Comparative Studies on Polyamine Levels in Plasma and Urine of Androgenic Alopecia Patients Treated with Finasteride for a Period of Three Years
Article Information
Yu Ra Lee1,2, Woo Young Sim3, Jongki Hong2,4, Bark Lynn Lew3,*, Bong Chul Chung1,2,*
1Molecular Recognition Research Center, Korea Institute of Science and Technology, Seoul, 02792, Korea
2KHU-KIST, Department of Converging Science and Technology, Kyung Hee University, Seoul, 02447, Korea
3Department of Dermatology, Kyung Hee University Hospital at Gangdong, Kyung Hee University, Seoul, 05278, Korea
4College of Pharmacy, Kyung Hee University, Seoul, 02447, Korea
*Corresponding Author: Bong Chul Chung, PhD, Molecular Recognition Research Center, Korea Institute of Science and Technology, Seoul, 02792, Korea; Department of Converging Science and Technology, Kyung Hee University, Seoul, 02447, Korea
Bark Lynn Lew, MD, Department of Dermatology, Kyung Hee University Hospital at Gangdong, Kyung Hee University, Seoul 05278, Korea
Received: 05 October 2020; Accepted: 16 October 2020; Published: 21 October 2020
Citation:
Yu Ra Lee, Woo Young Sim, Jongki Hong, Bark Lynn Lew, Bong Chul Chung. Comparative Studies on Polyamine Levels in Plasma and Urine of Androgenic Alopecia Patients Treated with Finasteride for a Period of Three Years. International Journal of Applied Biology and Pharmaceutical Technology 11 (2020): 245-255.
View / Download Pdf Share at FacebookAbstract
Abstract
Androgenic alopecia, characterized by hair loss, has been correlated with high androgen levels, especially dihydrotestosterone. Finasteride treatment inhibits 5-alpha reductase and blocks dihydrotestosterone production. Further, dihydrotestosterone inhibits hair cell proliferation. Polyamines are closely associated with proliferation. We aimed to identify differences in polyamine levels in plasma and urine samples from patients with androgenic alopecia receiving finasteride treatment for three years. Liquid chromatography-tandem mass spectrometry was used to quantify polyamines. Plasma samples were derivatized with dansyl chloride; urine samples were derivatized with isobutyl chloroformate. Patient plasma and urinary polyamine concentrations were followed up annually. There were significantly higher plasma levels of spermidine (P value, 0.01) and N-acetyl spermine (P value, 0.03) between baseline and baseline after one year (total two year) of finasteride treatment. After two years of treatment at baseline (total three years of treatment), plasma levels of 1,3-diaminopropane (P value, 0.005) and N-acetyl spermidine (P value, 0.01) were significantly higher than one year of treatment at baseline (total two years of treatment). However, there were no significant differences observed in urine samples. Our findings suggest that treatment with finasteride alters plasma polyamine concentrations but not urine polyamine concentrations. Different aspects in polyamine metabolites were detected between the urine and plasma samples following finasteride treatment. Our approach can be used to recognize the therapeutic effects of finasteride through polyamine metabolism.
Keywords
Androgenic alopecia; Finasteride; Polyamine; Plasma; Urine
Androgenic alopecia articles; Finasteride articles; Polyamine articles; Plasma articles; Urine articles
Article Details
Introduction
Hair loss occurs as the hair growth cycle rate gradually shortens, and the hair follicles gradually become smaller [1]. Androgenic alopecia (AGA) is the most common cause of hair loss and is caused by an increase in dihydrotestosterone (DHT), a male sex hormone. The mechanism of AGA involves high levels of DHT, which is synthesized from testosterone (T) catalyzed by 5α-reductase type II enzyme. Androgens are important in regulating hair growth. Therefore, substantial research has been performed investigating the relationship between AGA patients and androgens in laboratory studies. We determined that DHT and the DHT/T ratio were elevated in plasma samples of balding patients [2]. Further, we confirmed that the ratio of testosterone to epitestosterone was significantly higher in balding people [3]. We also confirmed that there were different amounts of androgen levels between patients with baldness of Caucasian and Koreans [4].
