Comparison of The Anti-Inflammatory and Immunomodulatory Mechanisms of Two Medicinal Herbs: Meadowsweet (Filipendula ulmaria) and Harpagophytum (Harpagophytum procumbens)
Article Information
Juliette Cholet1*, Caroline Decombat1†, Marjolaine Vareille-Delarbre1,†, Maël Gainche2, Alexandre Berry1, Clémence Ogéron2, Isabelle Ripoche2, Laetitia Delort1, Marion Vermerie1, Didier Fraisse1, Catherine Felgines1, Edwige Ranouille3, Jean-Yves Berthon3, Albert Tourette4, Yves Troin2, François Senejoux1, Pierre Chalard2, Florence Caldefie-Chezet1
1Université Clermont Auvergne, INRA, UNH, Unité de Nutrition Humaine, CRNH Auvergne, F-63000, Clermont-Ferrand, France
2Université Clermont Auvergne, CNRS, SIGMA Clermont, ICCF, F-63000 Clermont-Ferrand, France
3Greentech, Biopôle Clermont-Limagne, 63360 Saint-Beauzire, France
4AltoPhyto, 7 rue des gargailles, 63370 Lempdes, France
†Equal contributions
*Corresponding Author: Juliette Cholet, Université Clermont Auvergne, INRA, UNH, Unité de Nutrition Humaine, CRNH Auvergne, F-63000, Clermont-Ferrand, France
Received: 24 June 2019; Accepted: 10 July 2019; Published: 22 July 2019
Citation:
Juliette Cholet, Caroline Decombat, Marjolaine Vareille-Delarbre, Maël Gainche, Alexandre Berry, Clémence Ogéron, Isabelle Ripoche, Laetitia Delort, Marion Vermerie, Didier Fraisse, Catherine Felgines, Edwige Ranouille, Jean-Yves Berthon, Albert Tourette, Yves Troin, François Senejoux, Pierre Chalard, Florence Caldefie-Chezet. Comparison of The Anti-Inflammatory and Immunomodulatory Mechanisms of Two Medicinal Herbs: Meadowsweet (Filipendula ulmaria) and Harpagophytum (Harpagophytum procumbens). International Journal of Plant, Animal and Environmental Sciences 9 (2019): 145-163.
View / Download Pdf Share at FacebookAbstract
Background: Meadowsweet (Filipendula ulmaria) and Harpagophytum (H. procumbens) are two medicinal herbs traditionally used for their anti-inflammatory effect. Nonetheless, if the effects of the single compounds isolated from these plants have been well described, little is known about the molecular mechanisms behind whole extracts.
Methods: We studied and compared the effects of methanolic extracts from the aerial parts of F. ulmaria (FUE) and from the roots of H. procumbens (HPE) on different markers of inflammation such as antioxidant capacity, leukocyte ROS production, COX-2/PGE2 pathway or cytokine secretions.
Results: FUE proved to be better than HPE in terms of antioxidant capabilities. Even if their effect on COX-2/PGE2 were similar, we found that their immune-modulatory activities were quite different. In the basal state, the FUE favored cytokines associated with Th1 lymphocytes whereas the HPE decreased the secretion of IL-21 and IL-23, associated with Th17 cells. In PHA-stimulated cells, the HPE increased the characteristic cytokines of Th1 cells, whereas the effects of the FUE were more nuanced.
Conclusion: Though both plants are known as anti-inflammatory herbs, these results suggested that, apart from their similar anti-inflammatory effect on COX-2/PGE2, both could improve neutrophil and monocyte recruitment, as well as monocytes/macrophages and Th1, and presumably Th17, activation. Therefore, their impact on immune response was more likely immunostimulant.
Keywords
Harpagophytum procumbens; Filipendula ulmaria; Cytokines; Inflammation; Immune-modulation; Molecular mechanism; Natural products; Radical oxygen species; COX-2/PGE2
Harpagophytum procumbens articles, Filipendula ulmaria articles, Cytokines articles, Inflammation articles, Immune-modulation articles, Molecular mechanism articles, Natural products articles, Radical oxygen species articles, COX-2/PGE2 articles
Article Details
1. Introduction
Medicinal plants are one of the oldest sources of treatment used for healthcare, and for several years there is a reviving interest in them, especially in the context of chronic ailments and inflammation. Most of the well-known traditional medicinal plants have been found through empirical observations, the trial and error process. Among them, Harpagophytum procumbens DC. (Pedaliaceae), Devil’s claw, and Filipendula ulmaria (L.) Maxim. (Rosaceae), also known as Meadowsweet, have been extensively used for their anti-inflammatory and antirheumatic properties [1,2].
