Clinical Characteristics and Incidence of Spontaneous Preterm Birth in Symptomatic COVID-19 Pregnant Women: An Observational Study
Article Information
Shruti Yarra1, Sindy Carolina Moreno1, Justin K To1, David Garry2, Ivan M Ngai1*
1Department of Obstetrics and Gynecology, Flushing Hospital Medical Center, New York, United States
2Department of Obstetrics, Gynecology and Reproductive Medicine, Stony Brook Medicine, Health Sciences Center, New York, United States
*Corresponding Author: Ivan M Ngai, Assistant Professor, New York Institute of Technology College of Osteopathic Medicine, Maternal-Fetal Medicine, Director of Obstetric Quality Improvement, Flushing Hospital Medical Center, Flushing New York, 4500 Parsons Blvd, Queens, NY 11355, United States
Received: 28 August 2020; Accepted: 04 September 2020; Published: 18 September 2020
Citation:
Shruti Yarra, Sindy Carolina Moreno, Justin K To, David Garry, Ivan M Ngai. Clinical Characteristics and Incidence of Spontaneous Preterm Birth in Symptomatic COVID-19 Pregnant Women: An Observational Study. Obstetrics and Gynecology Research 3 (2020): 199-206.
View / Download Pdf Share at FacebookAbstract
Background: There is limited data regarding COVID-19 and preterm birth. Most published studies do not exclude induced preterm deliveries or women at higher risk for preterm birth.
Objective (s): To determine the incidence of spontaneous preterm delivery in pregnancies complicated by maternal COVID-19 infection with no other independent risk factors for preterm birth.
Study design: This was an observational study of symptomatic women diagnosed with COVID-19 during the third trimester of pregnancy who delivered at Flushing Hospital Medical Center in Queens, New York between March 1 and May 1, 2020. IRB approval was obtained. Charts of symptomatic women who were confirmed COVID-19 positive by RT-PCR in the third trimester were reviewed. Patient information collected included gestational age at diagnosis of COVID-19, obstetrical history, comorbidities, x-ray findings, and blood work. Women with a history of preterm birth, short cervix in current pregnancy, and multiple gestations were excluded. Statistical evaluation for normally distributed continuous variables was done using a student’s t-test, and for dichotomous variables, a Chi-square analysis or Fisher’s exact test was used. A multiple regression analysis model was used to correlate the relationship of clinical variables to the gestational age at delivery.
Results: 24 pregnant women met initial criteria, 17 of which delivered in the time period stated. Preterm delivery rate in our sample size was 29% (n=5). Our institution’s preterm delivery rate for a similar time-period in 2019, using the same exclusion and inclusion criteria, was 2.7%. When comparing COVID-19 positive women that delivered preterm to COVID-19 positive women who delivered at term, the gestational age on admission was lower in the preterm cohort (weeks 34.1 ± 1.5 weeks versus 38.0 ± 1.7; p<0.001). M
Keywords
Preterm Delivery, Birth, COVID-19, Pregnancy, Risk Factors
Preterm Delivery articles Preterm Delivery Research articles Preterm Delivery review articles Preterm Delivery PubMed articles Preterm Delivery PubMed Central articles Preterm Delivery 2023 articles Preterm Delivery 2024 articles Preterm Delivery Scopus articles Preterm Delivery impact factor journals Preterm Delivery Scopus journals Preterm Delivery PubMed journals Preterm Delivery medical journals Preterm Delivery free journals Preterm Delivery best journals Preterm Delivery top journals Preterm Delivery free medical journals Preterm Delivery famous journals Preterm Delivery Google Scholar indexed journals Birth articles Birth Research articles Birth review articles Birth PubMed articles Birth PubMed Central articles Birth 2023 articles Birth 2024 articles Birth Scopus articles Birth impact factor journals Birth Scopus journals Birth PubMed journals Birth medical journals Birth free journals Birth best journals Birth top journals Birth free medical journals Birth famous journals Birth Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Pregnancy articles Pregnancy Research articles Pregnancy review articles Pregnancy PubMed articles Pregnancy PubMed Central articles Pregnancy 2023 articles Pregnancy 2024 articles Pregnancy Scopus articles Pregnancy impact factor journals Pregnancy Scopus journals Pregnancy PubMed journals Pregnancy medical journals Pregnancy free journals Pregnancy best journals Pregnancy top journals Pregnancy free medical journals Pregnancy famous journals Pregnancy Google Scholar indexed journals Risk Factors articles Risk Factors Research articles Risk Factors review articles Risk Factors PubMed articles Risk Factors PubMed Central