Finasteride is one of the most commonly used medications for treating AGA. Finasteride influences the hair-growth cycle and increases the length, diameter [5], and weight [6] of existing hair. In addition, in AGA patients, treated with finasteride 1 mg/day, decreased the progression of hair loss and increased hair growth in clinical trials over two years [7]. As a competitive inhibitor of the enzyme 5α-reductase type II, finasteride decreased DHT levels in serum samples [8]. Thus, we investigated androgen profiles in AGA patients who had been treated with finasteride for five months and confirmed the decreased DHT/T ratio in human plasma samples [9]. In addition, we conducted steroid profiling to compare normal controls and patients with AGA who had been treated with finasteride for a year in human urine samples [10].
We suggest that other metabolites, such as polyamines, are potentially correlated with AGA. Polyamines are associated with cell proliferation. Thus, polyamines are potential indicators of malignant growth. Unbalanced polyamine metabolism could lead to several pathological conditions, such as cancer and inflammation [11]. However, another disease correlated with polyamine is hair loss. As hair follicles are highly proliferative, polyamines would be correlated with hair growth. Thus, we performed polyamine profiling in human hair samples among AGA patients, alopecia areata patients, and normal controls [12]. In addition, we analyzed the polyamine profile of AGA patients distributed across the human scalp (vertex and occipital) [13].
There is evidence of a correlation between androgens and polyamines. DHT levels correlate with the proliferation in hair cells, which inhibits hair cell proliferation [14] and induces the apoptosis signaling pathway [15]. In addition, we previously confirmed the presence of androgen and polyamine concentrations in urine samples of alopecia areata patients. We detected the ratio of dehydroepiandrosterone to spermine was significantly lower than in controls [16]. However, to the best of our knowledge, no studies have provided evidence of the precise change in polyamine expression with finasteride treatment. Finasteride treats hair loss by inhibiting the production of DHT, which inhibits hair cell proliferation. Therefore, we hypothesize that treatment with finasteride will inhibit DHT and hair cell proliferation will occur. This can be observed with changes in polyamine concentrations related to cell proliferation. We believe that polyamine levels and finasteride treatment may be correlated. Thus, we also hypothesize that polyamine levels will be higher because of hair loss treatment using finasteride. Through this preliminary study, we want to confirm whether polyamine metabolism was correlated with finasteride treatment.
Materials and methods
Chemicals
N-Acetyl putrescine (N-PUT), N-acetyl cadaverine (N-CAD), N-acetyl spermidine (N-SPD), N-acetyl spermine (N-SPM), 1,3-diaminopropane (DAP), cadaverine (CAD), putrescine (PUT), spermidine (SPD), spermine (SPM), 1,6-diaminohexane (DAH) (used as an internal standard), formic acid (ACS reagent), sodium carbonate, sodium bicarbonate, isobutyl chloroformate and dansyl chloride were purchased from Sigma-Aldrich. High-performance liquid chromatography-grade acetonitrile (ACN), methanol, pentane, and diethyl ether were acquired from Burdick and Jackson (Muskegon, MI, USA). Distilled water was prepared using a Milli-Q purification system (Millipore, Billerica, MA, USA).
Sample information and ethics statement
Plasma and urine samples were obtained from 20 patients (aged 19–56 years, mean ± standard deviation (SD) (31.3 ± 10.4). Our experiment was conducted in patients who had taken finasteride every day for at least one year (baseline), and continued finasteride treatment for a total of three years. Plasma and urine samples were collected annually for measurements. We collected three groups, baseline (one year treatment), after one year of baseline (total two years treatment) and after two years of baseline (total three years treatment). Patients received 1 mg of finasteride daily. Exclusion criteria included the presence of hormone-related diseases other than male pattern baldness (MPB) or severe inflammatory disease and a history of past treatment with 5α-reductase inhibitors. The inclusion criteria enabled the selection of MPB patients who were 18 years of age and older and did not meet the exclusion criteria. Quantitative profiling of polyamines was performed for 60 human plasma and urine samples separately. Early morning urine and plasma samples were collected, and urine samples were normalized with creatinine. Human plasma and urine samples were collected from the Department of Dermatology at Kyung Hee University Hospital at Gangdong and plasma stored at -80°C; urine was stored at -20°C until analysis. Written informed consent was obtained from all patients and the study was approved by institutional review board (IRB No. 2015-11-022).
