Associated with Point Bars and Islands in a Costa Rican Estuary. [PhD Thesis]. Louisiana State University.
- Dharani, N. (2019). Field Guide to common Trees and Shrubs of East Africa (3rd ed.). Struik Random House Publishers.
- Duarte, C. M, Geertz-Hansen, O, Thampanya, U, Terrados, J, Fortes, M. D, Kamp-Nielsen, L, Borum, J, & Boromthanarath, S. (1998). Relationship between sediment conditions and mangrove Rhizophora apiculata seedling growth and nutrient status. Marine Ecology Progress Series, 175, 277–283.
- Duke, N, & Schmitt, K. (2015). Mangroves: Unusual Forests at the Seas Edge. Tropical Forestry Handbook, 1–24.
- Elmqvist, T, & Cox, P. A. (1996). The evolution of vivipary in flowering plants. Oikos, 3–9.
- (1994). Mangrove forest management guidelines. Food and Agriculture Organization of the United Nations.
- (2005). Global Forest Resources Assessment 2005: Progress towards sustainable forest management (No. 147; FAO Forestry Paper). Food and Agriculture Organization of the United Nations.
- (2008). Loss of mangroves alarming: 20 percent of mangrove area destroyed since 1980 – rate of loss slowing. FAO Newsroom.
- (2016). Valuing Coastal Ecosystems as Economic Assets: The Importance of Mangroves for Food Security and Livelihoods Among Communities in Kilifi Country and the Tana Delta, Kenya. FAO and UNEP.
- Farnsworth, E. J, & Ellison, A. M. (1997). The global conservation status of mangroves. Ambio (Sweden).
- Feller, I. C, & Sitnik, M. (Eds.). (1996). Mangrove Ecology: A Manual for a Field Course. Smithsonian Environmental Research Center.
- Fourqurean, J. W, & Robblee, M. B. (1999). Florida Bay: A history of recent ecological changes. Estuaries, 22(2), 345–357.
- Gallagher, S. B, & Reid, G. K. (1979). Population Dynamics and Zonation in the Periwinkle Snail, Littorina Angulifera, of the Tampa Bay, Florida Region. Population Dynamics and Zonation in the Periwinkle Snail, Littorina Angulifera, of the Tampa Bay, Florida Region, 93(4), 162–178.
- Gang, P. O, & Agatsiva, J. L. (1992). The current status of mangroves along the Kenyan coast: A case study of Mida Creek mangroves based on remote sensing. Hydrobiologia, 247(1), 29–36.
- Giri, C, Ochieng, E, Tieszen, L. L, Zhu, Z, Singh, A, Loveland, T, Masek, J, & Duke, N. (2011). Status and distribution of mangrove forests of the world using earth observation satellite data. Global Ecology and Biogeography, 20(1), 154–159.
- GHOSE, H. J. (2003). Forest structure and species distribution along soil salinity and pH. India: International Society for Tropical Ecology.
- Gul, B, Böer, B, Khan, M. A, Clüsener-Godt, M, & Hameed, A. (Eds.). (2019). Sabkha Ecosystems: Volume VI: Asia/Pacific. Springer.
- Gwada, P, & Kairo, J. G. (2001). Litter production in three mangrove stands of Mida Creek, Kenya. South African Journal of Botany, 67(3), 443–449.
- Hamilton, S. E, & Casey, D. (2016). Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Global Ecology and Biogeography, 25(6), 729–738.
- Havanon, S, Chukwamdee, J, Anunsiriwat, A, & Meepol, W. (1995). Study on mangrove forest structure at Samut Songkram Province. In The ninth national seminar on mangrove ecology, mangrove conservation for Thai society in the next decade (p. ap No. 111-02). National Research Council of Thailand.
- Hoorweg, J, & Muthiga, N. (2009). Advances in coastal ecology: People, processes and ecosystems in Kenya.
- Hutchings, P, & Saenger, P. (1987). Ecology of mangroves. University of Queensland Press.
- Hwang, Y.-H, & Chen, S.-C. (2001). Effects of Ammonium, Phosphate,and Salinity on Growth, Gas Exchange Characteristics, and Ionic Contents of Seedlings of Mangrove Kandelia Candel (L.) Druce. Botanical Bulletin of Academia, 42(2), 131–139.
- (2020). Mangrove restoration. Forest Landscape Restoration.
- Joshi, H. H, & Ghose, M. (2003). Forest structure and species distribution along soil salinity and pH gradient in mangrove swamps of the Sundarbans. Tropical Ecology, 44(2), 197–206.
- Kairo, J. G, Dahdouh-Guebas, F, Gwada, P. O, Ochieng, C, & Koedam, N. (2002). Regeneration status of mangrove forests in Mida Creek, Kenya: A compromised or secured future? AMBIO: A Journal of the Human Environment, 31(7), 562–568.
- Kathiresan, K, & Bingham, B. L. (2001). Biology of Mangroves and Mangrove Ecosystems. Advances in Marine Biology, 40(2001), 81–251.
- (2017). National Mangrove Ecosystem Management Plan 2017—2027 (p. 115). Kenya Forest Service (KFS).