Although the beneficial effects of both Devil's Claw and Meadowsweet have been observed on inflammation in vivo, little is known about their underlying mechanisms. Indeed, most studies have been focused on their capacities to reduce inflammation in vivo, in models such as the paw edema or hot plate, or, in particular, on their action on the arachidonic acid pathway in vitro [3-7]. Thus, their inhibitory effect on the generation of leukotrienes and prostaglandins, considered as their main mechanism of action, has been well documented. However, their broad mechanism, and eventual side effects at the cellular level, remains unclear. In particular, some authors have pointed out that these plants, or certain compounds (such as quercetin or harpagoside) isolated from them, might impact the secretion of some cytokines during inflammation [8-10]. Nevertheless, the inflammation process is highly complex as is the interplay between immune cells via cytokines, prostaglandins or chemokines.
Therefore, the current study was conducted to investigate and compare the potential antioxidant, anti-inflammatory and immunomodulatory effects of a methanol extract of Harpagophytum procumbens root (Harpagophytum procumbens extract – HPE) and of a methanol extract of the aerial parts from Filipendula ulmaria (Filipendula ulmaria extract – FUE). We studied the extracts on peripheral blood mononuclear cells (PBMCs) and human leukemic monocytic cells THP-1, through different markers of inflammation: leukocyte radical oxygen species (ROS) production, monocyte differentiation, cytokine secretion, cyclooxygenase-2 (COX-2) expression and PGE2).
2. Materials and Methods
2.1 Plant material and preparation of the extract
The dried roots of Harpagophytum procumbens and the dried aerial parts of Filipendula ulmaria were obtained in a pharmaceutical dispensary (Pharmacie Fontgiève, Clermont-Ferrand, France) and were powdered and macerated 3 times in methanol for 24h. After each filtration, the solvent was removed in a rotary evaporator under reduced pressure. The yield obtained was found to be 41.2% and 29.2% for Harpagophytum procumbens extract (HPE) and Filipendula ulmaria extract (FUE), respectively.
2.2 Identification of the major compounds of the methanolic extracts
The major constituents of the FUE and the HPE were determined using LC-MS (UHPLC Ultimate 3000 RSLC chain) and an Orbitrap Q-Exactive (Thermo Scientific) with an Uptisphere C18-3 (250 x 4.6 mm, 5 µm) column from Interchim, by comparison with analytical standard (Extrasynthèse, France) or literature data.
2.3 Phenolic content and antioxidant activity
The total phenolic content was estimated using the Folin-Ciocalteu method as previously described by Dubost et al. This same method was modified (precipitation of tannins involving skin powder) in order to estimate tannin content [11]. The total flavonoid content was assessed with the Dowd method [12,13].
An oxygen radical absorbance capacity (ORAC) assay was done in 96-well plates with a final volume of 200 µL as described by Gillespie et al. [14]. Fluorescein was used as the fluorescent probe and 2,2'-azobis (2-methylpropionamidine) dihydrochloride (AAPH) as the peroxyl radical generator. The decrease in fluorescence was measured every minute for 1 h (excitation/emission: 485/530 nm) using a microplate reader (TECAN infinite F200 PRO, Männedorf, Switzerland). The ORAC value of the FUE or the HPE were calculated using the regression equation between Trolox equivalent (TE) and the net area under the curve. The results were expressed as μmol TE per gram of dry extract.
The DPPH scavenging activity was evaluated according to the method of Meda et al. [15] with slight modifications. Briefly, 10 µL of a solution of the HPE or the FUE (1 mg/mL in methanol) was mixed with 2.5 mL of fresh DPPH solution (25 µg/mL in methanol). After 30 min of incubation, DPPH absorbance was recorded at 515 nm using a spectrophotometer (JASCO V-630). A standard curve of Trolox, in the range of 100 µM to 3000 µM, was plotted. The results were expressed as µmol of Trolox equivalents (µmol TE) per gram of dry extract.
The nitric oxide scavenging activity was performed according to a modified method of Balakrishnan et al. [11]. Briefly, sodium nitroprusside solution (SNP) (10 mM) in phosphate buffer saline (PBS 50 mM) was mixed with different concentrations of the extract (1-250 µg/mL for FUE and 100-600 µg/mL for HPE) dissolved in methanol and water and incubated at room temperature for 2 hours. Griess reagent (1% sulphanilamide, 0.1% naphthyl ethylenediamine dichloride and 5% phosphoric acid) in PBS (50mM) was added to these preparations. The absorbance of the chromophores formed during the diazotization of nitrite with sulphanilamide and subsequent coupling with naphthyl ethylenediamine dichloride was read at 540 nm (TECAN infinite M200 PRO) against two blanks (one with SNP and PBS and the other with SNP, extract and PBS) in 96-well plates (same as above).