Sample preparation
For plasma samples analysis, we added 1 mL of ACN for protein precipitaion to 200 µL of each plasma sample, followed by the addition of an internal standard (1 μg/mL × 20 µL). After protein precipitation, samples were centrifuged at 1200 × g for 5 min. The supernatants were transferred to a new 10 mL tube, and 100 µL dansyl chloride (4 mg/mL in acetone) and 100 µL of sodium bicarbonate buffer (0.1 M) were added. The mixture was incubated at 60°C for 10 min. The mixture was then evaporated under a gentle nitrogen stream. The residue was reconstituted with 100 µL of methanol, and a 10 µL aliquot was injected into the HPLC-MS/MS system. The derivatized procedure used to analyze plasma has been previously described [17].
For urine samples analysis, urine samples (200 μL) were extracted for 10 min with 1 mL pentane, and the organic phase was discarded by centrifugation (1300 g for 5 min). After addition of 20 μL 1,6-diaminohexane (1 μg/mL), samples were adjusted to pH 9.0 by the addition of 70 μL sodium-carbonate buffer (0.1 M, pH 9.0). Amine carbamoylation was performed by adding 20 µL isobutyl chloroformate, followed by incubation at 35°C for 15 min. After cooling, the solution was extracted twice with 2 mL diethyl ether, and the organic layer was combined and then evaporated under N2. The residue was reconstituted in 100 µL methanol, and a 10 µL aliquot was injected into the LC-MS/MS system. The method used to analyze urine has been previously described [18].
High-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS)
Chromatography was performed with a Shiseido nanospace SI-2 HPLC system (Osaka Soda, Osaka, Japan) coupled with an LTQ XL ion trap MS (Thermo Fisher Scientific, Waltham, MA, USA). A gradient eluent (A, 0.1% formic acid in 5% ACN; B, 0.1% formic acid in 95% ACN) was used with a Hypersil GOLD C18 column (150 × 2.1 mm inside diameter, 3 µm). For plasma analysis, the gradient elution system was controlled as follows: time = 0 min, 12% B; time = 0–17 min, 12 to 88% B (hold for 8 min); time = 25–28 min, 88 to 12% B for flow rate 200 µL. The gradient was finally returned to the initial condition (12% B) and was held for 7 min before running the next sample. For urine analysis, gradient elution was controlled as follows: time = 0 min, 50% B; time = 0–12 min, 50 to 95% B (hold for 5 min); and time = 17–18 min, 95 to 50% B. The gradient was returned to the initial condition (50% B) and was held there for 10 min before running the next sample at 100 µL/min. Details of MS detection as follows: Column temperature, 40°C; Ionization mode, Electrospray ionization; Capillary voltage, 26V; Capillary temperature, 350°C; Spray voltage, 5.2 kV; Sheath gas flow rate, 20 arb.
Statistical testing
Polyamines concentrations were obtained from calibration curves. The ion peak area of the metabolite was normalized by dividing its value by the internal standard. Polyamine levels were expressed as the mean ± SD. Comparisons between baseline, 1-year after baseline (total two years treatment), and 2-years after baseline (total three years treatment) were performed using Mann-Whitney U test. The threshold of significance was set to P < 0.05. The receiver operating characteristic (ROC) curves were drawn by MedCalc software (MedCalc, Ostend, Belgium) to identify candidate biomarkers. The threshold of candidate biomarker was set to an area under the curve (AUC) > 0.7.