- Kokwaro, J. O. (1985). The Distribution and Economic Importance of the Mangrove Forests of Kenya. Journal of the East Africa Natural History Society and National Museum, 75(188), 1–10.
- Kristensen, E, Bouillon, S, Dittmar, T, & Marchand, C. (2008). Organic carbon dynamics in mangrove ecosystems: A review. Aquatic Botany, 89(2), 201–219.
- Lee, S. Y. (1998). Ecological role of grapsid crabs in mangrove ecosystems: A review. Marine and Freshwater Research, 49(4), 335–343.
- Lugo, A. E, & Snedaker, S. C. (1974). The Ecology of Mangroves. Annual Review of Ecology and Systematics, 5(1), 39–64.
- Machiwa, J. F, & Hallberg, R. O. (1995). Flora and Crabs in a Mangrove Forest Partly Distorted by Human Activities, Zanzibar. Ambio, 24(7/8), 492–496. JSTOR.
- Macintosh, D. J, & Ashton, E. C. (2003). Draft code of conduct for the sustainable management of mangrove ecosystems. World Bank, ISME, cenTER Aarhus.
- Mainoya, J. R, Mesaki, S, & Banyikwa, F. F. (1986). The distribution and socio-economic aspects of mangrove forests in Tanzania. United Nations University website.
- McGuinness, K. A. (1996). Dispersal, establishment and survival of Ceriops tagal propagules in a north Australian mangrove forest. Oecologia, 109(1), 80–87.
- Mchenga, I. S, & Ali, A. I. (2014). Natural regeneration of mangroves in a degraded and non-degraded tropical forest of Zanzibar Island. Journal of Global Biosciences, 3(1), 334–344.
- McKee, K. L. (1995). Mangrove Species Distribution and Propagule Predation in Belize: An Exception to the Dominance-Predation Hypothesis. Biotropica, 27(3), 334–345. JSTOR.
- Mendelssohn, I. A, & McKee, K. L. (2000). Saltmarshes and mangroves. In M. G. Barbour & W. D. Billings (Eds.), North American Terrestrial Vegetation (2nd ed, pp. 501–536). Cambridge University Press.
- Middleton, B. (2005). Propagule deposition and landscape characteristics of source forests of Rhizophora mangle in coastal landscapes in Florida. Landscape Ecology, 20, 63–72.
- Miyagi, T. (2013). Environmental Characteristics of Mangroves for Restoration in the Yucatan Peninsula, Mexico. International Society for Mangrove Ecosystems (ISME), 4, 1–21.
- Nagelkerken, I, Blaber, S. J. M, Bouillon, S, Green, P, Haywood, M, Kirton, L. G, Meynecke, J.-O, Pawlik, J, Penrose, H. M, Sasekumar, A, & Somerfield, P. J. (2008). The habitat function of mangroves for terrestrial and marine fauna: A review. Aquatic Botany, 89(2), 155–185.
- Natividad, R. A, & Jimenez Jr, J. P. (2015). Development of laminated buho (Schizostachyum lumampao (Blanco) Merr.) lumber. Philippine Forest Products Journal (Philippines).
- (2009). State of the Coast Report: Towards Integrated Management of Coastal and Marine Resources in Kenya. (p. 104) [Report]. National Environment Management Authority.
- Onrizal, O, Amelia, R, Amri, K, Sulistiyono, N, & Mansor, M. (2019). Stand structure and diversity of restored mangroves at abandoned pond in eastern coast of North Sumatra. IOP Conference Series: Earth and Environmental Science, 305(2019), 012050.
- Paulay, G. (2007). Metopograpsus oceanicus (Crustacea: Brachyura) in Hawai‘i and Guam: Another Recent Invasive?1. Pacific Science, 61(2), 295–300.
- Pereira, F. R. de S, Kampel, M, & Cunha-Lignon, M. (2016). Mangrove vegetation structure in Southeast Brazil from phased array L-band synthetic aperture radar data. Journal of Applied Remote Sensing, 10(3), 1–16.
- Peria, L. C. S, Fernandes, P. P. C. P, Menezes, G. V, Grasso, M, & Tognella, M. M. P. (1990). Estudos estruturais comparativos entre bosques de manguezais impactados (Canal da Bertioga) e não impactados (Ilha do Cardoso), estado de São Paulo. II Simpósio de Ecossistemas da costa brasileira: Estrutura, função e manejo. ACIESP, 2.
- Rawangkul, S, Angsupanich, S, & Panitchart, S. (1995). Prelirninary study of barnacles damaging the mangrove plantation Rhizophora mucronata at Tha Phae canal, Nakorn Si Thammarat. In The ninth national seminar on mangrove ecology, mangrove conservation for Thai society in the next decade. National Research Council of Thailand Bangkok.
- Robertson, A. I. (1991). Plant-animal interactions and the structure and function of mangrove forest ecosystems*. Australian Journal of Ecology, 16(4), 433–443.
- Robertson, A. I, Daniel, P. A, & Dixon, P. (1991). Mangrove forest structure and productivity in the Fly River estuary, Papua New Guinea. Marine Biology, 111(1), 147–155.