The iron (II)-chelating activity of the extracts was measured according to Wang et al. [16]. A volume of 100 µL of the HPE or the FUE (5 mg/mL) was blended with 135 µL of distilled water and 5 µL of FeCl2 (2 mM) in microplate wells. After 10 min of incubation, the reaction was initiated by the addition of 10 µL of ferrozine (5 mM). The decrease of the purple ferrozine-Fe2+ complex was estimated 10 min later by reading the absorbance at 562 nm (TECAN infinite M200 PRO). Distilled water (100 µL) was used as a control and 10 µL of distilled water was used for the blank. A standard curve of EDTA in the range of 3 to 50 µg/mL was performed. The results were expressed as nmol of EDTA equivalents (nmol EDTAE) per gram of dry extract.
2.4 Kinetics of ROS production and viability assay
Blood was collected from healthy human volunteers (n = 6; Etablissement Français du Sang, EFS, Clermont-Ferrand, France). All donors gave their written informed consent for the use of blood samples for research purposes under EFS contract n°16-21-62 (in accordance with the following articles L1222-1, L1222-8, L1243-4 and R1243-61 of the French Public Health Code). Whole blood leukocytes were obtained by hemolytic shock using ammonium chloride solution (NH4Cl, 155 μM; NaHCO3 12 μM, EDTA 0.01 μM). Leukocytes were then washed with RPMI, centrifuged (400 g, 10 min) and suspended in RPMI. The cell preparations were adjusted to 106 cells/mL with supplemented RPMI (fetal bovine serum (FBS) 10%, gentamicin 50 μg/mL and glutamine (Gln) 2 mM). Cells were placed in 96-well polystyrene plates (Cell Wells™, Corning, NY), incubated with the FUE or the HPE at 0 or 50 µg/mL and dihydrorhodamine 123 (DHR 123, 1 μM, Cayman Chemical Company, Ann Arbor, MI), and stimulated, or not, by 1 µM PMA for 120 min. The fluorescence intensity of rhodamine 123, which is the product of dihydrorhodamine 123 oxidation by ROS, was recorded every 5 min for 120 min (excitation/emission: 485/538 nm) using the Fluoroskan Ascent FL® apparatus (ThermoFisher Scientific, Illkirch, France). Concurrently, cells from same donors were placed in 96-well polystyrene plates, incubated with an extract of 0 or 50 µg/mL, PMA (0 or 1 µM) and resazurin (25 µg/mL) to track their viability. Fluorescence (excitation/emission: 544/590 nm) was recorded every 30 min for 2 h using the Fluoroskan Ascent FL® apparatus.
2.5 Arachidonic acid pathway
Blood buffy coats were collected from healthy human volunteers (n = 3 donors) and carefully layered on a double gradient of Ficoll-Histopaque 1119® and 1077®. After centrifugation (400 g, 40 min at 20°C), the first layer of plasma was aspirated, yielding a phase of monocytes and lymphocytes (PBMCs) just above the 1.077 g/mL layer. The phase with the PBMCs was washed with RPMI and centrifuged (5 min, 400 g) twice and then suspended in 5 mL of supplemented RPMI (FBS 10%, gentamicin 50 μg/mL and Gln 2 mM). PBMCs (5x106 cells/mL) (n=3) were incubated with or without lipopolysaccharide (LPS) (1 µg/mL, LPS O26:B26, Sigma-Aldrich) and the FUE or the HPE (0 or 50 µg/mL) for 4 h. Cell lysates were then harvested and the expression of COX-2 mRNA was detected by quantitative real-time PCR. To measure the secretion of the PGE2, PBMCs (106 cells/mL) (n = 5 donors) were incubated with or without lipopolysaccharide (LPS) (1 µg/mL, LPS O26:B26, Sigma-Aldrich) and the FUE or the HPE (0 or 50 µg/mL) for 24 h. The PGE2 in the culture media was assessed by ELISA, using the PGE2 assay kit from Invitrogen (Invitrogen™; Thermo Fisher Scientific).
2.6 Immunomodulatory activity
We investigated the effect of the FUE and the HPE on macrophage activation. The human monocytic leukemia cell line, THP-1 (American Type Culture Collection) was cultured and propagated at 37 °C in a humidified atmosphere of 5% CO2 in a RPMI 1640 medium (GIBCO, ThermoFisher Scientific) supplemented with 10% FBS, gentamicin 50 μg/mL and Gln 2 mM. For differentiation, THP-1 cells (2 x 105 cells/mL) were incubated in electrosensing plates in a complete growth medium containing 16.2 nM phorbol 12-myristate 13-acetate (PMA) and the FUE or the HPE (0 or 50 µg/mL). The adhesion and the spreading of the cells were monitored continually every 15 min using the real-time cell electronic sensing (RT-CES, ACEA Biosciences Inc., San Diego, CA) system for a period of three days. The RT-CES system detected impedance change derived from cell reactions on the electrodes as the change of cell index (CI). For this assay, four independent experiments were conducted, each time in duplicate.
