Association of Cirrhosis and Increased Risk of Cardiovascular Events in a VA Patient Population, A Retrospective Cohort Study
Article Information
Leila Hashemi1*, Joseph Pisegna2, Mathew Budoff3, Ning Li4, Minh Thu Thi Nguyen5, Ramin Ebrahimi3, Emily Chu6, Elani Streja7, David Elashoff8, Tomas Ganz9
1Assistant Professor of Medicine, Department of General Internal Medicine, University of California Los Angeles, VA Greater Los Angeles, USA
2Chief, Division of Gastroenterology, Hepatology and Parenteral Nutrition, Professor of Medicine and Human Genetics, California, USA
3Professor of Medicine, University of California Los Angeles, California, USA
4Associate Professor, Department of Medicine Statistics Core and Computational Medicine, University of California Los Angeles, David Geffen School of Medicine, California, USA
54th year Medical student, David Geffen School of Medicine, University of California Los Angeles, California, USA
63rd year Medical student, David Geffen School of medicine, University of California Los Angeles, California, USA
7Health Science Specialist, Assistant Professor in Residence, University of California Irvine, Long Beach VA Medical Center, Long Beach, California, USA
8Professor of Medicine, Biostatistics and Computational Medicine, Director, Department of Medicine Statistics Core, University of California Los Angeles, California, USA
9Distinguished Professor of Medicine and Pathology, Department of Medicine, David Geffen School of Medicine, University of California Los Angeles, California, USA
*Corresponding author: Leila Hashemi, Assistant Professor of Medicine, David Geffen School of Medicine, University of California Los Angeles, Ambulatory care clerkship Director, West Los Angeles VA hospital, 11301 Wilshire Blvd. Los Angeles, Mail code 111A California, 90073, USA
Received: 01 October 2020; Accepted: 08 October 2020; Published: 21 October 2020
Citation:
Leila Hashemi, Joseph Pisegna, Mathew Budoff, Ning Li, Minh Thu Thi Nguyen, Ramin Ebrahimi, Emily Chu, Elani Streja, David Elashoff, Tomas Ganz. Association of Cirrhosis and Increased Risk of Cardiovascular Events in a VA Patient Population, A Retrospective Cohort Study. Archives of Internal Medicine Research 3 (2020): 210-229.
View / Download Pdf Share at FacebookAbstract
Background: There is conflicting evidence regarding prevalence and incidence of atherosclerotic cardiovascular disease (ASCVD) in patients with liver cirrhosis. The risk factors associated with ASCVD within this group of patients have not been investigated previously.
Methods and Results: This is a retrospective longitudinal study utilizing the Veterans Affairs (VA) Greater Los Angeles Electronic Medical Record of 623 patients with diagnosis of liver disease. We investigated the incidence of ASCVD events and risk factors associated with ASCVD in these patients. We observed an increase in prevalence of ASCVD events in patients with cirrhosis compared to liver disease patients without cirrhosis (19.12% vs 2.46%). Although the cirrhosis group patients were older but, in our Cox-regression model, after adjusting for traditional ASCVD risk factors especially age, cirrhosis remained a major risk factor for ASCVD events with a hazard ratio (HR) of 5.73 (CI: 2.74-12.72). In the subgroup analysis of cirrhosis group, transferrin saturation greater or equal to 40% had 4.27 times higher risk of ASCVD events than lower transferrin saturation.
Conclusion: We propose that the liver damage and subsequent decrease in hepcidin production in patients with cirrhosis would cause a major increase in Non-Transferrin-Bound Iron (NTBI) in circulation. Circula-ting NTBI promotes endothelial dysfunction, produces reactive oxygen species (ROS), and exposes important biomolecules, like low density lipoprotein (LDL), to oxidative stress to facilitate atherosclerosis. Accordingly, these epidemiological results provide evidence for further translational investigations to identify factors to mitigate the development of ASCVD.
Keywords
Cirrhosis, Atherosclerotic Cardiovascular Disease, Inflammation, Transferrin Saturation (T-Sat), Survival Analysis
Article Details
1. Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality globally [1]. According to the American Heart Association, at least 48% of adults in the United States (US) have some degree of cardiovascular disease [1]. Liver cirrhosis (LC) is also a major health problem, affecting about 1.8% of adults in the US and contributing to 12.8% of total US mortalities each year [2]. Although there is mutual interaction between the function of the cardiovascular system and the liver, the association between LC and CVD remains unclear. It had been previously believed that patients with LC have a more favorable cardiovascular risk profile due to depletion of clotting factors, thrombocytopenia, lower cholesterol level, lower blood pressure, and malnutrition [3]. However, patients with LC also tend to have an increased inflammatory state, triggered by cytokines and growth factors that can play a role in atherosclerosis [4].
Recent studies on the association between LC and CVD have incongruous results. Some studies have shown increased prevalence of CVD in patients with LC [5-6] while others have shown decreased rate [7] or no difference [8-9]. A recent study showed that the overall incidence rates of acute coronary syndrome (ACS) and peripheral arterial disease (PAD) in cirrhotic patients were 2.81 and 2.97 per 1000 person-years, respectively. These patients had a significantly higher risk of ACS and PAD compared with those without liver disease [5]. Conversely, a study that assessed the prevalence and severity of atherosclerosis in the carotid and vertebral arteries of cirrhotic patients showed a lower prevalence of cerebrovascular accident (CVA) and ischemic CVD in cirrhosis compared to non-cirrhosis. Interestingly, although both cohorts had the same severity of atherosclerotic plaques, cirrhotic patients had less abnormal flow pattern [7]. A different study examining the prevalence of coronary artery disease (CAD) found no significant difference between cirrhotic patients and controls (77% vs. 65%) [8]. Moreover, another study showed no significant difference in prevalence of obstructive CAD between cirrhotic and non-cirrhotic patients (7.2% vs. 7.9%). However, cirrhotic patients had a higher prevalence of non-obstructive CAD compared to non-cirrhotic patients (30.6% vs. 23%) [9].
Despite discordant results on the association between LC and CVD, it is well established that cardiovascular complications in patients with LC are a major cause of perioperative mortality following major surgeries, especially liver transplant surgery [10]. Therefore, it is important to understand the clinical significance of LC in relation to CVD. This study aims to assess incidence and prevalence of CVD and risk factors in patients with LC compared to those without cirrhosis in Veterans.
