Childhood Asthma-Pattern, Severity and Control Among Children Seen in an Outpatient Respiratory Clinic in River State University Teaching Hospital, Port Harcourt, Nigeria
Article Information
Onubogu UC1*, West BA2
Department of Paediatrics, Rivers State University Teaching Hospital, Port Harcourt, Nigeria
*Corresponding Author: Onubogu UC, Department of Paediatrics, Rivers State University Teaching Hospital, Port Harcourt, Nigeria
Received: 14 July 2020; Accepted: 04 August 2020; Published: 07 August 2020
Citation:
Onubogu UC, West BA. Childhood Asthma-Pattern, Severity and Control Among Children Seen in an Outpatient Respiratory Clinic in River State University Teaching Hospital, Port Harcourt, Nigeria. Journal of Pediatrics, Perinatology and Child Health 4 (2020): 073-085.
View / Download Pdf Share at FacebookAbstract
Background: Asthma, the commonest respiratory disorder globally is often a cause of emergency room visits, school and work absenteeism thus affects the quality of life of children and their caregivers.
Objective: To assess the pattern of asthma severity and level of control among children attending the respiratory clinic in Rivers State University Teaching Hospital, Nigeria.
Methods: It was a hospital based cross-sectional study carried out at the Paediatric respiratory clinic in the Rivers State University Teaching Hospital, Nigeria over a 6-year period.
Results: Of 307 patients recruited into the study, males predominated with a M: F ratio of 1.47:1. There was family history of atopy in 195 (63.5%) and cough was the commonest symptom reported 234 (96.3%). Median age at first diagnosis was 4(IQR:2-8) years and mean duration of illness, 1(IQR:0.2-3) year. Majority had intermittent asthma 92 (37.9%) while only 55 (29.6%) had controlled asthma. Malnutrition, living in households with domestic fuel being kerosene or firewood only were significantly associated with having severe persistent asthma while living in households in which gas is the primary source of domestic fuel, upper socioeconomic class and duration of illness being ≤ 2 years were significantly associated with not having severe persistent asthma. Overweight was significantly associated with having controlled asthma while age <11 years and physician-diagnosed asthma at ≤ 2yrs of age were significantly associated with not having controlled asthma.
Conclusion: Use of clean domestic fuel and reduction of the effects of deprivation of social and economic determinants of health among the low socioeconomic class can improve control and reduce severity of asthma in children.
Keywords
Childhood Asthma, Severity, Control, Pattern, Nigeria
Article Details
1. Introduction
Asthma is a chronic inflammation of the lung airways leading to episodic airflow obstruction [1]. Children present with recurrent episodes of cough, breathlessness, chest tightness as well as exercise intolerance and wheezing with the symptoms resolving spontaneously or with the use of bronchodilators [2]. Asthma which has high levels of preventable morbidity and mortality is the commonest respiratory disorder globally accounting for more than 300 million people being affected and has a prevalence rate ranging between 1% - 18% [2-5]. In Nigeria, it is estimated that about 3.1% of children in any given community have asthma [6] and asthma ranks second to pulmonary tuberculosis among chronic respiratory diseases [7]. It is one of the commonest causes of emergency room visits, school absenteeism and absence from work by parents, thereby affecting the quality of life of children as well as their parents/caregivers [2, 8, 9].
Severity of asthma is determined by the disease activity and the patients’ phenotype while control is the extent of reduction or removal of the manifestation of the disease by treatment [10]. The assessment of the severity of asthma, which is in accordance with the national guidelines is important in the management of asthma as this guideline links the assessed level of asthma severity to appropriate action plan [11]. Integral to the management of asthma is the achievement of optimal control which refers to the degree to which symptoms, on-going functional impairments and risk of adverse events are minimized, thus enabling children with asthma to live normal lives and achieve their potentials [1, 2]. The present study was therefore carried out to assess the pattern, severity and level of control of asthma among children attending the respiratory clinic in Rivers State University Teaching Hospital in Nigeria as similar study has not been carried out in Port Harcourt, Nigeria.
