Clinical Response to Osimertinib Compared to Other Tyrosine Kinase Inhibitors and Chemotherapy in Non-Small Cell Lung Cancer
Article Information
Sánchez-Ríos Carla Paola1*, Martínez-Herrera José Fabián2, Guzmán-Casta Jordi1, Riera-Sala Rodrigo1, Guzmán-Huesca Jorge3, Carrasco-CaraChards Sonia4, Martínez-Barrera Luis Manuel1, Alatorre-Alexander Jorge Arturo1, Rodríguez-Cid Jerónimo Rafael1
1Clinic of Thoracic Oncology, Instituto Nacional de Enfermedades Respiratorias, Dr. Ismael Cosio Villegas, Ciudad de México, México
2Cancer Center of Medical Center ABC (American British Cowdray)
3Bonita Community Health Center, Inc. Bonita Springs, Florida, USA
4Facultad de Medicina, Universidad Nacional Autónoma de México, Ciudad de México, México
*Corresponding Author: Dr. Sánchez-Ríos Carla Paola, Clinic of Thoracic Oncology, Instituto Nacional de Enfermedades Respiratorias, Dr. Ismael Cosio Villegas, Ciudad de México, México
Received: 10 August 2020; Accepted: 10 September 2020; Published: 20 November 2020
Citation: Sánchez-Ríos Carla Paola, Martínez-Herrera José Fabián, Guzmán-Casta Jordi, Riera-Sala Rodrigo, Guzmán-Huesca Jorge, Carrasco-CaraChards Sonia, Martínez-Barrera Luis Manuel, Alatorre-Alexander Jorge Arturo, Rodríguez-Cid Jerónimo Rafael. Clinical Response to Osimertinib Compared to Other Tyrosine Kinase Inhibitors and Chemotherapy in Non-Small Cell Lung Cancer. Archives of Clinical and Medical Case Reports 4 (2020): 1108-1115.
View / Download Pdf Share at FacebookAbstract
Osimertinib targets the same activating mutations in the EGFR (exon 19 and L858R of exon 21) as other target therapies but it also has action over the T790M mutation (the most frequently resistance mutation found in these tumors) and has better efficacy than 1st and 2nd generation TKI at the Central Nervous System (CNS) level. In Mexico has a higher incidence alterations of the EGFR gene are higher, being the most frequent molecular alteration in up to forty percent of patients with advanced NSCLC and the mutation resistance in T790M is 50% in this group. Here we present 3 cases of patients who received Osimertinib as a 2nd line therapy for metastatic NSCLC obtaining a complete response in one of them.
Keywords
Osimertinib; Non-small lung cancer; Tyrosine kinase inhibitors; Chemotherapy
Osimertinib articles; Non-small lung cancer articles; Tyrosine kinase inhibitors articles; Chemotherapy articles
Osimertinib articles Osimertinib Research articles Osimertinib review articles Osimertinib PubMed articles Osimertinib PubMed Central articles Osimertinib 2023 articles Osimertinib 2024 articles Osimertinib Scopus articles Osimertinib impact factor journals Osimertinib Scopus journals Osimertinib PubMed journals Osimertinib medical journals Osimertinib free journals Osimertinib best journals Osimertinib top journals Osimertinib free medical journals Osimertinib famous journals Osimertinib Google Scholar indexed journals Non-small lung cancer articles Non-small lung cancer Research articles Non-small lung cancer review articles Non-small lung cancer PubMed articles Non-small lung cancer PubMed Central articles Non-small lung cancer 2023 articles Non-small lung cancer 2024 articles Non-small lung cancer Scopus articles Non-small lung cancer impact factor journals Non-small lung cancer Scopus journals Non-small lung cancer PubMed journals Non-small lung cancer medical journals Non-small lung cancer free journals Non-small lung cancer best journals Non-small lung cancer top journals Non-small lung cancer free medical journals Non-small lung cancer famous journals Non-small lung cancer Google Scholar indexed journals cancer articles cancer Research articles cancer review articles cancer PubMed articles cancer PubMed Central articles cancer 2023 articles cancer 2024 articles cancer Scopus articles cancer impact factor journals cancer Scopus journals cancer PubMed journals cancer medical journals cancer free journals cancer best journals cancer top journals cancer free medical journals cancer famous journals cancer Google Scholar indexed journals Tyrosine kinase inhibitors articles Tyrosine