In a second phase, to determine the impact of the extracts on cytokine profiles, PBMCs (106 cells/mL, n=3 donors) were incubated with or without phytohemagglutinin (PHA, 5 µg/mL) and the FUE or the HPE (0 or 50 µg/mL) for 24 h. Milliplex® MAP kits (EMD Millipore™; Merck; Darmstadt, Germany) were used for all assays. All samples were run in triplicate and were assayed for 14 human cytokines (IFNγ, IL-10, IL-12 p70, IL-1β, IL-2, IL-21, IL-4, IL-23, IL-5, IL-6, IL-8, MIP-1α, MIP-1β, and TNFα). Cytokine levels were measured using optimal concentrations of standards and antibodies according to the manufacturer's instructions. After completion of all the steps in the assay, the plates were read in the Luminex Bio-Plex 200 System (Biorad, Marnes-la-Coquette, France) and the data analyzed using BioPlex Manager™ 4.1 software with a five-parameter logistic regression (5PL) curve fitting.
2.7 Statistical analysis
All statistical analyses were performed using R software (version 3.5.1). The normality of the variables was assessed by the Shapiro–Wilk test and their homoscedasticity by Bartlett’s test. Multiple comparisons between groups were made by the Kruskal–Wallis test followed by Dunn’s test. Comparisons between the two groups were made by a t-test or the Wilcoxon-Mann-Whitney test when normality was rejected. Values with p < 0.05 were considered significant. Cytokine modulation of the extracts was plotted using a heatmap with a two-way hierarchical clustering (“gplots” package, with Pearson correlation used to build the distance matrix instead of Euclidean distance).
3. Results
3.1 Direct antioxidant potential and antiradical activities
3.1.1 Composition and direct ROS scavenger potential
The Harpagophytum procumbens extract complied to European pharmacopeia with 1.5% of harpagoside in the root. We also detected classical compounds of Harpagophytum roots, such as harpagide and harpagide derivatives and verbascoside (Table 1).
|
RTa (min) |
[M-H]- (m/z) |
MS² fragments (m/z) |
Compound |
|
4.08 |
341.1087 |
89b / 59 / 341 / 71 / 119 |
Saccharosec |
|
6.80 |
191.0186 |
111b / 87 / 85 / 191 / 129 |
Citric acidc |
|
8.24 |
361.1141 |
181b / 137 / 119 / 361 / 59 |
Procumbided |
|
9.76 |
363.1299 |
183b / 89 / 201 / 165 / 363 |
Harpagided |
|
11.00 |
461.1667 |
461b / 113 / 135 / 71 / 315 |
Decaffeoylverbascosided |
|
18.33 |
623.1985 |
161b / 623 / 113 / 461 / 179 |
Verbascosidec |
|
20.67 |
623.1985 |
161b / 623 / 461 / 113 / 179 |
Isoverbascosidec |
|
26.34 |
509.1663 |
147b / 163 / 119 / 509 / 304 |
8-O-p-coumaroylharpagided |
|
28.51 |
539.1774 |
193b / 175 / 134 / 539 / 160 |
8-O-p-feruloylharpagided |
|
31.33 |
491.1562 |
163b / 119 / 147 / 311 / 491 |
Pagosided |
|
34.04 |
521.1667 |
193b / 147 / 121 / 178 / 341 |
Pagided |
|
39.55 |
493.1718 |
147b / 165 / 89 / 179 / 345 |
Harpagosidec |
aRetention time; bFragment with the highest relative intensity; cIdentification by comparison with a standard; dIdentification by comparison with publication.
Table 1: Major constituents of the methanolic extract of Harpagophytum procumbens roots.
Major constituents of the aerial parts of Filipendula ulmaria, such as quercetin derivatives, chlorogenic acid and salicylic acid, were determined using LC-MS analysis (Table 2).
|
RTa (min) |
[M-H]- (m/z) |
MS² fragments (m/z) |
Compoundc |
|
3.84 |
191.0553 |
191b / 85 / 192 / 127 / 93 |
Quinic acid |
|
6.81 |
191.0189 |
111b / 87 / 85 / 191 / 129 |
Citric acid |
|
8.53 |
169.0133 |
125b / 169 / 126 / 170 / 97 |
Gallic acid |
|
12.97 |
353.0882 |
191b / 353 / 85 / 161 / 179 |
Chlorogenic acid |
|
13.12 |
289.0721 |
289b / 245 / 109 / 125 / 203 |
Catechin |
|
17.11 |
609.1469 |
300b / 609 / 301 / 271 / 255 |
Rutin |
|
18.04 |
300.9992 |
301b / 302 / 229 / 257 / 283 |
Ellagic acid |
|
18.30 |
463.0889 |
300b / 463 / 301 / 271 / 255 |
Isoquercitrin |
|
18.67 |
463.0887 |
300b / 463 / 301 / 271 / 255 |
Hyperoside |
|
22.25 |
447.0937 |
284b / 447 / 285 / 151 / 107 |
Astragalin |
|
23.88 |
463.0885 |
301b / 151 / 300 / 463 / 178 |
Spiraeoside |
|
30.42 |
137.0231 |
93b / 137 / 94 / 138 / 65 |
Salicylic acid |
|
38.60 |
301.0356 |
301b / 151 / 179 / 121 / 107 |
Quercetin |
aRetention time; bFragment with the highest relative intensity; cIdentification by comparison with a standard.