2. Methodology
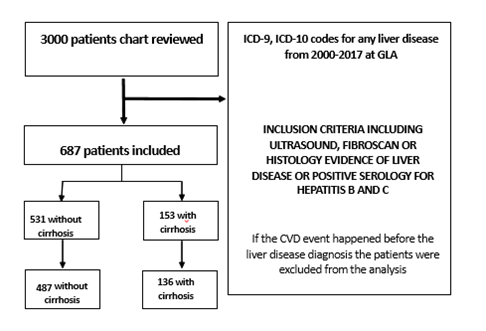
This is a retrospective cohort study utilizing the Veterans Affairs (VA) Greater Los Angeles Electronic Medical Record of 3000 patients with International Classification of Disease 9 (ICD-9) and International Classification of Disease 10 (ICD-10) codes of chronic liver disease from January 1 2000 to December 30 2017 from inpatient and outpatient visits. Each patient’s chart was reviewed to extract data used for analyses. Patients without a histology report, imaging report, or a liver clinic note confirming their liver disease diagnoses were excluded from the analyses. The sample size after excluding these patients was 684 (Figure 1). This population included patients with confirmed diagnosis of cirrhosis by Fibroscan, liver biopsy, complications of cirrhosis or a liver clinic note with an ICD9/10 code for cirrhosis. The final cohort included 153 patients with a confirmed diagnosis of cirrhosis and a non-cirrhotic cohort of 531 control patients with either non-alcoholic fatty liver disease (NAFLD), hepatitis C, hepatitis B and alcoholic liver disease (supplement table 3). Patients who had their atherosclerotic cardiovascular disease (ACSVD) event before the diagnosis of liver disease or unknown date of ASCVD event were also excluded from the analysis (Figure 1). ASCVD events extracted from the chart review and included ST-elevation MI (STEMI) and non-ST- elevation MI (NSTEMI) needing interventions like percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG), coronary artery disease status PCI or CABG, ischemic stroke, transient ischemic event and peripheral vascular disease requiring revascularization (Table 1). Type two myocardial infarction due to anemia, shock and hypovolemia was excluded.
|
ASCVD diagnosis |
After censoring |
|
pad s/p angioplasty |
3 (7.89%) |
|
MI |
2 (5.26%) |
|
STEMI |
4 (10.52%) |
|
NSTEMI |
5 (13.15%) |
|
CVA |
10 (26.31) |
|
unstable angina |
10 (26.31%) |
|
TIA |
4 (10.52%) |
Table 1: ASCVD events (this table shows the ASCVD events included in the study).
The values used for the analysis included systolic blood pressure, lipid panel, medication list, lab values including liver function test and body mass index (BMI). These were extracted from the last visit during the timeframe of the study. ASCVD events were collected from the chart by reviewing the problem list, hospital admission and discharge notes, cardiology, neurology, vascular and primary care notes. Each diagnosis was confirmed by reviewing brain, cardiac, and vascular imaging and procedure notes, including angiography note and surgical note. If a patient had dual care in the community or he/she was on active duty, his/her outside charts were accessed via Joint Legacy Viewer (JLV) to confirm the diagnosis of liver disease and ASCVD events. Patients were considered to have type 2 diabetes mellitus (T2DM) if they had two recorded HgA1c higher than 6.5 or they were prescribed any blood sugar lowering medications during the timeframe of the study. The medication list was extracted from the pharmacy databases. Only one patient had type 1 diabetes. Patients were considered to have hypertension if the diagnosis of hypertension was part of the problem list in the chart and hypertension was included in the assessment and plan section of the primary care notes or their average systolic blood pressure was higher than 140 mm-hg in last the 12 months of the study timeframe.
Free text search was used to search the entire chart for family history of early cardiovascular disease and history of smoking, drug or alcohol abuse. Alcohol abuse is defined as having either more than 14 drinks per week, a DUI, or participation in substance use disorder programs. Drug abuse is defined as any documented use of recreational or prescription drugs for purposes other than those for which they were prescribed. For smoking history, pack years (multiplying the number of packs of cigarettes smoked per day by the number of years the person has smoked) was recorded when available. The sex, race, and age of the patient were obtained from the chart face sheet. Free text search was employed if there was any doubt about the patient demographic. Race was unable to be confirmed in 10% of the patients.
Iron markers including ferritin, transferrin, serum iron and TIBC were collected from the time of cardiovascular events or cirrhosis diagnosis. Only 189 patients without cirrhosis and 94 patients with cirrhosis had iron markers recorded in their chart. International Normalized Ratio (INR) values were missing for most patients. The inflammatory markers Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP) were not available for the majority of patients in both groups. This study was approved by Greater Los Angeles Veterans Affairs Medical Centers’ Institutional Review Boards. Due to the non-invasive nature and patient anonymity, this study was exempt from written consent. This data was provided under contract by the US Department of Veterans Affairs (VA) and cannot be made available to other researchers for replication or reproducibility purposes. Therefore, details about the study methods and procedures can be provided upon request.
2.1 Statistical analysis
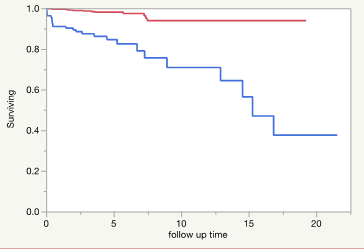
Patient’s level characteristics were reported using mean and standard deviation for continuous variables and frequency and percentage for categorical variables. A student-t test or Wilcoxon rank-sum test was used to compare continuous variables and a Chi-square test or Fisher’s exact test was used to compare categorical variables between groups. Kaplan-Meier curves were used to estimate the survival function for time to ASCVD events in cirrhosis and non-cirrhosis groups. Log-rank test was used to compare the survival function between the 2 groups (Figure 2). If ASCVD status was unknown, the event time was censored at the date when the patient was last known to be free of ASCVD. Univariate cox-regression analysis was used to explore the association between traditional cardiovascular risk factors (age, sex, race, history of DM, HTN, active smoking and family history of early CVD, low density lipoprotein (LDL), high density lipoprotein (HDL), total cholesterol, systolic and diastolic blood pressure (BP), body mass index (BMI), and cirrhosis) with ASCVD events.
Figure 2: Shows the Kaplan-Meier Curve for the cirrhosis (blue) vs no-cirrhosis cohort (red). Log-Rank test: Chi-Square 48.72 (pvalue <0.0001) Wilcoxon Test: ChiSquare: 43.69, (P-value: <0.0001. The median survival time for cirrhosis is 15 years while the non-cirrhosis patients have not reached their median survival time per year yet during the time frame of the study.
Multivariable cox-regression models were used to evaluate the association between cirrhosis and ASCVD while adjusting for traditional cardiovascular risk factors. All traditional cardiovascular risk factors and cirrhosis were included in the full model. Covariates were evaluated and selected using backward stepwise elimination, and some of them were dropped from the final model due to collinearity and model fitting issues (Supplement Table 2). We forced the following variables: cirrhosis, age, history of DM, HTN and active smoking, LDL and HDL into the parsimonious model because they are established risk factors of ASCDV events in the ASCVD risk calculator and also Framingham risk scores (Table 5). Family history of early CVD was excluded from the final analysis due to high rate of missing values (23%). Sex was eliminated from the final analysis due to no event rate in the female sex (table 4, yellow mark). We checked the proportional hazards assumption for all the covariates. The linearity assumption between continuous variables and log hazard was also checked. The linearity assumption was violated when age was treated as a continuous variable, and therefore, age was dichotomized at age 65 before included into the Cox regression models. All the analysis was done using JMP-14 and R version 3.6.2 (2019-12-12).