kinase inhibitors Research articles Tyrosine kinase inhibitors review articles Tyrosine kinase inhibitors PubMed articles Tyrosine kinase inhibitors PubMed Central articles Tyrosine kinase inhibitors 2023 articles Tyrosine kinase inhibitors 2024 articles Tyrosine kinase inhibitors Scopus articles Tyrosine kinase inhibitors impact factor journals Tyrosine kinase inhibitors Scopus journals Tyrosine kinase inhibitors PubMed journals Tyrosine kinase inhibitors medical journals Tyrosine kinase inhibitors free journals Tyrosine kinase inhibitors best journals Tyrosine kinase inhibitors top journals Tyrosine kinase inhibitors free medical journals Tyrosine kinase inhibitors famous journals Tyrosine kinase inhibitors Google Scholar indexed journals Chronic kidney disease articles Chronic kidney disease Research articles Chronic kidney disease review articles Chronic kidney disease PubMed articles Chronic kidney disease PubMed Central articles Chronic kidney disease 2023 articles Chronic kidney disease 2024 articles Chronic kidney disease Scopus articles Chronic kidney disease impact factor journals Chronic kidney disease Scopus journals Chronic kidney disease PubMed journals Chronic kidney disease medical journals Chronic kidney disease free journals Chronic kidney disease best journals Chronic kidney disease top journals Chronic kidney disease free medical journals Chronic kidney disease famous journals Chronic kidney disease Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Chemotherapy articles Chemotherapy Research articles Chemotherapy review articles Chemotherapy PubMed articles Chemotherapy PubMed Central articles Chemotherapy 2023 articles Chemotherapy 2024 articles Chemotherapy Scopus articles Chemotherapy impact factor journals Chemotherapy Scopus journals Chemotherapy PubMed journals Chemotherapy medical journals Chemotherapy free journals Chemotherapy best journals Chemotherapy top journals Chemotherapy free medical journals Chemotherapy famous journals Chemotherapy Google Scholar indexed journals EGFR gene articles EGFR gene Research articles EGFR gene review articles EGFR gene PubMed articles EGFR gene PubMed Central articles EGFR gene 2023 articles EGFR gene 2024 articles EGFR gene Scopus articles EGFR gene impact factor journals EGFR gene Scopus journals EGFR gene PubMed journals EGFR gene medical journals EGFR gene free journals EGFR gene best journals EGFR gene top journals EGFR gene free medical journals EGFR gene famous journals EGFR gene Google Scholar indexed journals
Article Details
1. Introduction
Non-Small Cell Lung Cancer (NSCLC) is the leading cause of death among malignancies in the world and in advanced stages carries a somber prognosis [1]. Around 15 years ago the first therapies targeting alterations in the EGFR gene, like a group of deletions of the exon 19 or the punctual mutation of the exon 21 (L858R) were approved, changing the outlook of this sub-group of patients [2]. Current results of a Phase III multicenter research clinical trial reported that Osimertinib, a 3rd generation inhibitor of the tyrosine kinase (TKI) is superior in terms of survival and toxicity compared to 1st generation TKI therapy as a standard of treatment [3]. In the FLAURA trial, patients with advanced NSCLC who received Osimertinib as the initial treatment lived in average 7 months longer than patients who received Erlotinib or Gefitinib. Moreover the improvement in survival did not have a negative impact on the safety of the treatment, and an increase in the occurrence of significant side effects was not observed in patients treated with Osimertinib [4]. The FDA (Food and Drug Administration in the United States) approved as a first line treatment for people with advanced NSCLC with specific mutations in the EGFR gene [5].
Osimertinib targets the same activating mutations in the EGFR (exon 19 and L858R of exon 21) as other target therapies but it also has action over the T790M mutation (the most frequently resistance mutation found in these tumors) and has better effcacy than 1st and 2nd generation TKI at the Central Nervous System (CNS) level [6].