articles Risk Factors 2023 articles Risk Factors 2024 articles Risk Factors Scopus articles Risk Factors impact factor journals Risk Factors Scopus journals Risk Factors PubMed journals Risk Factors medical journals Risk Factors free journals Risk Factors best journals Risk Factors top journals Risk Factors free medical journals Risk Factors famous journals Risk Factors Google Scholar indexed journals Preterm labor articles Preterm labor Research articles Preterm labor review articles Preterm labor PubMed articles Preterm labor PubMed Central articles Preterm labor 2023 articles Preterm labor 2024 articles Preterm labor Scopus articles Preterm labor impact factor journals Preterm labor Scopus journals Preterm labor PubMed journals Preterm labor medical journals Preterm labor free journals Preterm labor best journals Preterm labor top journals Preterm labor free medical journals Preterm labor famous journals Preterm labor Google Scholar indexed journals prostaglandins articles prostaglandins Research articles prostaglandins review articles prostaglandins PubMed articles prostaglandins PubMed Central articles prostaglandins 2023 articles prostaglandins 2024 articles prostaglandins Scopus articles prostaglandins impact factor journals prostaglandins Scopus journals prostaglandins PubMed journals prostaglandins medical journals prostaglandins free journals prostaglandins best journals prostaglandins top journals prostaglandins free medical journals prostaglandins famous journals prostaglandins Google Scholar indexed journals pregnant women articles pregnant women Research articles pregnant women review articles pregnant women PubMed articles pregnant women PubMed Central articles pregnant women 2023 articles pregnant women 2024 articles pregnant women Scopus articles pregnant women impact factor journals pregnant women Scopus journals pregnant women PubMed journals pregnant women medical journals pregnant women free journals pregnant women best journals pregnant women top journals pregnant women free medical journals pregnant women famous journals pregnant women Google Scholar indexed journals preterm birth articles preterm birth Research articles preterm birth review articles preterm birth PubMed articles preterm birth PubMed Central articles preterm birth 2023 articles preterm birth 2024 articles preterm birth Scopus articles preterm birth impact factor journals preterm birth Scopus journals preterm birth PubMed journals preterm birth medical journals preterm birth free journals preterm birth best journals preterm birth top journals preterm birth free medical journals preterm birth famous journals preterm birth Google Scholar indexed journals Obstetrical articles Obstetrical Research articles Obstetrical review articles Obstetrical PubMed articles Obstetrical PubMed Central articles Obstetrical 2023 articles Obstetrical 2024 articles Obstetrical Scopus articles Obstetrical impact factor journals Obstetrical Scopus journals Obstetrical PubMed journals Obstetrical medical journals Obstetrical free journals Obstetrical best journals Obstetrical top journals Obstetrical free medical journals Obstetrical famous journals Obstetrical Google Scholar indexed journals
Article Details
1. Background
Preterm labor is the leading cause of perinatal morbidity and mortality worldwide [1]. Infants born prematurely, defined as less than 37 weeks of pregnancy, not only have numerous health complications, but also add substantial short-term and long-term costs to the health care system [2]. Approximately 40% of preterm labor is a result of intraamniotic infection [3]. Viral infections can lead to a release of pro-inflammatory cytokines, chemokines, prostaglandins, and other effector molecules that result in the characteristic phenomena of labor such as uterine contractions and rupture of membranes. Unfortunately, there is limited data regarding preterm birth and symptomatic pregnant women who test positive for COVID-19, a viral infection that was declared a pandemic by the World Health Organization (WHO) in March of 2020. A meta-analysis by Di Mascio et al reported a preterm birth rate as high as 41.1% in the COVID-19 pregnancy population [4], but did not focus on a low-risk population. Flushing Hospital Medical Center (FHMC) is located in Queens, New York, which was an epicenter of the coronavirus pandemic in the United States [5]. As of August 2020, with 3028 positive cases of coronavirus per 100000, it was one of the worst affected areas in the state of New York. Given the limited but concerning data regarding preterm birth, our study objective was to determine the incidence of spontaneous preterm delivery in pregnancies complicated by maternal COVID-19 with no risk factors for preterm birth.