3. Results
Table 2 shows the patient’s level characteristics stratified by cirrhosis. Most patients were white and male. All patients had some degree of liver disease while 22% of the patients had cirrhosis. Cirrhotic patients were older (mean age: 64 (SD:10) vs 48 (SD: 15.2). The ASCVD event rates were much higher in cirrhotic patients (19.2% vs 2.46%). Additionally, patients with cirrhosis had higher rates of diabetes (DM) and hypertension (HTN), yet lower body mass index (BMI), and more favorable lipid profiles. The liver markers, platelet, and white blood cell count were also significantly different in both groups with higher liver values in non-cirrhosis patients and lower platelet and white blood cell count in cirrhosis group. Heavy drinking status was more common in non-cirrhosis group. Other lab values, such as HgA1c and mean systolic blood pressure (BP), were similar across both groups. Cirrhotic patients were more often taking beta-blockers or potassium-sparing diuretics than non-cirrhotic group, likely due to their underlying portal hypertension from cirrhosis (Supplement Table1). Non-alcoholic fatty liver disease (NAFLD) was the most common etiology of liver disease in non-cirrhotic patients, while hepatitis C and alcoholic liver disease were more prevalent in cirrhotic patients (Supplemental Table 3). Etiology of liver disease among the patient with ASCVD event is Hepatitis C (12 patients), NAFLD (10 patients) and Alcohol (7 patients) (Supplement table 4).
Table 3 shows the patient characteristics stratified by ASCVD events. Patients in ASCVD group were older, had higher rates of hypertension, and were more often prescribed beta-blockers, aspirin, and statins than patients without ASCVD events (Supplement Table 2). In univariate Cox- regression analysis cirrhosis, age, HTN history, family history of early CVD, BMI and race (white vs others) were found to be associated with higher ASCVD event risk (Table 3). In the univariate Cox regression analysis with cirrhosis as an explanatory variable, cirrhotic patients had 8 times higher ASCVD event risk than patients without cirrhosis (HR 8.01, CI: 4.11 -16.52) (Table 4). In the multivariate Cox analysis, cirrhosis remains a major risk factor for ASCVD event after adjusting for the traditional CVD risk factors. (Table 5). The risk of developing ASCVD event in patients with cirrhosis is 5.73 times (HR: 5.73, CI (2.74-12.72) higher than patients without cirrhosis after adjusting for age, hypertension and diabetes history, low- and high-density lipoprotein (LDL and HDL).
In the subgroup analysis of cirrhotic patients with the covariates including TSAT (transferrin saturation) as categorical variables (level 2: Iron saturation greater and equal to 40 and level 1: iron saturation lower than 40) and traditional ASCVD risk factors (age, race, DM, HTN, lipid markers, systolic and diastolic BP, and BMI), iron saturation category 2 and age were major risk factors for ASCVD event. The level 2 iron saturation in patient with cirrhosis increases the risk of ASCVD event 4.78 times after adjusting for all the other covariates (Tables 6-1 and 6-2). Figure 2 shows the Kaplan Meier curve for time to ASCVD events in cirrhosis vs non-cirrhotic patients. Cirrhotic patients have a median survival time of 15 years, whereas the non-cirrhotic patients have not reached their median survival time during the time frame of the study. The proportions of the cirrhotic patients that stay disease free in 5 and 10 years is 84% vs 70% while in non-cirrhotic patients, the proportions respectively are 98% vs 93% (Supplement Table 6). The median follow-up time was 3.6 years vs 3.4 years in cirrhotic and non-cirrhotic patients, respectively (Supplement Table 6). The patient’s military branch was also stratified by both cirrhosis and ASCVD events shown in Supplemental Table 5.
|
Baseline Characteristic stratified by Cirrhosis |
|||
|
Participants' characteristics |
Cirrhosis |
P-value |
|
|
No (N=487 (78.17%)) |
Yes (N=136 (21.83%)) |
||
|
CVD |
12 (2.46) |
26 (19.12) |
< 0.001 |
|
Age >65 |
84 (17.25) |
59 (43.38%) |
<0.001 |
|
Age =< 65 |
403 (82.75) |
77 (56.62) |
|
|
Male |
466 (95.69%) |
129 (94.85%) |
0.64 |
|
White race |
309 (70.39%) |
94 (74.02%) |
0.01 |
|
Black race |
49 (11.16%) |
22 (17.32%) |
|
|
Other race |
81 (18.45%) |
11 (8.66%) |
|
|
Non-smoker (yes) |
192 (38.39%) |
44 (34.88%) |
0.13 |
|
Former smoker (yes) |
175 (36.23%) |
50 (37.04%) |
0.91 |
|
current smoker (yes) |
116 (24.02%) |
42 (30.88%) |
0.11 |
|
heavy drinker (yes) |
187 (39.62%) |
89 (66.42%) |
<0.001 |
|
FHx of early CVD (yes) |
50 (13.26%) |
14 (14%) |
0.86 |
|
DM (yes) |
102 (10.94%) |
48 (35.29%) |
0.005 |
|
Hypertension (yes) |
189 (38.81%) |
90 (66.18%) |
<0.001 |
|
LDL |
113.58 (38.73) |
92.91 (35.35) |
<0.001 |
|
HDL |
43.04 (14.83) |
44.25 (17.00) |
0.46 |
|
Total cholesterol |
190.09 (45.62) |
162.22 (42.52) |
<0.001 |
|
Triglyceride |
181.22 (120.94) |
118.84 (68.35) |
<0.001 |
|
TSAT |
29.05 (13.33) |
33.85 (23.16) |
0.06 |
|
HgA1c |
5.8 (1.2) |
5.7 (1.2) |
0.49 |
|
PLT |
239 (63) |
96 (42) |
<0.001 |
|
ALT |
59 (47) |
35 (26) |
<0.001 |
|
Total bilirubin |
0.92 (1.75) |
1.75 (2.72) |
0.003 |
|
AST |
41 (32) |
45 (34) |
0.33 |
|
Systolic BP |
128 (13) |
129 (16) |
0.78 |
|
Diastolic BP |
76 (10) |
71 (10) |
<0.001 |
|
BMI |
32.31 (5.91) |
29.18 (5.79) |
<0.001 |
|
Median Follow-up time |
5.2 |
4.7 |
0.28 |
Table 2: Descriptive statistics: in parenthesis percentage has been shown for categorical variables and standard deviation for the continuous variables. ASCVD= Atherosclerotic Cardiovascular disease, FHx=Family history, LDL= low density lipoprotein, HDL= high density lipoprotein, DM=Diabetes, PLT= platelet, ALT= alanine aminotransferase, AST= aspartate aminotransferase, BP= blood pressure, BMI= body mass index, TSAT (transferrin saturation) calculated by the following formula: (serum iron: TIBC)*100. International Normalised Ratio (INR) was missing for most patients. P-values are from 2-sided test. Units: systolic and diastolic BP: millimeter of mercury. Total cholesterol, HDL, LDL and Triglyceride: Milligram per deciliter (mg/dl). HgA1c: percentage. AST and ALT: units/litter, PLT: * 10^9 per litter, White blood cell: *10^9 per litter, Albumin: gram per deciliter (gr/dl), BMI: kilogram per square meter (kg/m^2), median follow-up time: per year.