In Mexico has a higher incidence alterations of the EGFR gene are higher than those in Caucasian populations, being the most frequent molecular alteration in up to forty percent of patients with advanced NSCLC [7]. Here we present 3 cases of patients who received Osimertinib as a 2nd line therapy for metastatic NSCLC obtaining a complete response in one of them.
2. Clinical Cases
|
Case # 1 |
Case # 2 |
Case # 3 |
|
|
Demographics and clinical data |
Age at Diagnosis 64 Male No Smoking history No other smoke exposure Histopathology: Well differentiated Lung Adenocarcinoma |
Age at Diagnosis: 60 Female 40 pack year history No other smoke exposure Histopathology: Large cell, micropapillary non mucinous Lung Adenocarcinoma CK7+ TTF1+ |
Age at Diagnosis: 79 Female 20 pack year history No other smoke exposure Histopathology: Acinar Lung Adenocarcinoma CK7+ Napsin A+ TTF1+ |
|
date of initial diagnosis |
May 15th,2014 |
April 23rd, 2018 |
October 31st, 2016 |
|
EGFR mutation |
Exon 19 |
Exon 21 |
Exon 19 |
|
Site of metastasis |
Multiple pulmonary lesions |
T4aNxM1c, CNS, Bone |
T4NxM1b, Pleura, Bone |
|
previous therapy |
First line Gefitinib Second line Paclitaxel + Carboplatin + Gefitinib |
Afatinib for 3 months and Brain Radiotherapy, posterior G1 and G3 toxicity. Starts Gefitinib on October 2nd, 2018. In March 2019 GGO positive, Bronchoalveolar Lavage (BAVL) with biopsy and cultures are obtained due to the suspicion of an infectious process. T790M was documented. In July of 2019 progression of the disease in the lungs is observed. |
Gefitinib for 2 months with dose adjusted due to cutaneous toxicity G2. September of 2017 due to progression of the disease in the lungs patient is given 4 cycles of chemotherapy. Gefitinib was also given from January 2017 through December 2018. In January of 2019 chemotherapy is started again and signs of progression of the disease are found in September of 2019. |
|
time to response to first line of treatment |
3 months |
6 months (Partial Response) |
4 months (Partial Response 90%) |
|
Toxicity to first line of treatment |
Gefitinib – Rash G1 |
Afatinib (Acneiform rash G2, Diarrhea G3-4) Gefitinib (Acneiform Rash G1, Diarrhea G2, Transaminitis G1) |
Gefitinib Acneiform Rash G3 Diarrhea G3 Mucositis G1 |
|
date of progression of the disease |
09.12.2016 |
07.03.2019 (Progression Free Survival 12 months) |
09.31.2017 (Progression Free Survival 10 months) |
|
progression site |
Lung and Brain |
Contralateral thoracic lymph nodes |
Bilateral Pulmonary lesions |
|
start date of osimertinib |
9.26.2017 |
07.01.2019 |
10.28.2019 |
|
radiographic response to osimertinib |
Partial Response |
Complete Response |
Partial Response at 80% |
|
Ttime to response to OSIMERTINIB |
3 Months |
1 Month |
1.5 Months |
|
ToXICITY OF OSIMERTINIB |
None |
Acneiform Rash G1 Diarrhea G2 Epigastric Pain G3 |
Fatigue G1 Diarrhea G3 |
|
PROGRESSION FREE SURVIVAL TO juNE 2020 |
21 Months |
NA |
NA |
|
OVERALL SURVIVAL TO junio 2020 |
72 Months |
26 Months |
41 Months |
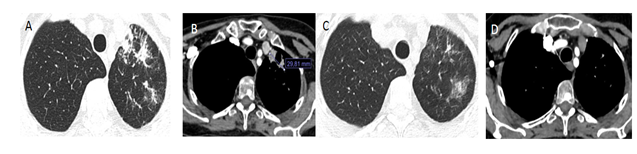
Figure 1 A and B: Images of Chest CAT Scan with pulmonary and soft tissue windows of patient number 2, both at the start of treatment with Osimertinib. C and D. Images of Chest CAT Scan with pulmonary and soft tissue windows showing complete response to treatment at month 12. Patient remains asymptomatic.