2. Methods
This was an observational study of symptomatic women diagnosed with COVID-19 during the third trimester of pregnancy who delivered at FHMC between March 1, 2020 and May 1, 2020. This study was reviewed and approved by the Institutional Review Board of Flushing Hospital Medical Center. All pregnant women who presented to FHMC labor and delivery units with signs or symptoms concerning for COVID-19 had nasopharyngeal swab samples taken after obtaining New York State Department of Health (NYSDOH) authorization. Results were available by the following day. COVID-19 status was diagnosed via SARS-CoV-2 rRT-PCR test, performed by the New York City Public Health Laboratory. Positive patients were then followed until delivery. Obstetrical management was provided by the standard of care. All sample collection, processing, and laboratory testing complied with WHO guidance [6]. Charts of these patients were reviewed for symptoms, vitals, white blood cell counts and other lab results, medications used for COVID-19 such as azithromycin or hydroxychloroquine, and chest x-rays. Additional neonatal collected information included neonatal intensive care unit (NICU) admission, number of days in NICU, comorbidities such as transient tachypnea of newborn, respiratory distress syndrome, and necrotizing enterocolitis.
Inclusion criteria included viable pregnancies greater or equal to 24 weeks of gestation where the mother was symptomatic (for example fever, cough, shortness of breath, and/or myalgias) and tested positive for COVID-19 on PCR. Exclusion criteria included women who were diagnosed with COVID-19 post discharge from FHMC, women who underwent labor induction at less than 37 weeks gestation, patients with risk factors for entering preterm labor such as a history of preterm labor [7], women with a history of short cervix [8], and multiple gestation pregnancies [9, 10]. Statistical evaluation for normally distributed continuous variables used a student’s t test and for dichotomous variables a Chi square analysis or Fisher’s exact test was used. For variables which were not normally distributed, Mann-Whitney testing was utilized. Multiple regression analysis model was used to correlate the relationship of clinical variables to the gestational age at delivery. Statistical analysis used MedCalc version 19.2.1 (MedCalc Software, Ostend, Belgium). P <0.05 was considered significant.
3. Results
During the study period, 24 symptomatic COVID-19 positive pregnant women were evaluated and 7 women did not deliver within the time frame stated. There were 17 women with complete pregnancy outcome data who met inclusion criteria. The maternal age range was 23 to 41 years old and the mean gestational age of the cohort was 36.9 ± 2.4 weeks at diagnosis. Spontaneous preterm birth, with delivery < 37 weeks, occurred in 5 women (29%). None of these women had risk factors for preterm birth, including a history of preterm delivery, short cervix, or multiple gestations. When comparing COVID-19 positive women that delivered preterm to those that delivered at term, the gestational age on admission was lower in the preterm cohort (38.0 ± 1.7 weeks versus 34.1 ± 1.5 weeks; p<0.001) (Table 1, Figure 1). The remainder of maternal demographics were similar between cohorts including maternal white blood cell count (median values 7.1 thousand/µL versus 7.7 thousand/µL; p=0.52) and maternal lymphocyte percentage (19.4 ± 9.1% versus 13.8 ± 4.1%; p=0.21). Mode of delivery and neonatal outcomes were similar between groups. The preterm group birth weight was smaller (3322.5g ± 541 versus 2245g ± 250; p<0.01) which was expected. The preterm group had more transient tachypnea then the term group and no neonates tested positive for COVID-19. One neonate, born at 32 weeks, had sepsis and was in NICU for 19 days (Table 1).