2. Materials and Methods
It was a hospital based cross-sectional study carried out at the Paediatric respiratory clinic in the Rivers State University Teaching Hospital, Nigeria over a 6-year period from April 2014 to March 2020. Rivers State University Teaching Hospital is a tertiary health facility owned by the Rivers State Government in the South-South geo-political zone in Nigeria. It is a 375 bedded hospital and serves as a referral center for all the government owned primary health centers, general hospitals as well as private health facilities in the state. The Paediatric respiratory clinic which is one of the specialist clinics in the department of Paediatrics is run once every week on Fridays by a consultant specialized in respiratory paediatrics, with paediatric trained nurses. Referrals are gotten from the children emergency room, other clinics in the department as well as primary health centers, general hospitals and private health facilities in the state. Children seen in the clinic are recorded in an asthma register as part of the clinic protocol and the scope of details entered in the register progressively increased over the years. Ethical approval was obtained from the Rivers State Health Research Ethics committee and informed consent was obtained from the parents/caregivers and/or from older children respectively. Children aged less than 17 years with diagnosis of asthma made by a consultant with history of recurrent episodes of cough, wheezing, chest tightness and shortness of breath which resolves spontaneously or with the use of bronchodilators were consecutively recruited.
Severity was assessed for all who had physician diagnosed asthma for up to 2 months and who were able to retrospectively recall symptoms frequency. They were then given a symptom diary to prospectively document their symptom and a 6 weeks’ clinic appointment to reconfirm the classification after which an asthma action plan was drawn up for each patient. The severity of asthma before commencement of management was assessed and categorized using frequency of day and nighttime symptoms into intermittent, mild persistent, moderate persistent and severe persistent based on the National Asthma Education and Prevention Program (NAEPP) guidelines [1, 12].
Control was assessed for all those with a physician diagnosed asthma for up to 2 months before presenting to our facility in order to assess the efficacy of their asthma management intervention before commencing treatment. Newly diagnosed asthma, those who could not recall symptoms, those lost to follow up, those who did not give consent were excluded. Asthma control test (ACT) was done using the Childhood ACT (C-ACT) questionnaire [13] for children aged 11 years and below while ACT for adult questionnaire was done for children aged 12 years and above [14]. For the C-ACT a total of 7 questions were asked with the first 4 covering the child’s perception of their symptoms while the remaining 3 quantified patients’ symptoms, a maximum of 27 could be scored. The Adult version of the ACT consisted of 5 questions with a maximum score of 25. For both ACTs, patients were classified as well controlled with ACT of ≥ 20, partial control with ACT score of 16-19, and poor control with ACT score of <15. Sociodemographic information such as age, sex, age at diagnosis of asthma and parental and family history of asthma were obtained. Other history obtained were presence of adult smoker(s) in the house, types of cooking fuel, symptoms reported by the patients/caregivers and the duration of their asthma.
Social class of the parents/caregivers were determined using the classification by Olusannya et al. [15]. The total class score ranged from 1 to 5 in order of descending privileges and divided into 3 equal parts to get upper, middle and lower socioeconomic classes. Nutritional status of each child was classified using the weight for age Z score [16-18]. Data collected was entered into Excel spreadsheet and analysis was done using IBM SPSS Statistics version 23. Descriptive statistics was used to express patient’s characteristics while bivariate association test was done using chi-squared test. P value was set at ≤ 0.05 with 95% confidence interval.
3. Results
3.1 Characteristics of the study population
Three hundred and seven patients with asthma were recruited into the study. There were more males 185 (59.6%) than females with a M: F ratio of 1.47: 1 and the median age of the patients being 7 (IQR: 4-10) years. Majority had normal nutritional status 238 (77.5%), lived in households with no adult smoker 247 (80.5%), used cooking gas as their domestic fuel source 211 (68.7%) and belonged to the upper socioeconomic class 132 (43%). Positive family history of atopy was identifiable in 195 (63.5%). The most common symptom reported was cough in 234 (96.3%) of the children. At least 3 of the cardinal symptoms of asthma were present in 123 (50.6%) and the median age at first diagnosis was 4 (IQR: 2 - 8) years while the median duration of illness was 1 (IQR: 0.2 - 3) year. Majority came for their first scheduled outpatient visit in stable clinical state not having acute exacerbations 213 (69.4%), Table 1.