Table 2: Major constituents of the methanolic extract of Filipendula ulmaria aerial parts.
The HPE had a phenolic content of 56 mg of gallic acid equivalent (GAE)/g of dry extract, with 5 mg of gallic acid equivalent (GAE)/g of tannins and 1.9 mg of rutin equivalent (RE)/g of flavonoids. By comparison, with 419 mg of GAE of phenols per gram of dry extract, the phenolic content was seven times higher in the Filipendula ulmaria extract (Table 3). Flavonoid content was also higher in the FUE than in the HPE (70 times higher), and, in addition, represented a greater percentage of the total phenols (31% in the FUE against 3% in the HPE).
The HPE exhibited mild radical scavenging properties, considering the oxygen radical absorbance capacity (ORAC) value obtained (2888 µmol trolox equivalent/g). However, the DPPH value, an assay that measures the capacity of an anti-oxidant to reduce an oxidant, was only 247 µmol TE/g, which is consistent with its low phenolic content [11]. As for the FUE, its ORAC value as well as its DPPH value were higher (6157 and 4950 µmol TE/g, respectively), in line with its phenol and flavonoid contents. As for nitric oxide (NO) scavenging activity (EC50 NO), the EC50 of the FUE was seven times lower than the EC50 of the HPE, with 14 µg/mL against 95 µg/mL, respectively. The FUE was thus better at scavenging NO than the HPE.
We also measured the iron (II) chelating capacity of our extracts. The FUE was shown to be more effective at chelating ferrous iron than the HPE (220.9 nmol EDTA equivalent vs 72.9 nmol EDTA equivalent, respectively).
|
Composition |
ORAC c |
DPPH c |
EC50 NOd |
Fe2+ chelation e |
|||
|
Flavonoids a |
Tannins b |
Total phenols b |
|||||
|
FUE |
131 |
227 |
419 |
6157 |
4950 |
14 |
220.9 |
|
HPE |
1.9 |
5 |
56 |
2888 |
247 |
95 |
72.9 |
a mg RE/g of dry extract; b mg GAE/g of dry extract; c µmol TE/g ; d µg/mL ; e nmol EDTA equivalent. Effect on leukocyte ROS production
Table 3: FUE and HPE phenolic composition and antioxidant properties.
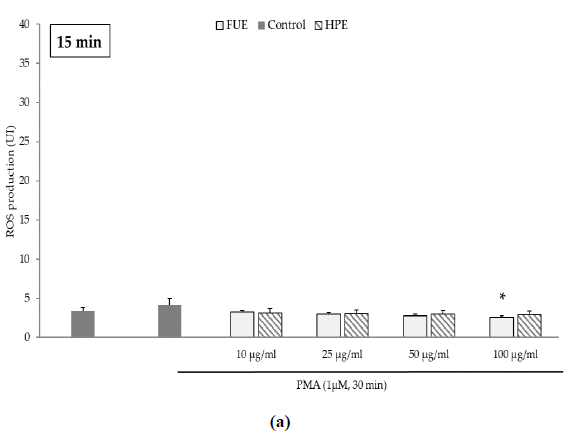
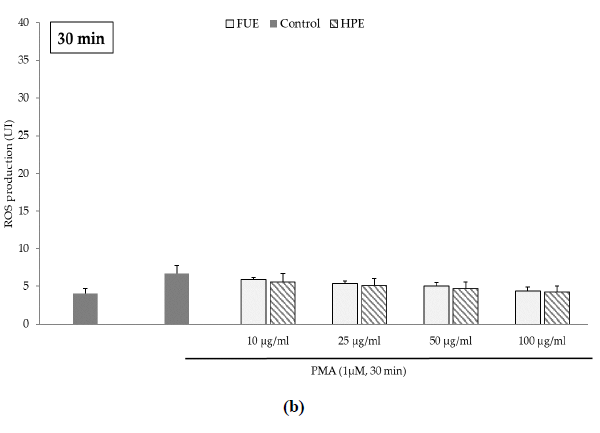
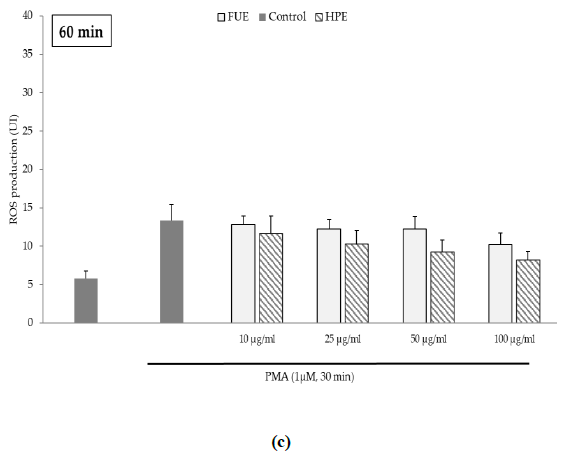
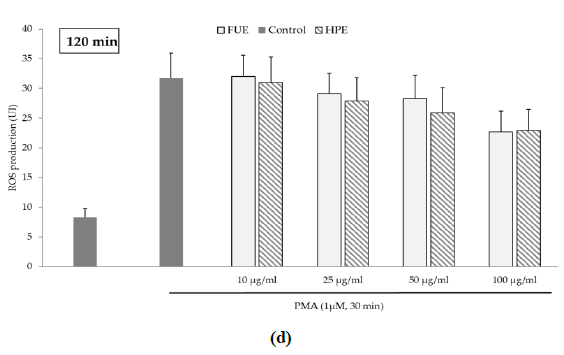