|
Baseline Characteristic stratified by CVD |
||||
|
Patient's characteristics |
CVD |
P-Value |
||
|
Yes (N=38,6.10%) |
No (N=585,93.90%) |
|||
|
Age>65 |
25 (65.79%) |
118 (20.17%) |
<0.001 |
|
|
Age=<65 |
13 (34.21%) |
467 (79.83%) |
||
|
Male |
38 (100%) |
557 (95.21%) |
0.4 |
|
|
White Race |
28 (82.35%) |
375 (70.49%) |
0.21 |
|
|
Black Race |
4 (11.76%) |
67 (12.59%) |
||
|
Other Race |
2 (5.88%) |
90 (16.92%) |
||
|
Non-smoker (yes) |
9 (23.68) |
227 (39.07)) |
0.06 |
|
|
Former smoker (yes) |
20 (52.63%) |
205 (35.28%) |
0.03 |
|
|
Current smoker (yes) |
9 (23.68%) |
149 (25.65%) |
1 |
|
|
Heavy Drinker (yes) |
23 (71.05%) |
253 (43.08%) |
0.04 |
|
|
DM (yes) |
14 (36.84) |
136 (23.25) |
0.07 |
|
|
Hypertension (yes) |
27 (4.33%) |
252 (40.45%) |
0.001 |
|
|
LDL |
94.72 (37.67%) |
110.08 (38.87) |
0.02 |
|
|
HDL |
40.01 (11.21) |
43.51 (15.52) |
0.08 |
|
|
Total Cholesterol |
165.13 (45.29) |
185.26 (46.23) |
0.01 |
|
|
Triglyceride |
162.25 (1353.46) |
167.91 (113.18) |
0.83 |
|
|
TSAT |
42.25 (24.08) |
29.47 (16.10) |
0.01 |
|
|
BMI |
28.17 (5.86) |
31.85 (5.97) |
0.0005 |
|
|
Systolic BP |
125 (15) |
129 (14) |
0.16 |
|
|
Diastolic BP |
69 (10) |
75 (10) |
0.001 |
|
Table 3: Descriptive statistics: in parenthesis percentage has been shown for categorical variables and standard deviation for the continuous variables. ASCVD= Atherosclerotic Cardiovascular disease, FHx=Family history, LDL= low density lipoprotein, HDL= high density lipoprotein, DM=Diabetes, PLT= platelet, ALT= alanine aminotransferase, AST= aspartate aminotransferase, BP= blood pressure, BMI= body mass index, TSAT (transferrin saturation) calculated by the following formula: (serum iron: TIBC)*100. International Normalised Ratio (INR) was missing for most patients. P-values are from 2-sided test. Units: systolic and diastolic BP: millimeter of mercury. Total cholesterol, HDL, LDL and Triglyceride: Milligram per deciliter (mg/dl). HgA1c: percentage. AST and ALT: units/litter, PLT: * 10^9 per litter, White blood cell: *10^9 per litter, Albumin: gram per deciliter (gr/dl), BMI: kilogram per square meter (kg/m^2), median follow-up time: per year.
|
Univariate analysis of risk factors vs ASCVD events |
||
|
Characteristics |
HR (95%CI) |
P-Value |
|
Cirrhosis |
||
|
Yes vs No |
8.01 (4.11-16.52) |
<0.001 |
|
Age category |
||
|
Older than 65 vs 65 or younger |
6.97 (3.63-14.06) |
<0.001 |
|
Diabetes |
||
|
Yes vs No |
1.91 (0.96-3.63) |
0.06 |
|
Hypertension |
||
|
Yes vs No |
2.65 (1.34-5.6) |
0.004 |
|
Current smoking |
||
|
Yes vs No |
0.89 (0.39-1.82) |
0.77 |
|
Race |
||
|
Other vs AA |
0.38 (0.05-1.95) |
0.24 |
|
White vs AA |
1.31 (0.51-4.45 |
1.31 |
|
White vs other |
3.46 (1.03-21.46) |
0.04 |
|
Family History of early CVD |
||
|
Yes vs No |
3.16 (1.21-7.43) |
0.02 |
|
Continuous variables |
||
|
Total Cholesterol |
0.99 (0.98-0.99) |
0.02 |
|
LDL |
0.99 (0.98-0.99) |
0.03 |
|
HDL |
0.98 (0.96-1) |
0.18 |
|
Systolic BP |
0.98 (0.96-1) |
0.15 |
|
Diastolic BP |
0.94 (0.91-0.97) |
0.007 |
|
BMI |
0.9 (0.84-0.95) |
0.008 |
|
SEX |
||
|
Male vs female |
0.23E+08 (0.85-0.85) |
0.07 |
Table 4: This table shows the univariate analysis of risk factors vs ASCVD events, HR: Hazard ratio, CI: confidence interval, all p-values from Effect Wald Tests. For race category all the other races except African American (AA) and white have been combined in other category due to low count. Due to lack event rate in female category, (showing in yellow), therefore sex has been excluded from the final analysis.
|
Multivariate cox-regression analysis, all risk factors vs ASCVD event simplified model |
||
|
Characteristics |
HR (95% CI) |
P value |
|
cirrhosis |
||
|
Yes vs No |
5.73 (2.74-12.72) |
<0.001 |
|
Age |
||
|
Older than 65 vs 65 and younger |
4 (1.95-8.57) |
<0.001 |
|
Diabetes |
||
|
Yes vs No |
1.21 (0.58-2.46) |
0.59 |
|
Hypertension |
||
|
Yes vs No |
1 (0.46-2.29) |
0.98 |
|
Continuous variables |
||
|
LDL |
0.99 (0.98-1) |
0.63 |
|
HDL |
0.97 (0.94-0.99) |
0.04 |
Table 5: Parsimonious model, HR=hazard ratio, CI= confidence interval. All P-values are from effect-Wald tests. Cirrhosis stays a strong risk factor for ASCVD event even after adjusting for other covariates. LDL: low density lipoprotein (mg/dl), HDL: high density lipoprotein (mg/dl).