Results
Quantification of polyamines in plasma and urine samples of androgenic alopecia patients treated with finasteride
Between baseline and one year after baseline (total two years of treatment), the levels of SPD and N-SPM were significantly higher (SPD: mean 83.9 ng/mL, range 17.53-210.03 ng/mL, P = 0.001; N-SPM: mean 61.91 ng/mL, range 5.02-124.18 ng/mL, P = 0.03) than the baseline (SPD: mean 50.12 ng/mL, range 16.77-94.27 ng/mL; N-SPM: mean 20.79 ng/mL, range 4.92-96.4 ng/mL). The levels of DAP and N-SPD were significantly higher after two years of baseline (total three years of treatment) (DAP: mean 9.28 ng/mL, range 1.32-21.95 ng/mL, P = 0.005; N-SPD: mean 29.53 ng/mL, range 3.89-80.69 ng/mL, P = 0.01) when compared to one year after baseline (total two years treatment) (DAP: mean 4.39 ng/mL, range 0.7-18.49 ng/mL; N-SPD: mean 15.17 ng/mL, range 4.82-40.69 ng/mL) (Table 1). However, in urine samples, there were no significant differences during finasteride treatment (Table 2).
Table 1: Concentration of polyamines in plasma samples obtained from patients with androgenic alopecia treated with finasteride (ng/mL)
|
Baseline Mean ± SD Median, range |
Baseline+1 yr Mean ± SD Median, range |
Baseline+2 yr Mean ± SD Median, range |
p value |
|||
|
b vs. b+1 yr |
b vs. b+2 yr |
b+1 yr vs. b+2 yr |
||||
|
N-PUT |
32.6±11.11 31.97,13.28-62.62 |
39.78±14.24 40.03,14.8-63.84 |
38.58±19.62 30.85,14.54-74.58 |
0.08 |
0.25 |
0.83 |
|
N-CAD |
7.92±6.97 5.15,1.81-24.86 |
7.06±5.47 5.44,0.7-18.49 |
9.4±9.16 6.62,0.45-37.27 |
0.67 |
0.57 |
0.34 |
|
DAP |
4.73±2.47 4.1,1.82-11.84 |
4.39±2.97 3.98,0.87-12.29 |
9.28±5.9 9.14,1.32-21.95 |
0.7 |
0.01 |
0.005 |
|
PUT |
9.04±5.71 7.77,0.65-23.94 |
8.47±4.16 7.54,3.11-18.99 |
13.25±16.62 7.69,2.61-59.84 |
0.72 |
0.3 |
0.23 |
|
CAD |
20.86±23.98 7.19,2.61-91.78 |
22.57±14.68 18.8,6.36-49.94 |
89.39±133.16 35.5,5.89-416.21 |
0.84 |
0.12 |
0.13 |
|
N-SPD |
14.81±6.9 12.04,8.72-37.93 |
15.17±9.09 12.98,4.82-40.69 |
29.53±21.57 26.06,3.89-80.69 |
0.89 |
0.008 |
0.01 |
|
SPD |
50.12±22.4 48.76,16.77-94.27 |
83.9±48 73.92,17.53-210.03 |
93.27±51.54 84.39,4.31-237.48 |
0.01 |
0.002 |
0.56 |
|
N-SPM |
20.79±26.25 10.36,4.92-96.4 |
61.91±49.1 46.87,5.02-124.18 |
31.93±21.57 23.97,16.19-69.81 |
0.03 |
0.42 |
0.23 |
|
SPM |
160.82±181.2 104.68,18.55-588.55 |
224.11±144.13 200.69,52.72-588.5 |
263.85±176.5 251.76,69.83-603.55 |
0.38 |
0.23 |
0.56 |
N-PUT, N-acetyl putrescine; N-CAD, N-acetyl cadaverine; DAP, 1,3-diaminopropane; PUT, Putrescine; CAD, Cadaverine; N-SPD, N-acetyl spermidine; SPD, Spermidine; N-SPM, N-acetyl spermine; SPM, Spermine; b, baseline; baseline, patients with androgenic alopecia treated with finasteride for 1 year daily