3.2 Classification of asthma severity
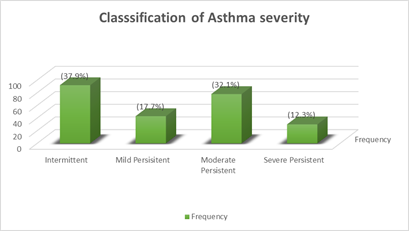
The presence of the cardinal symptoms of asthma identifiable by the patients/caregivers was assessed in 243 patients among whom majority had intermittent asthma 92 (37.9%), severe persistent asthma was seen in 30 (12.3%) while persistent asthma constituted 151 (62.1%) of the types of asthma seen. Initial classification from retrospective recall of symptoms changed in 26 (10.7%) of the patients after prospective symptom monitoring, 23 (9.5%) were reclassified to a more severe type while 3 (1.2%) were reclassified to a less severe type Figure 1.
3.3 Level of asthma control
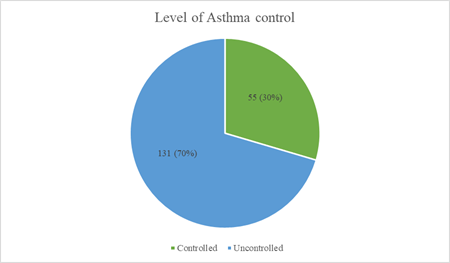
Asthma control test (ACT) was done in 186 of the patients, the mean ACT score of the patients was 16.9 ± 4.38 out of which only 55 (29.6%) had controlled asthma with an ACT score > 19 (Figure 2). Among the uncontrolled asthma, 80 (61.1%) had partial control with ACT score of 16-19, while 51 (38.9%) had poor control with ACT score of <15 Figure 2.
3.4 Factors associated with severe persistent asthma
Malnutrition ( OR :11.7, 95%CI;1.8 – 73.1 P=0.001), living in households in which the domestic fuel is only kerosene or firewood (OR: 2.9, 95%CI;1.13 – 7.8, P=0.02) were significantly associated with having severe persistent asthma while living in households in which gas was the primary source of domestic fuel (OR :0.4, 95%CI; 0.18 – 0.89, P=0.02), belonging to upper socio economic class (OR :0.3, 95%CI; 0.14 – 0.81, P=0.01) and having the duration of illness ≤ 2 years (OR :0.3, 95%CI; 0.1 – 0.83, P=0.01) were significantly associated with not having severe persistent asthma (Table 2).
3.5 Factors associated with controlled asthma
Being overweight (OR: 2.1, 95%CI; 1.04 – 4.3, P=0.03) was significantly associated with having controlled asthma while age <11 years (OR:0.48, 95%CI; 0.2 – 0.994, P=0.04), and having a physician-diagnosed asthma at ≤ 2 years of age (OR :0.1, 95%CI; 0.03 – 0.26, P=0.000004) were significantly associated with not having controlled asthma (Table 3).