Further to the direct antioxidant activity, we investigated the ability of the extracts to decrease cellular ROS production. Blood leukocytes from healthy donors were incubated with 12-myristate 13-acetate (PMA), a direct PKC activator. The Addition of PMA in culture wells dramatically and rapidly increased ROS production by blood leukocytes (Figure 1). After 15 min of incubation, the production of ROS was significantly decreased by the FUE at a dose of 100 µg/mL. Interestingly, both extracts failed to reduce PMA-induced ROS production by PBMCs beyond 15 min of incubation.
Figure 1(a): ROS production of blood leukocytes. Data were shown as means ± SEM,* p < 0.05 compared with Positive Control (cells incubated with PMA and without extract). Cells were incubated with FUE or HPE (10, 25, 50 and 100 µg/mL) and stimulated with PMA (1 µM) for: (a) 15 min; (b) 30 min; (c) 60 min and (d) 120 min. Six independent experiments were performed, each performed in triplicate.
Figure 1(b): ROS production of blood leukocytes. Data were shown as means ± SEM,* p < 0.05 compared with Positive Control (cells incubated with PMA and without extract). Cells were incubated with FUE or HPE (10, 25, 50 and 100 µg/mL) and stimulated with PMA (1 µM) for: (a) 15 min; (b) 30 min; (c) 60 min and (d) 120 min. Six independent experiments were performed, each performed in triplicate.
Figure 1(c): ROS production of blood leukocytes. Data were shown as means ± SEM,* p < 0.05 compared with Positive Control (cells incubated with PMA and without extract). Cells were incubated with FUE or HPE (10, 25, 50 and 100 µg/mL) and stimulated with PMA (1 µM) for: (a) 15 min; (b) 30 min; (c) 60 min and (d) 120 min. Six independent experiments were performed, each performed in triplicate.
Figure 1(d): ROS production of blood leukocytes. Data were shown as means ± SEM,* p < 0.05 compared with Positive Control (cells incubated with PMA and without extract). Cells were incubated with FUE or HPE (10, 25, 50 and 100 µg/mL) and stimulated with PMA (1 µM) for: (a) 15 min; (b) 30 min; (c) 60 min and (d) 120 min. Six independent experiments were performed, each performed in triplicate.