Table 2: Concentration of polyamines in urine samples obtained from patients with androgenic alopecia treated with finasteride (ng/mg creatinine)
|
Baseline Mean ± SD Median, range |
Baseline+1 yr Mean ± SD Median, range |
Baseline+2 yr Mean ± SD Median, range |
p value |
|||
|
b vs. b+1 yr |
b vs. b+2 yr |
b+1yr vs. b+2 yr |
||||
|
N-PUT |
258.3±338.77 117.79,29.53-1245.89 |
280.4±346.54 179.59,14.47-1455.58 |
337.72±241.32 323.03,19.31-901.79 |
0.85 |
0.43 |
0.57 |
|
CAD |
15.43±17.92 8.4,1.77-63.09 |
20.62±40.79 6.35,0.97-166.86 |
15.47±29.57 6.11,1.19-115.86 |
0.64 |
0.99 |
0.68 |
|
N-SPM |
3.11±4.2 1.68,0.3-17.62 |
2.21±3.13 0.67,0.1-10.95 |
1.78±1.71 0.96,0.13-5.46 |
0.49 |
0.25 |
0.64 |
|
PUT |
16.63±11.7 12.18,1.91-39.05 |
19.25±16.2 11.35,3.26-51.48 |
20.04±26.24 11.53,2.56-111.15 |
0.59 |
0.62 |
0.91 |
|
SPD |
55.65±46.8 37.12,6.35-166.81 |
59.06±100.84 34.44,4.26-450.09 |
35.43±26.09 30.98,4.05-81.31 |
0.9 |
0.11 |
0.35 |
|
DAP |
11.95±9.02 9.12,2.56-39.92 |
11.12±8.75 7.12,0.78-28.51 |
12.78±11.1 8.29,3.23-42.99 |
0.77 |
0.8 |
0.61 |
|
N-SPD |
246.44±152.73 210.51,9.94-450.68 |
178.31±132.32 153.45,8.11-466.1 |
178.42±208.26 127.47,5.44-885.68 |
0.19 |
0.32 |
0.99 |
|
N-CAD |
108.02±92.15 69.92,1.81-310.72 |
56.63±59.26 29.34,0.7-188.45 |
73.19±65.04 41.46,12.64-248.03 |
0.05 |
0.2 |
0.42 |
|
SPM |
189.05±152.79 145.97,39.65-592.36 |
154.55±223.24 81.15,12.92-992.65 |
76.78±55.96 76.81,8.63-213.12 |
0.6 |
0.02 |
0.16 |
N-PUT, N-acetyl putrescine; N-CAD, N-acetyl cadaverine; DAP, 1,3-diaminopropane; PUT, Putrescine; CAD, Cadaverine; N-SPD, N-acetyl spermidine; SPD, Spermidine; N-SPM, N-acetyl spermine; SPM, Spermine; b, baseline; baseline, patients with androgenic alopecia treated with finasteride for 1 year daily.
Receiver operating characteristic curve analysis
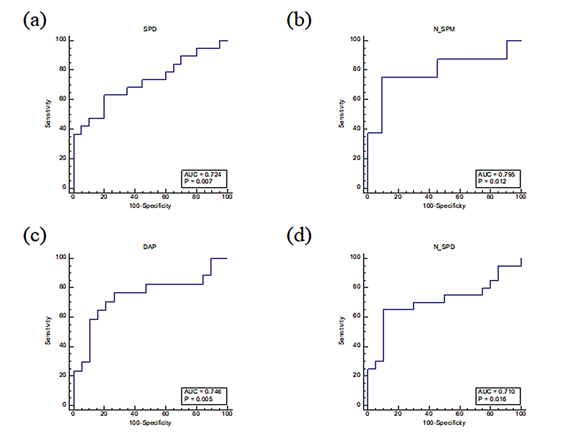
The results of receiver operating characteristic (ROC) curve analysis were based on the result of univariate and multivariate analyses, thus ensuring the reliability of potential candidate markers tested for independent validation. The area under the curve (AUC) values of plasma samples for all metabolites with significantly different concentration levels were greater than 0.7. On comparing baseline and one year after baseline (total two years of treatment), the AUC of SPD was 0.724 and N-SPM was 0.795. Between one year after baseline (total two years of treatment) and two years after baseline (total three years of treatment), the AUC of DAP was 0.7 and N-SPD was 0.710 (Figure 1).