A multiple regression analysis using clinical parameters
available on admission for the prediction of gestational age of delivery was modeled. The clinical admission variables which were significant in suggesting the gestational age at birth were the gestational age on admission (Coefficient 0.95, (95% CI 0.86 - 1.03; p<0.001), white blood count (Coefficient 0.142, 95% CI 0.07 - 0.22; p=0.002), and lymphocyte percentage (Coefficient 0.038, 95% CI 0.01 - 0.06; p=0.008). (Table 2) The same clinical variables obtained on admission, gestational age on admission, white blood count and lymphocyte percentile, remained significant when predicting preterm birth in the model.
|
All patients n=17 |
Full Term Deliveries n=12 |
Preterm Deliveries n=5 |
|
|
Age (years) |
32 ± 5.87 |
32.66 ± 5.33 |
30.4 ± 7.4 |
|
BMI (kg/m2) |
30.68 ± 3.62 |
29.94 ± 3.5 |
32.44 ± 3.4 |
|
Parity |
|||
|
Multiparous |
8(47%) |
5 (41%) |
3 (60%) |
|
Nulliparous |
9 (52%) |
7 (58%) |
2 (40%) |
|
Gravida |
|||
|
> 1 |
12(70%) |
9(75%) |
3(60%) |
|
= 1 |
5(29%) |
3(25%) |
2(40%) |
|
Gestational age at diagnosis (weeks) |
36.87± 2.4 |
38.0 ± 1.7 |
34.1 ± 1.5** |
|
Ethnicity |
|||
|
White |
1 (6%) |
0 (0%) |
1 (20%) |
|
Hispanic |
11 (65%) |
7 (58%) |
4 (80%) |
|
Asian |
5 (29%) |
5 (41%) |
0 (0%) |
|
Gestational age at delivery (weeks) |
37.38 ± 2.35 |
38.5 ± 1.38 |
34.54 ± 1.59 |
|
Co-morbidities |
|||
|
Diabetes |
1(5%) |
1(8%) |
0(0%) |
|
Hypertension |
1(5%) |
0(0%) |
1(20%) |
|
Asthma |
1(5%) |
0(0%) |
1(20%) |
|
Symptoms |
|||
|
Fever |
7(41%) |
5(41%) |
2(40%) |
|
Cough |
17(100%) |
12(100%) |
5(100%) |
|
Shortness of Breath |
2(11%) |
1(6%) |
1(20%) |
|
Medications in management |
|||
|
Plaquenil |
8(47%) |
5(41%) |
3(60%) |
|
Azithromycin |
15(88%) |
11(91%) |
4(80%) |
|
Rocephin |
9(52%) |
6(50%) |
3(60%) |
|
Pregnancy outcome |
|||
|
Cesarean Delivery |
7(41%) |
5(41%) |
2(40%) |
|
Vaginal delivery |
9(52%) |
6(50%) |
3(60%) |
|
VBAC |
1(5%) |
1(8%) |
0(0%) |
|
EBL |
441 ± 183.1 |
458 ± 191.7 |
400 ± 173.2 |
|
Postpartum days total |
2.12 ± 1.27 |
2.33 ± 1.23 |
1.09 ± 1.5 |
|
Neonatal outcome |
|||
|
Birth weight (grams) |
3053.1 ± 989 |
3322.5 ± 541 |
2245 ± 250** |
|
Apgar score at 5 minutes |
9 ± 2.1 |
9 ± 0 |
9 ± 4.02 |
|
TTN |
4(23%) |
1(8%) |
3(60%)** |
|
Sepsis |
1(5%) |
0 |
1(20%) |
|
IVH |
0 |
0 |
1(20%) |
|
NICU admission |
10(58%) |
6(50%) |
4(80%) |
Continuous variables are mean ± SD. Categorical variables are n (%). VBAC = Vaginal Birth After Cesarean Delivery. EBL = Estimated Blood Loss. TTN – Transient Tachypnea of Newborn. IVH = Intraventricular Hemorrhage. NICU = Neonatal Intensive Care Unit ** p < 0.01
Table 1: Patient demographics and pregnancy outcomes comparing term deliveries with those that delivered preterm (<37 weeks).