|
Variable |
Frequency n=307 (%) |
|
Age (years) |
|
|
<4 |
68 (22.1) |
|
4-<8 |
93 (30.3) |
|
8-<12 |
95 (30.9) |
|
12-16 |
(16.6) |
|
Gender |
|
|
Male |
183 (59.6) |
|
Female |
124 (40.4) |
|
Weight for Age Z score |
|
|
>2 (overweight) |
62 (20.2) |
|
2 to -2 (normal nutrition) |
238 (77.5) |
|
< -2 to -3(moderate malnutrition) |
5 (1.6) |
|
<-3 (severe malnutrition) |
2 (0.7) |
|
Adult smoker in child’s household |
|
|
Yes |
10 (3.3) |
|
No |
247 (80.5) |
|
Undisclosed |
50 (16.3) |
|
Cooking fuel used in household |
|
|
Gas |
211 (68.7) |
|
Kerosene |
101 (32.4) |
|
Firewood |
7 (2.3) |
|
Electric stove |
3 (1.0) |
|
Social class Score of families |
|
|
1 - 1.7 (upper) |
132 (43.0) |
|
>1.7 - 3.3 (Middle) |
122 (39.4) |
|
>3.3 - 5 (low) |
22 (7.2) |
|
Undisclosed |
31 (10.1) |
|
Known Family history of atopy |
|
|
Yes |
195 (63.5) |
|
No |
110 (35.8) |
|
Unsure |
2 (0.7) |
|
Symptoms reported by patient/care giver (n=243) |
|
|
Cough |
234 (96.3) |
|
Difficulty breathing |
234 (94.6) |
|
Wheeze |
215 (88.4) |
|
Chest pain |
116 (47.4) |
|
Number of symptoms per patient |
|
|
2 |
27 (11.1) |
|
3 |
123 (50.6) |
|
4 |
93 (38.3) |
|
Age at first diagnosis of asthma (years) |
|
|
<4 |
121 (39.4) |
|
4 - <8 |
101 (32.9) |
|
8 - <12 |
59 (19.2) |
|
12 - <16 |
21 (6.8) |
|
Undisclosed |
5 (1.6) |
|
Duration asthma since first diagnosis (years) |
|
|
≤ 3 |
241 (78.5) |
|
> 3-6 |
34 (11.1) |
|
> 6 -9 |
21 (6.8) |
|
> 9 |
11 (3.6) |
|
Clinical state at first consult |
|
|
Having acute exacerbation |
94 (30.6) |
|
Stable |
213 (69.4) |
Table 1: characteristics of study population.
|
Variable |
Severe Asthma n=30 (%) |
Odds ratio |
95% confi. interval |
P value |
|
|
Lower |
Upper |
||||
|
Age (years) |
|||||
|
< 11 |
18 (11.1) |
0.7 |
0.3 |
1.6 |
0.4 |
|
>11 |
12 (14.8) |
||||
|
Gender |
|||||
|
Female |
16 (16.5) |
1.8 |
0.86 |
4.0 |
0.1 |
|
Male |
14 (9.6) |
||||
|
Overweight (W/A Zscore >2) |
|||||
|
Yes |
5 (9.62) |
0.7 |
0.25 |
1.96 |
0.49 |
|
No |
25 (13.1 |
||||
|
Undernourished (W/A Zscore < -2) |
|||||
|
Yes |
3 (60) |
11.7 |
1.8 |
73.1 |
0.001* |
|
No |
27 (11.34) |
||||
|
Adult smoker in household |
|||||
|
Yes |
1 (10.0) |
0.8 |
0.09 |
6.62 |
0.65 |
|
No |
23 (34) |
||||
|
Household fuel includes Gas |
|||||
|
Yes |
15 (9.04) |
0.4 |
0.18 |
0.89 |
0.02* |
|
No |
15 (19.48) |
||||
|
Household fuel is kerosene and firewood only |
|||||
|
Yes |
8 (21.62) |
2.9 |
1.13 |
7.8 |
0.02* |
|
No |
13 (8.44) |
||||
|
Social class |
|||||
|
Upper |
8 (6.96) |
0.3 |
0.14 |
0.81 |
0.01* |
|
Lower |
17 (17.92) |
||||
|
Known family history of atopy |
|||||
|
Yes |
21 (13.6) |
1.3 |
0.6 |
3.175 |
0.4 |
|
No |
9 (10.3) |
||||
|
Clinical state at 1st scheduled consult |
|||||
|
Asthma exacerbation |
12 (15.38) |
1.4 |
0.67 |
3.2 |
0.32 |
|
Stable |
18 (10.9) |
||||
|
Diagnosed at ≤ 2years |
|||||
|
Yes |
10 (14.9) |
1.4 |
0.62 |
3.2 |
0.37 |
|
No |
19 (10.9) |
||||
|
Duration of illness ≤ 2years |
|||||
|
Yes |
14 (8.6) |
0.3 |
0.1 |
0.83 |
0.01* |
|
No |
16 (19.75) |
||||
*=Statistically significant
Table 2: Factors associated with severe persistent asthma.