3. Discussion
Osimertinib is an oral 3rd generation irreversible inhibitor of the EGFR receptor with significant activity against T790M and less activity against EGFRwt [11,12]. Osimertinib was first approved for the treatment of metastatic non microcytic lung cancer with T790M positive expression and progression to target therapy with first generation agents, based on the results of the AURA trial and suggesting increased effcacy in the CNS compared to platinum based chemotherapy [13, 14].
In that study, Osimertinib achieved an Objective Response Rate (ORR) of 61% and a Free Survival Progression of 9.6 months and established a standard dose of 80 mgs/day for posterior studies. Phase 2 of the AURA trial confirmed both safety and effcacy of Osimertinib as treatment for progression to TKI agents in patients with a T790M mutation [15, 16].
AURA 3 study compared Osimertinib with Platinum based chemotherapy and Pemetrexed in patients with NSCLC EGFR T790M with progression to first line therapy. Results obtained widely favored Osimertinib, reaching 71% ORR versus 31% for the other therapies and a Progression Free Survival of 10.1 months vs 4.4 (HR= 0.30; CI: 0.23-0.41 p<0.001). Osimertinib also had better tolerance as seen by a lower incidence of untoward effects grade 3 (G3) in comparison to chemotherapy (23% vs 47%) [9]. The joint analysis of the AURA and AURA 2 trials confirmed the effcacy of Osimertinib in patient with the T790M mutation reporting an overall survival of 26.8 months and an overall survival rate during the first year of treatment of 80% [17].
Given the results of previous studies and with the intention to avoid the development of resistance mediated by the T790M mutation, a new trial (FLAURA) was launched comparing Osimertinib versus first generation TKI (Gefitinib, Erlotinib) in 556 patients with NSCLC and EGFR mutations, no previously treated. All patients had deletion of exon 19 or mutation L858R during randomization. The primary objective was Progression Free Survival, which favored Osimertinib (18.9 versus 10.2 months, HR: 0.46, 95% CI: 0.37-0.57; p<0.0001) without difference in the rate of Objective Response (80% vs 76% OR 1.27; 95% CI: 0.85-1.90; p=0.24) and the recently reported benefit of overall survival (38.6 vs 31.8 months, HR 0.799; 95.05% CI; p=0.0462 and Overall survival at years 1, 2 and 3 were 89%, 83% and 74% in the Osimertinib group as compared to 59%, 54% and 44% respectively in the Gefitinib/Erlotinib group [10]. Toxicity profile also was better having less untoward effects grade 3 and 4 (34% vs 45%). With these results Osimertinib was granted an indication as a first line treatment independent of the T790M mutation status and also received approval as a potential option for second line treatment after progression of the disease on first generation TKI treated patients but in this instance only those with a T790M mutation [18].
The FLAURA trial also showed good effcacy of Osimertinib against lesions of the Central Nervous System (CNS). In patients with CSN lesions the Free Survival Progression was not reached vs 13.9 months obtained with Gefitinib/Erlotinib and the appearance of new lesions was also better in the Osimertinib arm (12% vs 30%) supporting the protective role of Osimertinib in regards with the appearance of new lesions [19].
The most common mechanism of resistance to the first generation TKI is the EGFR T790M mutation [20, 21]. In the case of Osimertinib, mechanisms dependent or independent of the EGFR have been identified [22-24]. The heterogeneity of the tumor promotes the coexistence of multiple molecular alterations that influence the possible mechanisms of resistance to Osimertinib. The identified mechanisms of resistance to Osimertinib differ whether this has been used as a first or as a second line of treatment [25].
When there is progression to the first line of treatment, the mutations that include the EGFR receptor are present in 6 to 10% of the cases, with C797, L718, G724S and S768I being the most common. Other mechanisms are independent of the EGFR as the amplification of the MET which is present between 7 and 15% of the cases or the amplification of HER2 in 2% of them. Up to 15% present histologic transformation to squamous cell or small cell carcinoma (the latter through the loss of the function of gene RB and alterations of the p53) while still in 40 to 50% of the cases of resistance to Osimertinib as a first line agent, no specific mechanism has been identified.