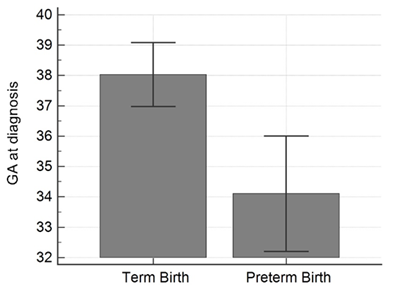
Figure 1: Gestational age on admission comparing those women that delivered full term versus those women delivering preterm (< 37 weeks).
|
Variable |
Coefficient |
95% Confidence Interval |
p-value |
|
|
Lower |
Upper |
|||
|
Parity |
-0.079 |
-0.316 |
0.158 |
0.475 |
|
Maternal Age |
0.031 |
-0.015 |
0.077 |
0.168 |
|
Gestational age at diagnosis |
0.945 |
0.858 |
1.031 |
<0.001 |
|
Lowest O2 saturation % (room air) |
-0.011 |
-0.083 |
0.062 |
0.746 |
|
Initial WBC Count |
0.142 |
0.065 |
0.219 |
0.002 |
|
Temperature maximum |
0.028 |
-0.142 |
0.198 |
0.722 |
|
Lymphocytes count % |
0.038 |
0.012 |
0.063 |
0.008 |
O2=oxygen
Table 2: Multiple regression analysis of clinical parameters available on admission for the prediction of gestational age of delivery.
4. Discussion
The preterm delivery rate in COVID-19 infected symptomatic pregnant women was higher than anticipated. Our institution’s preterm delivery rate for a similar time period (March through May 2019), using the same inclusion and exclusion criteria, was 2.7%. In 2018, preterm birth affected 1 of every 10 infants born in the United States [11] and 8.8% in New York City [12] and this rate did not exclude iatrogenic preterm birth or women with risk factors for preterm birth such as short cervix, history of preterm birth, infection, or multiple gestation. Though a small sample size, our finding of a 29% preterm delivery rate in a low-risk cohort is clinically significant and relevant to the current pandemic. COVID-19 infection diagnosed during the third trimester should be considered a significant risk factor for preterm birth in the low-risk population. Prior data regarding COVID-19 infection and preterm delivery is limited. Two case series studied preterm birth in pregnant women with COVID-19, [13, 14] but did not exclusively examine spontaneous preterm birth in low-risk populations. A meta-analysis by Di Mascio et al reported a preterm birth rate as high as 41.1% in the COVID-19 pregnancy population [4], but COVID-19 confirmation was variable and the study did not focus on a low-risk population.
Our observational study is important to the literature surrounding COVID-19 in pregnancy given its focus on a low-risk population for preterm birth. Analysis of the clinical admission parameters in symptomatic COVID-19 positive patients in our study shows that a lower gestational age on admission, lower maternal white blood cell count, and lower total lymphocyte count are possible risk factors for preterm delivery in this population. These same variables remain significant when predicting preterm birth in the multivariable regression model. These variables might be clinical indicators of poor prognosis in the COVID 19 pregnant population. Future prospective trials should focus on these variables further, and our findings suggest that a symptomatic COVID-19 positive pregnant woman presenting for evaluation during the third trimester may require prolonged observation and potential intervention to improve pregnancy outcome prognosis.