|
Variable |
Controlled Asthma n (%) |
Odds ratio |
95% confidence interval |
P value |
|
|
Lower |
Upper |
||||
|
Age(years) |
|||||
|
< 11 |
12 (20) |
0.48 |
0.2 |
0.994 |
0.04* |
|
>11 |
43 (34.1) |
||||
|
Gender |
|||||
|
Female |
19 (27.1) |
0.82 |
0.42 |
1.590 |
0.5 |
|
Male |
36 (31.03) |
||||
|
Overweight (W/A Zscore >2) |
|||||
|
Yes |
19 (42.2) |
2.1 |
1.04 |
4.3 |
0.03* |
|
No |
36 (25.5) |
||||
|
Undernourished (W/A Zscore < -2) |
|||||
|
Yes |
1 (25) |
0.7 |
0.08 |
7.76 |
0.8 |
|
No |
54 (29.6) |
||||
|
Adult smoker in household |
|||||
|
Yes |
4 (50) |
2.7 |
058 |
12.4 |
0.1 |
|
No |
47 (26.8) |
||||
|
Household fuel includes Gas |
|||||
|
Yes |
42 (27.1) |
0.58 |
0.26 |
1.3 |
0.1 |
|
No |
13 (39.3) |
||||
|
Household fuel is kerosene and firewood only |
|||||
|
Yes |
9 (29.03) |
1.04 |
0.4 |
2.4 |
0.09 |
|
No |
40 (28.1) |
||||
|
Social class |
|||||
|
Upper |
27 (27.6) |
0.84 |
0.4 |
1.6 |
0.61 |
|
Lower |
26 (30.9) |
||||
|
Known family history of atopy |
|||||
|
Yes |
36 (29.51) |
0.9 |
0.6 |
3.175 |
0.4 |
|
No |
19 (30.1) |
||||
|
Clinal state at 1st scheduled consult |
|||||
|
Asthma exacerbation |
15 (26.3) |
0.76 |
0.38 |
1.58 |
0.5 |
|
Stable |
40 (31.0) |
||||
|
Diagnosed at ≤ 2years |
|||||
|
Yes |
16 (30.7) |
0.1 |
0.03 |
0.26 |
0.000004* |
|
No |
39 (81.3) |
||||
|
Duration of illness ≤ 2years |
|||||
|
Yes |
37 (30.6) |
1.5 |
0.59 |
2.27 |
0.68 |
|
No |
18 (27.7) |
||||
*=Statistically significant
Table 3: Factors associated with controlled asthma in children.
4. Discussion
This study being the first of its kind in our center described the pattern of asthma, identified the risk factors associated with severe persistent asthma and the level of asthma control in our study cohort. The median age for physician diagnosed asthma in children in the present study was 4 years, which was comparable to the mean age at which asthma was diagnosed in the western world in the mid-nineties, although there has been a gradual decrease in age at which asthma is diagnosed overtime to 2.6 years in 2000 [19]. This decrease in age of first asthma diagnosis was attributed to an increase in awareness of asthma as a disease entity and improvement in guidelines for asthma diagnosis [19]. Malnourished children were significantly more likely to have severe persistent asthma than their well-nourished or overweight counterparts, while we found no association between asthma severity and overweight children with asthma. Taylor et al. [20] reported that obesity is associated with increased severity of asthma among adults with asthma. Another study [21] done among children found no association between nutritional status and severity of asthma, although in their cohort they only had one malnourished child and no patient with severe asthma, thereby limiting their ability to access severity of asthma among malnourished children with asthma. It is possible that increased severity of asthma in malnourished children seen in our study is an outcome of living with a severe chronic disease as opposed to malnutrition on its own increasing asthma severity. This could also explain our findings that children who were overweight were more likely to have controlled asthma. Although the literatures have shown that obesity has an intertwining relationship with asthma as it could lead to a decreased response to inhalational corticosteroids, severe asthma on the other hand can lead to exercise intolerance causing obesity asthma [22, 23].