|
6-1 Sub-group analysis in patients with cirrhosis, ASCVD as outcome, Univariate analysis |
||||
|
Characteristics |
HR (95%CI) |
P value |
||
|
TSAT category |
||||
|
category 2 vs 1 |
4.78 (1.82-13.29) |
0.001 |
||
|
age category |
||||
|
older than 65 vs younger than 65 |
2.65 (1.20-6.25) |
0.01 |
||
|
Race category |
||||
|
Other vs AA |
0.81 (0.04-6.38) |
0.85 |
||
|
White vs AA |
1.21 (0.4-5.22) |
0.75 |
||
|
white vs other |
1.49 (0.3-27) |
0.68 |
||
|
Diabetes |
||||
|
Yes vs No |
0.71 (0.27-1.62) |
0.43 |
||
|
Hypertension |
||||
|
Yes vs No |
0.73 (0.31-1.77) |
0.47 |
||
|
Continuous variables |
||||
|
Total cholesterol |
1 (0.9901.01) |
0.33 |
||
|
LDL |
1 (0.99-1.01) |
0.27 |
||
|
HDL |
0.98 (0.95-1.01) |
0.32 |
||
|
systolic BP |
0.97 (0.95-1) |
0.1 |
||
|
diastolic BP |
0.97 (0.94-1.01) |
0.24 |
||
|
BMI |
0.96 (0.89-1.03) |
0.29 |
||
|
6-2 Subgroup analysis in patients with cirrhosis, ASCVD as outcome, multivariate analysis |
||||
|
Characteristics |
HR (95% CI) |
P-value |
||
|
TSAT category 2 vs 1 |
4.27 (1.61-11.98) |
0.003 |
||
|
age category 1 vs 0 |
1.97 (0.79-5.33) |
0.14 |
||
Table 6: These tables show the relationship between higher transferrin saturation and ASCVD events in subgroup analysis of patients with cirrhosis. Iron saturation category 2: 40% and higher, TSAT category 1: less than 40%. LDL= low density lipoprotein, HDL= high density lipoprotein (unit: Milligram per deciliter (mg/dl). BP: blood pressure (unit: MMHG), BMI: body mass index (kg/m^2), HR: Hazard Ratio, CI: confidence Interval.
4. Discussion
The association between cirrhosis and relationship to cardiovascular disease remains an important question for the medical community given the increasing interest in identifying patients with cirrhosis. There was an assumption that because of higher estrogen levels, lower in cholesterol production, and lower systolic blood pressure (BP) from increases in vasodilatory peptides in cirrhotic patients, the incidence of ASCVD is lower [11-12]. However, recent studies have shown a higher prevalence of ASCVD in patients undergoing liver transplant than previously thought [13-14]. Plotkin and colleagues showed higher morbidity and mortality among patients with Coronary Artery Disease (CAD) on medical management who underwent liver transplantation compared to those who underwent angioplasty prior to their liver transplantation [15]. The overall prevalence of ASCVD events in the United States in the adult population is 7% [10] compared to 6% in our overall cohort. However, the prevalence of ASCVD events in the cirrhosis group was much higher at 19.5%. In our cohort, the prevalence of diabetes and hypertension were higher in cirrhotic patients, but neither was an independent risk factor for ASCVD events in the cirrhotic group. Although the prevalence of hypertension was higher amongst patients with cirrhosis, the mean systolic blood pressure was the same in both groups, with lower mean diastolic blood pressure in the cirrhosis group. Notably, there was a higher percentage of cirrhotic patients on blood pressure medications because they were on a beta-blocker or diuretics for treatment of their portal hypertension. There was no difference between the two groups when comparing other blood pressure lowering medication groups except calcium channel blockers. In the univariate analysis, systolic BP was not a risk factor for ASCVD event while diastolic BP had a small protective effect. These findings can be explained by pathophysiologic changes from cirrhosis. Blood pressure usually drops after development of cirrhosis, with such patients starting off as normotensive before becoming hypotensive later in the disease progression [12]. Therefore, the average BP measurement alone cannot be used to assess the association of systolic and diastolic BP and cardiovascular disease. As demonstrated in Table 3, patients who developed ASCVD events had lower incidence of hypertension. While mean systolic BP was the same in ASCVD vs non-ASCVD group, diastolic BP was much lower.
We also observed a more favorable lipid profile in patients with cirrhosis compared to those without, which is in agreement with prior studies [16] and could be due to severe illness, weight loss, and malnutrition often observed in this group of patients. We observed that only 14% of patients with ASCVD event were on ACE inhibitor or ARB medication, which are the contemporary medications in the treatment of cardiovascular disease that have been shown to decrease mortality in prior studies [17]. The reason for this could be electrolyte imbalances due to underlying severity of cirrhosis diagnosis and concurrent use of potassium-sparing medications and diuretics. Only 21 out of 36 patients with ASCVD events were on anti-platelet agents (ASA). Patients with ASCVD events who were not on ASA had history of upper or lower GI bleeding. One patient had GI bleeding due to aspirin alone and the rest had a history of varices or hemorrhoids. 17 patients with ASCVD events were not on a statin. We were unable to identify the reason for this in most patients, but four patients were off statin due to severe transaminitis and muscle pain. Per a primary care note, one patient was off statin due to “cirrhosis”. Our study showed that cirrhosis is an independent risk factor for ASCVD events even after adjusting for other traditional ASCVD risk factors (5.73 (2.74-12.72)). Although the patients in our cirrhosis cohort were older, but even after adjusting for age, cirrhosis remained a major risk factor for ASCVD events. This result parallels a recent study by Shih-Yi Lin et al. showing higher incidences of acute coronary syndrome and peripheral vascular disease in patients with chronic liver disease complicated by cirrhosis [6]. The higher incidence of cardiovascular event in patients with cirrhosis, therefore, cannot solely be explained by the traditional ASCVD risk factors. In the Cox regression model, shown in Tables 6-1 and 6-2, increases in the transferrin saturation (TSAT) could be a potential risk factor for cardiovascular events in patients with cirrhosis. Due to the nature of our study, we did not have access to hsCRP or IL-6 to adjust the iron markers for inflammation. Therefore, we chose transferrin saturation as the marker of body iron storage because this iron marker is affected by inflammation to lesser extent than other markers that are acute phase reactants, like ferritin and transferrin. Transferrin saturation usually decreases with increase in inflammatory markers, but we observed an increase in transferrin saturation in patients with cirrhosis. Also, patients with ASCVD events had higher mean transferrin saturation than those without (mean TSAT: 42 vs 29 with P-value <0.01, Table 3). This could partly be due to more severe liver damage in patients with ASCVD events, and therefore, transferrin saturation could possibly serve as a marker of cirrhosis severity.