We surmise that COVID-19 infection may act in a similar manner to other viral infections in causing preterm labor. Other viral infections can cause an inflammatory response in the host which results in the release of cytokines, chemokines prostaglandins, and other effector molecules. The inflammatory response is mediated by specific receptors on mononuclear phagocytes, decidual cells, cervical epithelia, and trophoblasts. Infection, even subclinical, can release cytokines such as interleukin-1 beta which can promote a series of responses that include increased synthesis of other cytokines such as IL-6, IL-8, and tumor necrosis factors alpha (TNF- alpha) 2) proliferation, activation, and migration of leukocytes. Release of cytokines can also cause modifications in extracellular matrix proteins and mitogenic cytotoxic effects such as fever and acute phase response [15]. IL-1 also promotes prostaglandin formation in many tissues, including myometrium decidua, and amnion [16]. This can lead to the characteristic phenomenon of labor, uterine contractions and rupture of fetal membranes. The importance of prostaglandins in infection-mediated preterm birth is supported by the observation that prostaglandin inhibitors can reduce the rate of LPS-induced preterm birth in both the mouse and non-human primate, and inhibition of cyclooxygenase-2 prevents inflammation-mediated preterm labor in mouse studies [17]. Though it is likely that COVID-19 infection may lead to a similar cytokine and prostaglandin release, future research will be necessary to elucidate the specific mechanisms. The strength of our study is that all COVID-19 infections were confirmed by nasopharyngeal RT-PCR testing, which is the gold standard [6]. Additionally, our study focused on the low-risk population for preterm birth. The weaknesses of our study include a small sample size at a single institution, which decreases the generalizability of our findings.
5. Conclusion
Symptomatic COVID-19 infection seems to be a significant risk factor for spontaneous preterm delivery, especially if the gestational age on admission is below 37 weeks, the white blood cell count is low, and the lymphocyte percentage is decreased. Special attention should be given to this population should they present with preterm labor symptoms.
References
- Richter LL, Ting J, Muraca GM, et al. Temporal trends in neonatal mortality and morbidity following spontaneous and clinician-initiated preterm birth in Washington State, USA: a population-based study. BMJ open (2020).
- Petrou S. OBGYN. Obstetrics and Gynecology (2020).
- Agrawal V, Hirsch E. Intrauterine infection, and preterm labor. Seminars in fetal & neonatal medicine (2012).
- Di Mascio D, Khalil A, Saccone G, et al. Outcome of Coronavirus spectrum infections (SARS, MERS, COVID 1 -19) during pregnancy: a systematic review and meta-analysis. American journal of obstetrics & gynecology MFM (2020).
- NYC Department of Heath. Coronavirus disease 2019 Data.
- World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. Interim guidance (2020).
- Kazemier BM, Buijs PE, Mignini L, et al. Impact of obstetric history on the risk of spontaneous preterm birth in singleton and multiple pregnancies: a systematic review. BJOG 121 (2014): 1197-1209.
- Ville Y, Rozenberg P. Predictors of preterm birth. Best Pract Res Clin Obstet Gynaecol 52 (2018): 23-32.
- Stock S, Norman J. Preterm and term labour in multiple pregnancies. Semin Fetal Neonatal Med 15 (2010) : 336-341.
- Fuchs F, Senat MV. Multiple gestations and preterm birth. Semin Fetal Neonatal Med 21 (2016): 113-120.
- Preterm Birth. Centers for Disease Control and Prevention (2020).
- March of Dimes (2020).
- Favre G, Pomar L, Musso D, et al. 2019-nCoV epidemic: what about pregnancies?. Lancet (London, England) (2020).
- Wong SF, Chow KM, Leung TN, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. American journal of obstetrics and gynecology (2020).
- Chapter 42: Preterm Labor. AccessMedicine (2020).
- Nadeau-Vallee M, Obari D, Beaudry-Richard A, et al. Preterm Birth and Neonatal Injuries: Importance of Interleukin-1 and Potential of Interleukin-1 Receptor Antagonists. Curr Pharm Des 23 (2017): 6132-6141.
- Gross G, Imamura T, Vogt SK, et al. Inhibition of cyclooxygenase-2 prevents inflammation-mediated preterm labor in the mouse. Am J Physiol Regul Integr Comp Physiol 278 (2000): R1415-R1423.


 Impact Factor: * 3.2
Impact Factor: * 3.2 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 