Passive smoking assessed by the presence of one adult smoker within the household was 3.3% among our cohort, which is relatively low compared to >37% rates reported in two studies in USA among parents whose children have asthma [24, 25]. The low prevalence of passive smoking among our sample size could have limited our ability to identify the effects of passive smoking on asthma. The reason for this relatively low prevalence is likely due to the warmer temperatures of African climate when compared to colder temperatures of the temperate region as a result the uptake of smoking among the general population is much lower. Tobacco exposure leads to a higher prevalence of asthma, especially the severe form of asthma by direct toxicity of the airways, increased inflammatory damage of the lung epithelium predisposing to respiratory infection, increased sensitization to allergens and also poor response to steroid control for asthma patients [26].
Kerosene and firewood were used as cooking fuel by more than a third of the households. Kerosene and firewood, which are not clean domestic fuel as their combustion is inefficient contribute to harmful levels of household air pollution. When combusting they emit nitric oxide, Sulphur oxide, high levels of PM 2.5 and polycystic hydrocarbons whereas liquefied petroleum gas combusts more efficiently and is relatively cleaner with barely detectable levels of PM 2.5 emission [27, 28]. It is therefore not surprising that use of kerosene and firewood in households is associated with having severe persistent asthma while use of only gas is associated with a reduced likelihood of having severe persistent asthma. Exposure to air pollution distorts normal lung developmental process, causes lung epithelial damage and airway narrowing all of which would increase severity of asthma [29, 30]. There is a continuous need to make clean domestic fuel available to low- and middle-income countries and a special need to ensure that clean domestic fuel is used in families that have persons with chronic respiratory disease like asthma in order to prevent their disease from progressively worsening.
There was a relatively higher proportion of children from upper socioeconomic class 43% in the present study when compared to the 24% seen in the general outpatient clinic in our hospital in a previous study [31]. Physician diagnosed asthma may be higher among the upper class, but severe forms of asthma is however more among those from low socioeconomic class. Other literatures have reported a higher prevalence of asthma among people from low socioeconomic privileges and explained that this could be due to high prevalence of deprivation of social and economic determinants of health among the low socioeconomic class [32]. The upper socioeconomic class are more likely to have access to better health care, afford their medications, have better living conditions that is devoid of common triggers like cockroaches, house dust mites, indoor air pollution. They are also more educated with the likelihood to understand and comply to asthma action plans and implement environmental modifications necessary to improve asthma symptom control.
Prevalence of asthma has been known to be higher among those with family history of atopy due to a genetic predisposition [33, 34]. This study however showed no association between family history of asthma and severity of asthma similar to findings in another study by Santos et al. [33]. Besides the fact that both studies were conducted over a long time in an outpatient clinic setting, both used the same GINA classification methods for classifying asthma severity. Although other studies [35, 36] found a positive association between asthma severity and positive family history of atopy, these studies had some differences in methodology with the present study. While one used a different inclusion criterion which included all children that had a wheezing episode without necessarily being diagnosed of asthma while the other classified all persistent asthma as severe.
This study highlights the fact that the four cardinal symptoms of asthma are not usually present in the patients for a diagnosis of asthma to be made. On the other hand, although cough is the commonest symptom of asthma, it is hardly an only presenting symptom as no child in the present study was diagnosed of asthma with only one of the cardinal symptoms.
Surprisingly, most of our patients had persistent asthma much higher than (62.1 vs 18.2%) was reported among children attending a chest clinic in another Nigerian study [37]. The reason for this disparity is not very clear but a possible explanation could be a difference in hospital protocol for recruitment into the asthma register as the study center waits for a recurrence of asthma symptom especially in children before they are registered for possible asthma diagnosis in order to exclude children that had an episode of wheeze from other causes.
We found a low rate of controlled asthma, 50 (30%) among children referred to the outpatient respiratory clinic of our hospital. This is similar to another Nigerian study [38] which reported a control rate of 29% using the ACT of <19. Although much lower than the 82.9% reported by another Nigerian study among children attending a pulmonology clinic, the difference however, could be because they used a different grading criterion to classify control [39]. Asthma control rates of 54% in the USA and 61% in Netherland were reported among children with asthma [40, 41] which is also much higher than reported in this study. Factors that we identified to be associated with poor asthma control were Age <11yrs, underweight children and asthma diagnosis made at ≤ 2 years. Asthma control for young children depends a lot on the commitment of the caregiver to carry out the asthma action plan and their surveillance to be able to identify when the children are having exacerbation of their symptoms before they can take their asthma medications. Although the small airways of growing children could make them have more severe symptoms when compared to their adult counterparts. It has also been documented that children with a longer duration of asthma are more likely to have poor pulmonary function and airway remodeling [42] all of which could result in poor asthma control. The relationship between underweight and poor control is likely consequential with the latter resulting in the former.