We speculate that the association between cirrhosis and higher incidence of ASCVD events could be due to an increase in inflammatory state in patients with cirrhosis accompanied by elevations in non-transferrin bound iron, which may act to catalyze the production of reactive oxygen species (ROS) and induce oxidative stress. ROS-induced oxidative stress plays an important role in pathology of both cirrhosis and ASCVD [18-20]. For liver disease, it has been shown in animal models that reduced bioavailability of nitric oxide is associated with increase in ROS level, which in turn increases the oxidative stress and inflammation in these patients [21]. Gut hyperpermeability in cirrhotic patients also plays an important role in increasing inflammatory markers [22]. Systemic inflammation and immune system deregulation, in turn, are major pathophysiological pathways in progression of cirrhosis. CAID syndrome (combination of immune deficiency and systemic inflammation) was recently described as the main culprit of cirrhosis progression. CAID is the consequence of immune cell activation through infectious and non-infectious causes [23]. Persistent immune system deregulation causes increase in the systemic inflammation by production of pro-inflammatory cytokines and upregulated expression of cell activation markers [24]. Oxidative stress is an established driver of atherosclerotic plaque formation, progression and rupture. Inflammatory markers, especially hsCRP and IL6, are emerging risk factors for atherosclerotic cardiovascular events [18-19, 37-38]. Endothelial cell dysfunction causes focal lipoprotein permeation, traps the lipoprotein particles, and promotes the recruitment of monocytes [18-19]. Oxidation of LDL activates secretion of Macrophage-Chemotactic protein-1 (MCP-1) in the arterial intima, where monocytes undergo transformation into macrophages [18-19]. Macrophages then express scavenger receptors that facilitates the uptake of oxidized LDL. Cholesterol-rich macrophages, or foamy cells, excrete matrix metalloproteinases (MMP) that facilitate breakdown of collagen and promote ROS production. The process could rupture the plaque and lead to acute myocardial infarction. Meanwhile, foamy cells that undergo necrosis will be unable to clear the dead cells from the area, leading to growth of plaques with necrotic cores.
Iron also may play a role in increasing the inflammation in cirrhotic patients. Iron overload is common in 8-30% of patients with end-stage liver disease [25], which could accelerate the rate of fibrosis [26]. Since our bodies lack a mechanism to excrete the iron, iron hemostasis is tightly regulated by the hepcidin-iron transporter ferroportin (FPN) axis [24]. FPN is a transporter located on enterocytes that control the movement of iron in the gut. FPN also regulates iron absorption in other cells types, like macrophages and hepatocytes [27]. Meanwhile, hepcidin, a liver hormone, controls the expression of the FPN in these cells. Patients with advanced chronic liver disease often have low hepcidin levels, which could partly be due to decreases in functional hepatocytes though the exact mechanism is not completely understood [27]. If one’s body iron supply is adequate, hepcidin level increases, which in turn reduces FPN; therefore, iron export. Most iron is transported in the circulation by binding to transferrin. However, iron can also circulate in non-transferrin bound form (NTBI), especially when serum iron is high and transferrin proteins are saturated. NTBI could trigger the development of atherosclerosis by inducing ROS production and exposing important biomolecules, such as lipoproteins, to oxidative stress. Circulating NTBI promotes endothelial activation by enhancing adhesion molecule expression and promoting endothelial cell dysfunction [28]. NTBI also affects vascular smooth muscle cell proliferation and migration into the atherosclerotic lesions, favoring progression of the plaque [28]. Multiple in vitro and in vivo studies in the past have shown the association between markers of iron overload and atherosclerotic cardiovascular disease [30-36].
4.1 Limitations
We recognized the limitations of our study. Our sample size is small, we evaluated only a single VA medical center and the event rate is relatively low. Also, given that this study is a retrospective design, we did not have access to the iron markers for most of the non-cirrhotic patients. Additionally, the inflammatory markers were not available for most patients because checking iron panel and inflammatory markers are not part of routine practice. Also, one should also take into account unknown residual confounding which might affect the results of the study. At the same time, because the information is not generated by computer and all variables have been verified by a physician, the risk of mistake and bias is very low which is one of the biggest strengths of this study. This study could be the steppingstone for future studies to explore the role of iron markers as one of explanatory variables in the process of atherosclerotic cardiovascular outcomes. We are suggesting the evaluation of the VA national database on a larger scale to verify the result of this study.
5. Conclusion
This study of Veterans in a large VA medical center found an increase in prevalence of ASCVD events in cirrhotic patients compared to liver disease patients without cirrhosis (19.12% vs 2.46%). Even after adjusting for traditional ASCVD risk factors including age, cirrhosis remained a major risk factor for ASCVD events with a hazard ratio of 5.73 (CI: 2.74-12.72). In the subgroup analysis of the cirrhosis group, patients with a transferrin saturation, a marker of total body iron stores, greater or equal to 40% had 4.27 times higher risk of ASCVD events than with lower saturations. We propose that increased non-transferrin bound iron (NTBI) concentrations could amplify the effect of inflammatory cytokines in the inflammation/ASCVD pathway. Therefore, given the complexity of this relationship, a more robust statistical model is needed to evaluate their association. Adjusting for only inflammation in a logistic or cox regression model would not be able to discriminate the effect of iron and inflammation on cardiovascular progression or event. A more comprehensive study with a prospective design and access to inflammatory markers is needed to explore the association of higher iron body storage,
atherosclerotic cardiovascular events, and inflammation.
Acknowledgement
I want to thank Dr Gregory Brent and Dr Neil Paige for their unlimited support and wisdom.
References
- Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-1029 update: a report from the American Heart Association. Circulation 139 (2019): e56-e528.
- Scaglione S, Kliethermes S, Cao G, et al. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol 49 (2015): 690-696.
- Erzigotti A, Bonfiglioli A, Muscari A, et al. Reduced prevalence of ischemic events and abnormal supraortic flow patterns in patients with liver cirrhosis. Liver Int 25 (2005): 331-336.
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 111 (2003): 1805-1812.
- Kalaitzakis E, Rosengren A, Skommevik T, et al. Coronary artery disease in patients with liver cirrhosis. Dig Dis Sci 55 (2010): 467-475.
- Lin SY, Lin CL, Lin CC, et al. Risk of acute coronary syndrome and peripheral arterial disease in chronic liver disease and cirrhosis: A nationwide population-based study. Atherosclerosis 270 (2018): 154-159.
- Berzigotti A, Bonfiglioli A, Muscari A, et al. Reduced prevalence of ischemic events and abnormal supraortic flow patterns in patients with liver cirrhosis. Liver Int 25 (2005): 331-336.
- Kazankov K, Munik K, Ovrehus KA, et al. High burden of coronary atherosclerosis in patients with cirrhosis. Eur J Clin Invest 47 (2017): 565-573.
- An J, Shim JH, Kim SO, et al. Prevalence and prediction of coronary artery disease in patients with liver cirrhosis: a registry-based matched case-control study. Circulation 130 (2014): 1353-1362.
- Katherine Gorcyca, PharmD, Irfan Khan, et al. Prevalence of atherosclerotic cardiovascular disease (ASCVD) and diabetes populations in the United States. Journal of Clinical Lipidology 9 (2015).