Figure 1: Univariate receiver operating characteristic (ROC) curve analyses for predicting usefulness of a marker’s performance in plasma and urine samples from patients with androgenic alopecia treated for three years. Samples were collected annually. The area under the ROC curve (AUC) is a measure of how well a parameter can be distinguished between treatment periods. Each point of the ROC curve represents a sensitivity/specificity pair corresponding to a particular decision threshold. On comparing baseline and one year after baseline (total two years of treatment), (a) spermidine and (b) N-acetyl spermine. On comparing one year after baseline (total two years of treatment) and two years after baseline (total three years of treatment), (c) 1,3-diaminopropane and (d) N-acetyl spermidine
Discussion
In this preliminary study, we quantitatively assessed polyamine profiles in plasma and urine samples of patients with AGA treated with finasteride for three years. In principle, quantification by LC-MS/MS using stable internal standards (1,6-diaminohexane) is the optimal method of quantitative analysis. Using the present method with aqueous extracts of plasma and urine, excellent separation of nine polyamines was achieved with no significantly interfering background peaks. We observed nine polyamines in plasma and urine sample, and confirmed significantly different concentrations of some metabolites in plasma samples, but not urine samples.
Since there are many studies evaluating androgen levels with finasteride, we chose to study other metabolites, such as polyamines that are known to be involved in cell proliferation and differentiation. Evidence for the correlation between polyamines and patients with AGA is provided from our previous studies [12, 13], and in the present study, our aim was to evaluate the relationship between finasteride treatment and polyamines. Our results show that the plasma polyamine concentrations are altered by treatment with finasteride; however, there were no significant differences between urine samples. This is first study to investigate the correlation between finasteride treatment and polyamines.
DHT inhibits hair cell proliferation [14]. Thereby, we hypothesized that the concentration of polyamines associated with cell proliferation will be increased during treatment. As we expected, most plasma polyamines concentrations were higher after three years of treatment when compared to baseline (one year of treatment). In plasma samples, among the polyamines with higher concentrations, significant increases were observed in SPD and N-SPM levels at baseline and the one-year follow-up (two years of treatment). SPD is a well-known metabolite associated with the promotion of hair growth (anagen phase) in human scalp hair follicle and human hair follicle epithelial stem cells [19]. In a previous study, we confirmed SPD levels were lower in AGA patients than normal controls [12]. The results from this study verify that the levels of SPD gradually increase in patients treated with finasteride. We observed N-acetyl polyamines levels, e.g., N-PUT, N-SPD and N-SPM, to be significantly higher in the vertex region, where hair loss predominantly occurs, than the occipital region in both males and females [13]. Higher spermidine/spermine-N1-acetyltransferase activity was related with hair loss [20]. Further detailed analysis between N-acetyl polyamines and finasteride should be done in the future.