4. Conclusion
In the case of progression to subsequent lines of treatment, the resistance to Osimertinib dependent of EGFR is present in 10 to 26% of the cases with the most frequent mutations being those in the C797, L792, G796 and L718 alleles and insertions in the exon 30. Of the mechanisms of resistance independent of the EGFR, the amplification of the MET, HER2 and PI3KCA are present in 15%, 5% and 5% respectively. The appearance of fusion genes including FGFR3, NTRK, RET, ALK and BRAF is present in 3-10% of the cases [25]. We present 3 cases of patients with NSCLC and EGFR mutations which developed progression to therapy with first and second generation TKI and posteriorly received Osimertinib as a subsequent line of systemic treatment with a good clinical and radiological response and progression free survival benefit similar to what has been reported in the literature.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68 (2018): 394-424.
- Lee DH. Treatments for EGFR-mutant non-small cell lung cancer (NSCLC): The road to a success, paved with failures. Pharmacol Ther 174 (2017): 1-2.
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 382 (2020): 41-50.
- Mezquita L, Varga A, Planchard D. Safety of osimertinib in EGFR-mutated non-small cell lung cancer. Expert Opin Drug Saf 17 (2018): 1239-1248.
- https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-osimertinib-first-line-treatment-metastatic-nsclc-most-common-egfr-mutations
- Shetty V, Babu S. Management of CNS metastases in patients with EGFR mutation-positive NSCLC. Indian J Cancer 56 (2019): S31-S37.
- Sánchez Ríos CP, et al. Clinical-epidemiological and molecular description of lung cancer in a national reference center. Neumol Cir Torax 78 (2019): 356-362.
- Arrieta O, Cardona AF, Martín C, et al. Updated Frequency of EGFR and KRAS Mutations in Non Small-Cell Lung Cancer in Latin America: The Latin-American Consortium for the Investigation of Lung Cancer (CLICaP). J Thorac Oncol 10 (2015): 838-843.
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 372 (2015): 1689-1699.
- Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 376 (2017): 629-640.
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N Engl J Med 378 (2018): 113-125.
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 4 (2014): 1046-1061.
- Mok T, Ahn M-J, Han J-Y, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: data from a randomized phase III trial (AURA3). J Clin Oncol 35 (2017): 9005.
- Mok TS, Wu Y-L, Ahn M-J et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 376 (2017): 629-640.
- Goss G, Tsai C-M, Sheperd F, et al. MA16.11 CNS response to osimertinib in patients with T790M-positive advanced NSCLC:pooled data from two phase II trials. J Thorac Oncol 12 (2017): S440-S441.
- Yang J. C-H, Ahn M-J, Kim D-W, et al. Osimertinib in pretreated T790M-positive advanced non-smal-cell lung cancer: AURA study phase II extension component. J Clin Oncol 35 (2017): 1288-1296.
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single arm, phase 2 study. Lancet Oncol 17 (2016): 1643-1652.
- Ahn MJ, Tsai CM, SHepherd FA, et al. Osimertinib in patients with T790M mutation-positive, advanced non-small cell lung cáncer: long term follow-up from a pooled analysis of 2 phase 2 studies. Cancer 125 (2019): 892-901.
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29 (2018): iv192-iv237.
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small cell lung cáncer. J Clin Oncol 36 (2018): 3290-3297.
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cáncer: distinct natural history of patients with tumours harboring the T790M mutation. Clin Cancer Res 17 (2011): 1616-1622.
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cáncer. Clin Cancer Res 19 (2013): 2240-2247.
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 21 (2015): 560-562.
- Kim TM, Song A, Kim DW, et al. Mechanisms of acquired resistance to AZD9291: a mutation-selective, irreversible EGFR inhibitor. J Thorac Oncol 10 (2015): 1736-144.
- Planchard D, Loriot Y, André F, et al. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann Oncol 26 (2015): 2073-2078.
- Leonetti A. Sharma S, Minari R, et al Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cáncer, Br J Cancer (2019).


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