3.1.2 Dual effect of HPE and FUE on arachidonic acid pathway
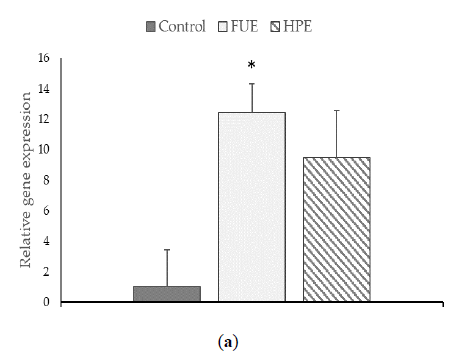
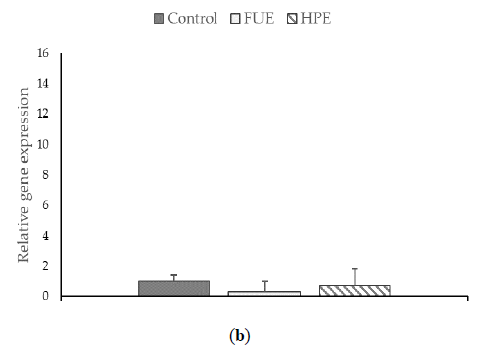
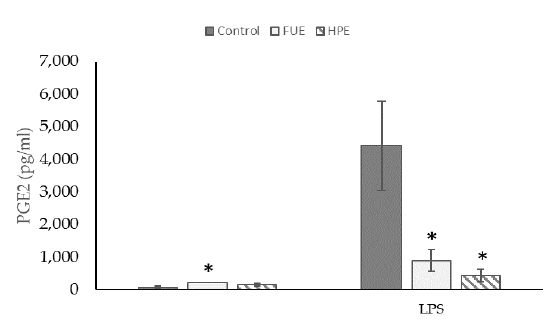
We then tested the extracts on COX-2 expression and PGE2 production in PBMCs. In the basal state, the HPE, and even more so the FUE, were shown to increase COX-2 relative expression (Figure 2) and also to induce the release of extracellular PGE2 (Figure 3), thus showing a slight pro-inflammatory effect in a physiological situation. When cells were incubated with lipopolysaccharide (LPS), the extracts had a tendency to decrease the COX-2 expression (although the effect was not significant) as well as PGE2 secretion. Thus, the FUE and the HPE had similar effects on COX-2 expression and PGE2 production.
3.2 Comparison of the immunomodulatory activities
3.2.1 Both extracts stimulated THP-1 differentiation
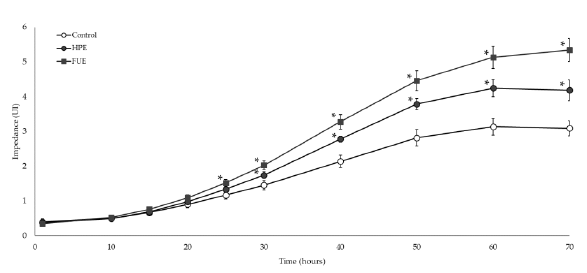
As shown in Figure 4, incubation with 10 ng/mL of PMA led to the adhesion and spreading of THP-1 cells in the wells, reflecting their differentiation from monocytes to macrophages. Without extract, impedance reached a plateau at around 60 h of incubation. In the presence of the HPE or the FUE (50 µg/mL), the slopes of the curves were higher. Moreover, the FUE proved to be highly effective at speeding up differentiation as the impedance started to be different from control at around 25 h of incubation (p<0.05). The effect of the HPE was significant shortly after, at around 30 h of incubation (Figure 4).
3.3 Difference in PBMC cytokine secretion profile
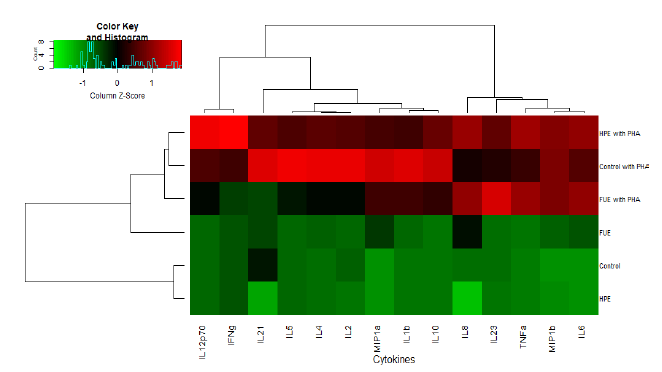
Lastly, the analysis of the secretion of 14 cytokines was performed in PBMC supernatants. As we can observe from Figure 5, the phytohemagglutinin (PHA) increased the secretions of all of the cytokines indiscriminately. Moreover, cluster analysis revealed that the HPE profile was closer to Control in the basal state (as we can conclude from the dendrogram on the side of the map). When PHA was added, the HPE has a profile closer to Control (Figure 5) once again. Therefore, the FUE had the most dramatic impact on the secretion profile.
Table 2 is a summary of the effect of the extracts on the cytokine profiles. In the basal state, the HPE showed an effect mostly on the cytokine characteristics of Th17 lymphocytes (ie IL-21 and IL-23). The IL-21 in particular was not detected in the supernatants of cells incubated with the HPE. In the same situation, the FUE stimulated the secretion of several cytokines, especially chemokines (MIP-1α, MIP-1β) and the cytokines associated with pro-inflammatory Th1 lymphocytes (IL-6, IL-1β and TNFα).