We also observed significantly higher DAP and N-SPD levels at one-year follow-up (two years of treatment) and two-years follow-up (three years of treatment). Previous studies suggest that DAP could be considered a marker of effective anti-cancer treatment of squamous cell carcinoma of the lung [21]. DAP is derived from S-adenosyl-L-methionine by S-adenosylmethionine decarboxylase and contributes to the formation of SPD and SPM. The increased levels of DAP by finasteride treatment could be interpreted as indicating that the interactions of spermidine and spermine with DNA analogues may change during the treatment of hair loss [21]. However, no studies have been conducted on the relationship with the treatment of hair loss and DAP. In addition, the values of N-SPD at the one- and two-year follow-up increased significantly. Acetylation leads to reduced interactions of polyamines with different negatively charged compounds, such as DNA and RNA [21]. Increased N-SPD levels may be one of the mechanisms by which the intracellular concentration of polyamines becomes dysregulated, which may affect the treatment outcomes with finasteride. Both DAP and N-SPD are both catabolic byproducts of SPD. SPD has been correlated with anagen phase promotion and hair follicle growth [19]. In addition, SPD is an important anagen prolongator [22] and it stimulates human hair growth [23]. Extra supply of spermidine prolongs life span [24]. Therefore, one may speculate that androgens stimulate ornithine decarboxylase mRNA expression, and increased the level of the main polyamine, thus, the concentrations of DAP and N-SPD change as by-products of spermidine, one of the main polyamines, via metabolism in the body. Checking polyamine levels in plasma showed a tendency for higher concentrations of polyamines with the longer treatment periods. Therefore, it is important to quantify SPD, N-SPM, DAP and N-SPD levels to confirm the therapeutic effects of finasteride treatment. Further study into the role of SPD, N-SPM, DAP and N-SPD are needed.
However, in urine samples, we had no significant differences during treatment. We found that the polyamine metabolites during finasteride treatment were different in urine and plasma. It is not possible to confirm why the polyamine concentrations in urine and plasma were different, but it suggests that further studies on the concentration of polyamines in urine and plasma during finasteride treatment are needed.
When treating with finasteride, it is important to check the concentration of polyamines in various biological samples. Although it is difficult to determine the clinical significance of polyamine levels in this study, as the comparisons described herein involved only 20 patients. Because this study was extended from its original one-year duration to two years, the sample size available for analysis decreased with time. Nonetheless, we can indirectly confirm that there is an association between polyamines and finasteride. In the future, it may be necessary to ensure that our experiments are clinically meaningful through various races and extended populations.
Conclusion
In the present study, we confirmed that there is a correlation between finasteride treatment and polyamine concentrations in plasma and urine. We suggest that increasing levels of plasma polyamines may alter during treat AGA. In addition, aspect of polyamines in urine and plasma were different. We suggest that the therapeutic effect of finasteride may be confirmed by the concentration of polyamine metabolites. These results provide insight in the finasteride-associated modifications of polyamine metabolism, which may help understand the treatment of AGA. However, further research is needed from larger scale studies to determine clinical relevance.
Declarations
Funding:
This study was supported by a grant from the Korea Institute of Science and Technology Institutional Program (Project No. 2E30480).
Conflicts of interest:
None declared
Ethics approval
All procedures performed in studies involving human participants were approved by the Ethics Committee of the Kyung Hee University Medical Center at Gangdong and approved by the institutional review board (IRB No. 2015-11-022). This study was performed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Written informed consent was obtained from all patients and controls prior to sample collection, and consent was obtained from a parent or legal guardian prior to participation by minors in this study.
Consent for publication:
Not applicable
Availability of data and material:
Not applicable
Code availability:
Not applicable
Author contributions:
YRL wrote the manuscript and performed the experiments. WYS and BLL contributed to the collection of essential samples. JH designed the experiments. BCC supervised the research. All authors approved the final version of the article.
References
- Schweikert HU, Wilson JD. Regulation of human hair growth by steroid hormones. I. Testosterone metabolism in isolated hairs. The Journal of Clinical Endocrinology & Metabolism 38 (1974): 811-81
- Bang HJ, Yang YJ, Lho DS, et al. Comparative studies on level of androgens in hair and plasma with premature male-pattern baldness. Journal of Dermatological Science 34 (2004): 11-1
- Choi MH, Yoo YS, Chung BC. Biochemical roles of testosterone and epitestosterone to 5α-reductase as indicators of male-pattern baldness. Journal of Investigative Dermatology 116 (2001): 57-61.