- Sanderman, J, Hengl, T, Fiske, G, Solvik, K, Adame, M. F, Benson, L, Bukoski, J. J, Carnell, P, Cifuentes-Jara, M, Donato, D, Duncan, C, Eid, E. M, Ermgassen, P. zu, Lewis, C. J. E, Macreadie, P. I, Glass, L, Gress, S, Jardine, S. L, Jones, T. G, … Landis, E. (2018). A global map of mangrove forest soil carbon at 30\hspace0.167emm spatial resolution. Environmental Research Letters, 13(5), 055002.
- Sanjay Deshmukh and V. Balaji (Ed.s). Conservation of Mangrove Forest Genetic Resources: A Training Manual. JTTO-CRSARD Project, M.S. Swaminathan Research Foundation, Madras, India, 1994.
- Satumanatpan, S, & Keough, M. J. (1999). Effect of barnacles on the survival and growth of temperate mangrove seedlings. Marine Ecology Progress Series, 181, 189–199.
- Schmitz, N. (2009). Growing on the Edge: Hydraulic Architecture of Mangroves: Ecological Plasticity and Functional Significance of Water Conducting Tissue in Rhizophora mucronata and Avicennia marina. ASP Editions.
- Schmitz, N, Robert, E. M. R, Verheyden, A, Kairo, J. G, Beeckman, H, & Koedam, N. (2008). A Patchy Growth via Successive and Simultaneous Cambia: Key to Success of the Most Widespread Mangrove Species Avicennia marina? Annals of Botany, 101(1), 49–58.
- Siddiqi, N. A. (1995). Role of crabs in the natural regeneration of mangroves in the Sundarbans forest of Bangladesh. Australian Journal of Ecology, 20(2), 340–343.
- Silveira Neto, S, Nakano, O, Barbin, D, & Villa Nova, N. (1976). Manual de ecologia dos insetos.
- Soares, M. L. G. (2009). A Conceptual Model for the Responses of Mangrove Forests to Sea Level Rise. Journal of Coastal Research, 56, 267–271. JSTOR.
- Sousa, W. P, & Mitchell, B. J. (1999). The Effect of Seed Predators on Plant Distributions: Is There a General Pattern in Mangroves? Oikos, 86(1), 55–66. JSTOR.
- Sousa, W. P, Quek, S. P, & Mitchell, B. J. (2003). Regeneration of Rhizophora mangle in a Caribbean mangrove forest: Interacting effects of canopy disturbance and a stem-boring beetle. Oecologia, 137(3), 436–445.
- Spalding, Marc, Blasco, F, & Field, C. (1997). World mangrove atlas. International Society for Mangrove Ecosystems.
- Spalding, Mark, Kainuma, M, & Collins, L. (2010). World atlas of mangroves. Washington, DC?: Earthscan.
- Steinke, T. D, Rajh, A, & Holland, A. J. (1993). The feeding behaviour of the red mangrove crab Sesarma meinerti De Man, 1887 (Crustacea: Decapoda: Grapsidae) and its effect on the degradation of mangrove leaf litter. South African Journal of Marine Science, 13(1), 151–160.
- Thampaya, U. (2006). Mangroves and Sediment Dynamics Along the Coasts of Southern Thailand [Ph.D Thesis]. Wageningen University and the Academic Board of the UNESCO-IHE Institute for Water Education.
- Thomas, N, Lucas, R, Bunting, P, Hardy, A, Rosenqvist, A, & Simard, M. (2017). Distribution and drivers of global mangrove forest change, 1996–2010. PLOS ONE, 12(6), e0179302.
- (1998). Eastern Africa Atlas of Coastal Resources: A project of the United Nations Environment Programme with the support of the Government of Belgium. United Nations Environmental Programme, Water Branch.
- (2013). Mangrove forest cover fading fast. UNEP Global Environmental Alert Service (GEAS).
- Van Leeuwen, M, & Nieuwenhuis, M. (2010). Retrieval of forest structural parameters using LiDAR remote sensing. European Journal of Forest Research, 129(4), 749–770.
- Van Speybroeck, D. (1992). Regeneration strategy of mangroves along the Kenya coast: A first approach. In V. Jaccarini & E. Martens (Eds.), The Ecology of Mangrove and Related Ecosystems (pp. 243–251). Springer Netherlands.
- Wairungu, S, Mumbu, D, Kimani, G, Welimo, M, Muthini, J, Ndungu, M, & Mukirae, P. (2009). Structure of Mangroves at Mida Creek. Kenya Forestry Research Institute. Retrieved from
- Warui, M. W. (2011). Current status, utilization, succession and zonation of mangrove ecosystem along Mida creek, Coast province, Kenya [Master’s Thesis]. Kenyatta University.
- Weru, S. M, Wakaba, G. M, Macharia, D, Mwakau, B. K, Njue, R. M, Verheij Koyo, A. O, Muthiga, N, Kavu, B. K, Kareko, J. K, & Litoro, M. (2000). Managment Plan: Malindi Watamu Marine Parks and Reserves. Kenya Wildlife Services.

 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 