5. Conclusion
The median age at first diagnosis of asthma in the present study was 4 years with majority of patients having intermittent asthma while the level of asthma control was poor. Use of clean domestic fuel and reduction of the effects of deprivation of social and economic determinants of health among the low socioeconomic class can improve control and reduce severity of asthma in children. There is a need for regular assessment of patients for effective institution of appropriate action plan in order to achieve good level of control of patients’ asthma.
References
- Liu AH, Covar RA, Spahn JD, et al. Childhood Asthma. In: Kliegman RM, Stanton BF, St Geme III, JN, Behrman RE, editor. Nelson Textbook of Paediatrics. 20th Philadelphia: Elsevier (2016): 1095-1115.
- Global Initiative for Asthma (GINA). Global strategy for Asthma management and prevention (2020).
- Asher MI, Montefort S, Björkstén, Bengt, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet (London, England) 368 (2006): 733-743.
- Masoli M, Fabian D, Holt S, et al. Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy 59 (2004): 469-478.
- Mannino DM, Homa DM, Akinbami LJ, et al. Survelliance for asthma: United States, 1980-1999. MMWR. MMWR Surveillance summaries 51 (2002): 1-13.
- Ozoh O, Aderibigbe S, Ayuk A, et al. The prevalence of asthma and allergic rhinitis in Nigeria: A nationwide survey among children, adolescents and adults. PLoS ONE (2019).
- Bousquet J KA. Global survelliance, prevention and control of chronic respiratory diseases: A comprehensive approach. Geneva: World Health Organization; Switzerland: WHO (2007).
- Dean BB, Calimlim BM, Kindermann SL, et al. The impact of uncontrolled asthma on absenteeism and health-related quality of life. The Journal of asthma 46 (2009): 861-866.
- Williams SA, Wagner S, Kannan H, et al. The association between asthma control and health care utilization, work productivity loss and health-related quality of life. Journal of occupational and environmental medicine 4 (2009): 780-785.
- Taylor DR, Bateman ED, Boulet L, et al. A new perspective on concepts of asthma severity and control. Eur Respir J European Respiratory Society 32 (2008): 545-554.
- Yawn BP, Brenneman SK, Allen-Ramey FC, et al. Assessment of asthma severity and asthma control in children. Pediatrics 118 (2006): 322-329.
- Colice GL. Categorizing asthma severity: an overview of national guidelines. Clinical medicine and research 2 (2004): 155-163.
- Liu AH, Zeiger R, Sorkness C, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol 119 (2007): 817-825.
- Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: A survey for assessing asthma control. J Allergy Clin Immunol 113 (2004): 59-65.
- Olusanya O, Okpere E, Ezimokhai M. The Importance of Social Class in Voluntary Fertility Control in a Developing Country. West African Journal of Medicine 4 (1985): 205-212.
- CDC Weight for Age Percentiles for Boys (2-20 years) Calculate Z-score and percentile (2020).
- CDC Weight for Age Percentiles for Girls (2 - 20 years) Calculate Z-score and percentile (2020).
- CDC/NCHS Infant Weight for Age Percentiles (2020).
- Radhakrishnan DK, Dell SD, Guttmann A, et al. Trends in the age of diagnosis of childhood asthma. J Allergy Clin Immunol 134 (2014): 1057-1062.
- Taylor B, Mannino D, Brown C, et al. Body mass index and asthma severity in the National Asthma Survey. Thorax 63 (2008): 14.
- Morishita RYM, Strufaldi MWL, Puccini RF. Clinical evolution and nutritional status in asthmatic children and adolescents enrolled in Primary Health Care. Rev. paul. Pediatr 33 (2015): 387-393.