- Vanecek R. Atherosclerosis and cirrhosis of the liver. Bull World Health Org 1976;53: 567-570.
- Henriksen J H, Fuglsang S, Bendtsen F, et al. Atrial hypertension in cirrhosis: arterial compliance, volume distribution, and central haemodynamics. Gut 55 (2006): 380-387.
- Carey WD, Dumot JA, Pimentel RR, et al. The prevalence of coronary artery disease in liver transplant candidates over age 50. Transplantation 59 (1995): 859-864.
- Godoy MF, Roveri PO, Santos MA, et al. Obstructive coronary disease in patients with chronic liver disease awaiting liver transplantation. Arq Bras Cardiol 96 (2011): 26-30.
- Plotkin JS, Scott VL, Pinna A, et al. Morbidity and mortality in patients with coronary artery disease undergoing orthotopic liver transplantation. Liver Transpl Surg 2 (1996): 426-430.
- D’Arienzo A, Manguso F, Scaglione G, et al. Prognostic value of progressive decrease in serum cholesterol in predicting survival in Child-Pugh C viral cirrhosis. Scand J Gastroenterol 33 (1998): 1213-1218.
- Stephan D Fihn, James C Blankenship, Karen P Alexander, et al. 2014 ACC/AHA/AATS/ PCNA/SCAI/STS Focused Update of the Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease. J Am Coll Cardiol 64 (2014): 1929-1949.
- Yousuf O, Mohanty B D, Martin S S, et al. High-sensitivity C-reactive protein and cardiovascular disease. A resolute belief or an elusive link?. Journal of the American College of Cardiology 62 (2013): 397-408.
- Balla G, Jacob HS, Eaton JW, et al. Hemin: a possible physiolog- ical mediator of low density lipoprotein oxidation and endothelial injury. Arterioscl Thromb 11 (1991): 1700-1711
- Halina Cicho?-Lach, Agata Michalak. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol 20 (2014): 8082-8091.
- Gracia-Sancho J, Lavin~a B, Rodri?guez-Vilarrupla A, et al. Increased oxidative stress in cirrhotic rat livers: a potential mechanism contributing to reduced nitric oxide bioavailability. Hepatology 47 (2008): e1248-e1256.
- Fukui H. Gut-liver axis in liver cirrhosis: How to manage leaky gut and endotoxemia. World J Hepatol 7 (2015): 425-442.
- Sipeki N, Antal-Szalmas P, Lakatos PL, et al. Immune dysfunction in cirrhosis. World J Gastroenterol 20 (2014): 2564-2577
- Dirchwolf M, Ruf AE. Role of systemic inflammation in cirrhosis: From pathogenesis to prognosis. World J Hepatol 7 (2015): 1974?1981.
- Kowdley KV. Iron Overload in Patients With Chronic Liver Disease. Gastroenterol Hepatol (N Y) 12 (2016): 695-698.
- Kowdley KV, Belt P, Wilson LA, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 55 (2012): 77-85.
- Ganz T, Nemeth E. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol 290 (2006).
- Cornelissen A, Guo L, Sakamoto A, et al. New insights into the role of iron in inflammation and atherosclerosis. EBioMedicine 47 (2019): 598-606.
- Cominacini L, Pasini AF, Garbin U, et al. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF- kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem 275 (2000): 12633-12638.
- Vela D. Low hepcidin in liver fibrosis and cirrhosis; a tale of progressive disorder and a case for a new biochemical marker. Mol Med 24 (2018): 5.
- Galesloot TE, Holewijn S, Kiemeney LA, et al. Serum hepcidin is associated with presence of plaque in postmenopausal women of a general population. Arterioscler Thromb Vasc Biol 34 (2014): 446-456.
- Li JJ, Meng X, Si HP, et al. Hepcidin destabilizes atherosclerotic plaque via overactivating macrophages after erythrophagocytosis. Arterioscler Thromb Vasc Biol 32 (2012): 1158-1166.
- Wolff B, Volzke H, Ludemann J, et al. Association between high serum ferritin levels and carotid atherosclerosis in the study of health in Pomerania (SHIP). Stroke 35 (2004): 453-457.
- Sempos CT, Looker AC, Gillum RE, et al. Serum ferritin and death from all causes and cardiovascular disease: The NHANES II Mortality Study. National Health and Nutrition Examination Study. Ann. Epidemiol 10 (2000): 441-448.
- Ki-Chul Sung, Seok-Min Kang, Eun-joo Cho, et al. Ferritine is independently associated with the presence of coronary artery calcium in 12033 men. Arterioscler Thromb Vasc Biol 32 (2012): 2525-2530.
- Silva G, Jeney V, Chora A, et al. Oxidized hemoglobin is an endogenous proinflammatory agonist that targets vascular endothelial cells. J Biol Chem 284 (2009): 29582-29595.
- Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 375 (2010):132-140.
- Simon TG, Trejo MEP, McClelland R, et al. Circulating Interleukin-6 is a biomarker for coronary atherosclerosis in nonalcoholic fatty liver disease: Results from the Multi-Ethnic Study of Atherosclerosis. Int J Cardiol 259 (2018): 198-204.
Supplement
|
Baseline Characteristic stratified by Cirrhosis |
|||
|
Medication |
Cirrhosis |
P-Value |
|
|
No (N=487 (78.17%) |
Yes (136 (21.83%) |
||
|
ACE Inhibitor (yes) |
92 (18.89%) |
29 (21.32%) |
0.52 |
|
ARB (yes) |
28 (5.75%) |
11 (8.09%) |
0.31 |
|
CCB (yes) |
51 (10.47%) |
28 (20.59%) |
0.003 |
|
Beta- Blocker (yes) |
64 (13.14%) |
33 (24.26%) |
0.003 |
|
Potassium sparing diuretics (yes) |
4 (0.82%) |
34 (25%) |
<0.001 |
|
Diuretics (yes) |
41 (8.24%) |
45 (33.09%) |
<0.001 |
|
ASA (yes) |
68 (13.96%) |
29 (21.32%) |
0.03 |
|
Plavix (yes) |
5 (1.03%) |
3 (2.21%) |
0.38 |
|
Metformin (yes) |
77 (15.81%) |
22 (16.18%) |
0.89 |
|
Statin (yes) |
141 (28.95%) |
42 (30.88%) |
0.67 |
|
Baseline Characteristic stratified by CVD |
|||
|
Medication |
CVD |
P-Value |
|
|
Yes (N=38,6.10%) |
No (N=585,93.90%) |
||
|
ACE inhibitor (yes) |
11 (28.95%) |
110 (18.80%) |
0.13 |
|
ARB (yes) |
3 (6.15%) |
36 (7.89%) |
0.72 |
|
CCB (yes) |
15 (39.47) |
64 (10.94) |
<0.001 |
|
Beta- Blocker (yes) |
18 (47.37%) |
79 (13.50%) |
<0.001 |
|
Potassium sparing diuretics (yes) |
7 (18.42) |
31 (5.30) |
0.005 |
|
Diuretics (yes) |
11 (28.95%) |
75 (12.82) |
0.01 |
|
ASA (yes) |
21 (55.26%) |
76 (12.99%) |
<0.001 |
|
Plavix (yes) |
6 (15.79%) |
2 (0.34%) |
<0.001 |
|
Metformin (yes) |
11 (28.95%) |
88 (15.04%) |
0.03 |
|
Statin (yes) |
21 (55.26%) |
162 (27.69%) |
0.0007 |
Supplement Table 1: This table shows the distribution of blood pressure, metformin, statin use and also anti-platelet agents stratified by both cirrhosis and ASCVD events. ARB; angiotensin II receptor blocker, ACE: angiotensin converting enzyme inhibitor, CCB: calcium channel blockers, B-blocker: beta blockers. ASA: aspirin, statin (Simvastatin, Atorvastatin, Rosuvastatin, Pravastatin).