- Choi MH, Kim SJ, Lew BL, et al. Hair steroid profiling reveals racial differences in male pattern baldness between Korean and Caucasian populations. The Journal of Investigative Dermatology 133 (2013):
- Price VH. Treatment of hair loss. New England Journal of Medicine 341 (1999): 964-9
- Price VH, Menefee E, Sanchez M, et al. Changes in hair weight and hair count in men with androgenetic alopecia after treatment with finasteride, 1 mg, daily. Journal of the American Academy of Dermatology 46 (2002): 517-5
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Journal of the American Academy of Dermatology 39 (1998): 578-5
- Drake L, Hordinsky M, Fiedler V, et al. The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia. Journal of the American Academy of Dermatology 41 (1999): 550-55
- Ryu HK, Kim KM, Yoo EA, et al. Evaluation of androgens in the scalp hair and plasma of patients with male-pattern baldness before and after finasteride administration. British Journal of Dermatology 154 (2006): 730-73
- Lee YR, Im E, Kim H, et al. Untargeted Metabolomics and Steroid Signatures in Urine of Male Pattern Baldness Patients after Finasteride Treatment for a Year. Metabolites 10 (2020):
- Russell DH. Increased polyamine concentrations in the urine of human cancer patients. Nature New Biology 233 (1971): 144-14
- Lee YR, Lew BL, Sim WY, et al. Altered polyamine profiling in the hair of patients with androgenic alopecia and alopecia areata. The Journal of Dermatology 46 (2019): 985-9
- Lee YR, Lee J, Lew BL, et al. Distribution of polyamines may be altered in different scalp regions of patients with hair loss. Experimental Dermatology 28 (2019): 1083-108
- Shin HS, Park SY, Hwang ES, et al. Ginsenoside F2 reduces hair loss by controlling apoptosis through the sterol regulatory element-binding protein cleavage activating protein and transforming growth factor-β pathways in a dihydrotestosterone-induced mouse model. Biological and Pharmaceutical Bulletin 37 (2014): 755-7
- Andrews P, Krygier S, Djakiew D. Dihydrotestosterone (DHT) modulates the ability of NSAIDs to induce apoptosis of prostate cancer cells. Cancer Chemotherapy and Pharmacology 49 (2002): 179-1
- Lee YR, Kim H, Lew BL, et al. Sex-related differences in urinary immune-related metabolic profiling of alopecia areata patients. Metabolomics 16 (2020):
- Wang HX, Zhou Y, Jiang QW. Enhanced screening efficiency for endocrine-disrupting chemicals in milk and powdered milk using UPLC/QTOF-MS by the introduction of dansyl chloride derivatisation. Food Additives & Contaminants: Part A 30 (2013): 166-1
- Byun JA, Lee SH, Jung BH, et al. Analysis of polyamines as carbamoyl derivatives in urine and serum by liquid chromatography–tandem mass spectrometry. Biomedical Chromatography 22 (2008): 73-80.
- Ramot Y, Tiede S, Bíró T, et al. Spermidine promotes human hair growth and is a novel modulator of human epithelial stem cell functions. PLoS One 6 (2011):
- Pegg AE. Spermidine/spermine-N 1-acetyltransferase: a key metabolic regulator. American Journal of Physiology-Endocrinology and Metabolism 294 (2008): E995-E
- Liu R, Li P, Bi CW, et al. Plasma N-acetylputrescine, cadaverine and 1, 3-diaminopropane: potential biomarkers of lung cancer used to evaluate the efficacy of anticancer drugs. Oncotarget 8 (2017):
- Rinaldi F, Marzani B, Pinto D, et al. A spermidine-based nutritional supplement prolongs the anagen phase of hair follicles in humans: a randomized, placebo-controlled, double-blind study. Dermatology Practical & Conceptual 7 (2017):
- Liyanage D, Sinclair R. Telogen effluvium. Cosmetics 3 (2016):
- Madeo F, Eisenberg T, Pietrocola F, et al. Spermidine in health and disease. Science 359 (2018).


 Impact Factor: * 3.0
Impact Factor: * 3.0 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