- Ramratnam SK, Bacharier LB, Guilbert TW. Clinical management Review; Severe Asthma in Children. J Allergy Clin Immunol Pract 5 (2017): 889-889.
- Forno E, Lescher R, Strunk R, et al. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol 127 (2011): 741-749.
- Mahabee-Gittens M. Smoking in parents of children with asthma and bronchiolitis in a pediatric emergency department. Pediatr Emerg Care 18 (2002).
- Farber HJ, Knowles SB, Brown NL, et al. Secondhand tobacco smoke in children with asthma: sources of and parental perceptions about exposure in children and parental readiness to change. Chest 133 (2008): 1367-1374.
- Gonzalez-Barcala F, Pertega S, Sampedro M, et al. Impact of parental smoking on childhood asthma. J Pediatr 89 (2013): 294-299.
- Puzzolo E, Zerriffi H, Carter E, et al. Supply Considerations for Scaling Up Clean Cooking Fuels for Household Energy in Low- and Middle-Income Countries. GeoHealth 3 (2019): 370-390.
- Shen G, Hays MD, Smith KR, et al. Evaluating the Performance of Household Liquefied Petroleum Gas Cookstoves. Environmental science and technology JID - 0213155 52 (2018): 904-915.
- Trasande L, Thurston GD. The role of air pollution in asthma and other pediatric morbidities. J Allergy Clin Immunol 115 (2005): 689-699.
- Plopper CG, Fanucchi MV. Do urban environmental pollutants exacerbate childhood lung diseases? Environ Health Perspect 108 (2000): A252-A253.
- West BA, Onubogu UC, Okari TG, et al. Prevalence, Knowledge, Practice and Problems Associated with Breastfeeding among Mothers/Caregivers Attending a Paediatric Out-Patient Clinic in Port Harcourt, Nigeria. Journal of Dental and Medical Sciences 19 (2020): 20-27.
- Uphoff E, Cabieses B, Pinart M, et al. A systematic review of socioeconomic position in relation to asthma and allergic diseases. Eur Respir J 46 (2015): 364.
- Santos HLBS, Möller LG, Duarte NP J, et al. Risk Factor for Asthma Severity in Children: Family History, Atopic Sensitization or Rhinitis. J Allergy Clin Immunol 117 (2006): S100.
- Bjerg A, Hedman L, Perzanowski MS, et al. Family History of Asthma and Atopy: In-depth Analyses of the Impact on Asthma and Wheeze in 7- to 8-Year-Old Children. Pediatrics 120 (2007): 741.
- Pamela SH, Wakefield D, Cloutier MM. Risk factors for asthma and asthma severity in nonurban children in connecticut*. Chest 128 (2005): 3846-3853.
- Wilson NM, Doré CJ, Silverman M. Factors relating to the severity of symptoms at 5 yrs in children with severe wheeze in the first 2 yrs of life. The European respiratory journal 10 (1997): 346-353.
- Kuti BP. Asthma co-morbidities in Nigerian children: prevalence, risk factors and association with disease severity and symptoms control. Pan African Medical Journal 35 (2020): 18470.
- Ozoh OB, Ayuk AC, Ukwaja KN, et al. Asthma management and control in Nigeria: the asthma insight and reality Nigeria (AIRNIG) study. Expert Review of Respiratory Medicine 13 (2019): 917-927.
- Kuti BP, Omole KO, Kuti DK. Factors associated with childhood asthma control in a resource-poor center. Journal of family medicine and primary care 6 (2017): 222-230.
- Kansen HM, Le TM, Uiterwaal CSPM, et al. Prevalence and Predictors of Uncontrolled Asthma in Children Referred for Asthma and Other Atopic Diseases. J Asthma Allergy 13 (2020): 67-75.
- Liu AH, Gilsenan AW, Stanford RH, et al. Status of Asthma Control in Pediatric Primary Care: Results from the Pediatric Asthma Control Characteristics and Prevalence Survey Study (ACCESS). J Pediatr 157 (2010): 276-281.e3.
- Sears MR. Lung function decline in asthma. Eur Respir J 30 (2007): 411.


 Impact Factor: * 3.8
Impact Factor: * 3.8 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 