|
Multivariate Cox-regression, all risk factors vs ASCVD events (Full model) |
||
|
Characteristics |
HR (95% CI) |
P-value |
|
Cirrhosis |
||
|
Yes vs No |
4.32 (1.94-10.19) |
0.003 |
|
Age |
||
|
65 and above |
4.39 (1.91-10.62) |
0.004 |
|
Race |
||
|
Other vs AA |
0.57 (0.7-3.19) |
0.53 |
|
White vs AA |
1.31 (0.44-4.96) |
0.63 |
|
white vs other |
2.29 (0.64-14.64) |
0.22 |
|
Diabetes category |
||
|
Yes vs No |
1.15 (0.49-2.61) |
0.72 |
|
Hypertension category |
||
|
Yes vs No |
1.26 (0.51-3.26) |
0.61 |
|
Current smoking history |
||
|
Yes vs No |
0.75 (0.28-1.75) |
0.52 |
|
Continuous variables |
||
|
Total Cholesterol |
1 (0.98-1.02) |
0.37 |
|
LDL |
0.98 (0.96-1.01) |
0.25 |
|
HDL |
0.96 (0.93-0.99) |
0.03 |
|
Systolic BP |
0.97 (0.94-1) |
0.08 |
|
Diastolic BP |
0.99 (0.95-1.04) |
0.82 |
|
BMI |
0.32 (0.01-4.48) |
0.41 |
Supplement Table 2: This table shows the full model adjusting for all the risk factors, cirrhosis, age 65 and above, white vs other race category, HDL are all significant. The model built by backward stepwise elimination included only cirrhosis and age categories. HR=hazard ratio, CI= confidence interval. All P-values are from effect-Wald tests. Cirrhosis stays a strong risk factor for ASCVD event even after adjusting for other covariates. LDL: low density lipoprotein (mg/dl), HDL: high density lipoprotein (mg/dl), Total cholesterol (mg/dl), BP: Blood Pressure (MMHG),
BMI: body mass index (kg/m^2).
|
Etiology of liver disease stratified by cirrhosis |
|||
|
Cirrhosis No |
Cirrhosis Yes |
||
|
NAFLD |
306 (64.01%) |
19 (13.97%) |
|
|
Alcoholic |
75 (15.69%) |
44 (32.35%) |
|
|
Hep C |
26 (5.43%) |
51 (37.5%) |
|
|
Hep B |
5 (1.04%) |
3 (2.20) |
|
|
NAFLD and alcoholic |
31 (6.48%) |
3 (2.20) |
|
|
Hep C and alcoholic |
9 (1.88) |
5 (3.67) |
|
|
Hep c and Hep B |
3 (0.62) |
0 |
|
|
Hep B, Hep c and alcohol |
1 (0.20) |
0 |
|
|
NAFLD , Hep c and alcohol |
1 (0.20) |
0 |
|
|
NAFLD and Hep C |
2 (0.41) |
4 (2.94) |
|
|
Idiopathic |
1 (0.20) |
1 (0.73) |
|
|
Primary Biliary cirrhosis |
0 |
1 (0.73) |
|
|
Hep B and alcohol |
3 (0.62) |
0 |
|
|
Hemochromatosis |
0 |
1 (0.73) |
|
|
Missing |
21 (4.39) |
4 (2.94) |
|
|
NAFLD and Hep B |
2 (0.41) |
0 |
|
|
NAFLD, Hep B and alcohol |
1 (0.20) |
0 |
|
Supplement Table 3: This table shows the etiology of liver disease stratified by cirrhosis, NAFLD (non-alcoholic fatty liver disease), Hep C (hepatitis c), Hep B (hepatitis B).
|
Etiology of liver disease stratified by ASCVD |
|
|
NAFLD |
10 |
|
Alcoholic |
7 |
|
Hep C |
12 |
|
Hep B |
1 |
|
NAFLD and alcoholic |
0 |
|
Hep C and alcoholic |
1 |
|
Hep c and Hep B |
0 |
|
Hep B, Hep c and alcohol |
0 |
|
NAFLD , Hep c and alcohol |
0 |
|
NAFLD and Hep C |
3 |
|
Idiopathic |
0 |
|
Primary Biliary cirrhosis |
0 |
|
Hep B and alcohol |
0 |
|
Hemochromatosis |
1 |
|
NAFLD and Hep B |
1 |
|
NAFLD, Hep B and alcohol |
0 |
Supplement Table 4: Etiology of liver disease in patients with ASCVD events.
|
Military branch by cirrhosis |
||
|
Cirrhosis |
No |
Yes |
|
Army |
177 (40.78%) |
61 (10.43%) |
|
Airforce |
38 (8.76%) |
12 (10.43%) |
|
Marines |
103 (23.73%) |
20 (17.39%) |
|
Navy |
106 (24.42%0 |
22 (19.13%) |
|
Others |
10 (2%) |
0.00% |
|
missing |
53 (10%) |
21 (15.4%) |
|
Military branch by ASCVD |
||
|
ASCVD |
No |
Yes |
|
Army |
224 (38.29%) |
14 (36.85) |
|
Airforce |
46 (7.86%) |
4 (10.53%) |
|
Marines |
117 (20%) |
6 (15.79%) |
|
Navy |
122 (20%) |
6 (15.79%) |
|
Others |
10 (1.70%) |
0.00% |
|
missing |
66 (11.28) |
4 (10.53%) |
Supplement Table 5: It shows patients’ military branch stratified by Cirrhosis and CVD events.
Supplement Table 6: These 3 tables are showing the survival analysis in patients with cirrhosis, patients without cirrhosis and combined. The highlighted rows in red in all tables show the median follow-up time in cirrhosis group, no-cirrhosis group and overall cohort. The highlighted rows in yellow in first and second table show the proportion of the patients without event at 5 and 10 year for cirrhosis and no-cirrhosis group.


 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 