In the presence of PHA, the FUE decreased the secretion of most of the Th1 cytokines, with the notable exception of TNFα, as well as the cytokines linked to Th2/Treg cells (although not significantly). However, there was a sharp increase in the IL-23. In contrast, the HPE did stimulate the secretion of Th1 cytokines in the presence of PHA, with the exception of IL-1β. In particular, it led to heightened secretion of IL12p70 and IFN-γ, two major pro-inflammatory cytokines. Finally, it should be noted that both extracts, in the presence of PHA, increased the secretion of the chemokine IL-8 significantly.
|
Without PHA |
PHA (5 µg/mL) |
|||||||||
|
Control |
FUE |
HPE |
Control |
FUE |
HPE |
|||||
|
IL-2 |
15.4 ± 7 |
13.39 ± 8 |
1.78 ± 0.8 |
328 ± 111 |
100 ± 44 |
186 ± 94 |
||||
|
IL-21 |
2.6 ± 0.4 |
1.77 ± 0.6 |
Und. |
7 ± 1.5 |
1.7 ± 0.2 |
(--) |
4.7 ± 1.7 |
|||
|
IL-23 |
21 ± 2.9 |
21.9 ± 2.5 |
13.3 ± 1.2 |
(--) |
386 ± 76 |
836 ± 80 |
(++) |
388 ± 173 |
||
|
IL-4 |
1.4 ± 0.6 |
7.6 ± 0.9 |
(++) |
1.9 ± 0.6 |
199 ± 57 |
59.9 ± 15.2 |
115 ± 42 |
|||
|
IL-10 |
14.4 ± 4.3 |
19.8 ± 6 |
11.6 ± 4.6 |
10355 ± 2253 |
5417 ± 2701 |
7231 ± 878 |
||||
|
IL-5 |
Und. |
Und. |
Und. |
33.9 ± 14 |
8.2 ± 3.1 |
17.5 ± 8.9 |
||||
|
IL12p70 |
0.73 ± 0.4 |
Und. |
Und. |
28.3 ± 9 |
15.5 ± 7 |
(--) |
53.2 ± 34 |
|||
|
IL-1β |
9.2 ± 4.8 |
114.6 ± 74 |
2.68 ± 2.4 |
2549 ± 237 |
1331 ± 8.7 |
1330 ± 26.2 |
||||
|
IL-6 |
34.8 ± 19.3 |
2928 ± 2658 |
27 ± 21.6 |
11129 ± 1247 |
13972 ± 42 |
14129 ± 153 |
||||
|
TNFα |
15.9 ± 5.4 |
287 ± 128 |
(++) |
30.3 ± 27.7 |
4637 ± 8934 |
7133 ± 234 |
(++) |
7171 ± 764 |
(++) |
|
|
IFNγ |
2.2 ± 0.7 |
1.4 ± 0.3 |
0.5 ± 0.4 |
3882 ± 1408 |
658 ± 472 |
9096 ± 4587 |
(++) |
|||
|
IL-8 |
624 ± 176 |
849 ± 0.9 |
434 ± 190 |
941 ± 101 |
1228 ± 1.4 |
(++) |
1238 ± 5.5 |
(++) |
||
|
MIP1α |
4.2 ± 1.6 |
450 ± 372 |
(++) |
13.9 ± 13.1 |
1758 ± 340 |
1046 ± 9.4 |
1087 ± 26 |
|||
|
MIP1β |
137 ± 63 |
1311 ± 405 |
(++) |
273 ± 170 |
6400 ± 356 |
6389 ± 153 |
6685 ± 324 |
|||
1(++): positive change between Control and 50 µg/mL of corresponding extract (p<0.05); (--): negative change between Control and 50 µg/mL of corresponding extract (p<0.05) (Mann-Whitney tests). Und. : Undetectable. Three independent experiments were performed.
Table 4: Cytokine secretion of PBMCs (pg/mL). Data were shown as means ± SEM 1.
4. Discussion
It is well established that oxidative stress is a key component of inflammation. Whilst ROS and nitric oxide (NO) are considered in physiological state as signaling molecules involved in several cell functions, they are deleterious in case of prolonged or chronic inflammation [17]. Thus, the antioxidant capability of a compound can be an indicator of its interest as an anti-inflammatory agent. Hence, we first tested the direct antioxidant activities of our extracts. The ORAC and DPPH values obtained were congruent with the differences of composition of our two extracts. Indeed, flavonoids were found in large quantities, the values being in line with those obtained in previous studies [18], as well as ellagitannins whose presence in F. ulmaria has already been highlighted in other studies. Both of these have been reported as good radical scavengers [19,20]. The FUE was also more efficient than the HPE at scavenging nitric oxide. The FUE was again the most effect extract in connection with free ferrous iron, an important generator of ROS in vivo as it enters the Fenton reaction. However, both extracts failed to reduce PMA-induced ROS production by PBMCs beyond 15 min of incubation. Thus, the capacity of the FUE to reduce ROS production seems entirely linked to its direct antioxidant i.e. scavenger capacities, rather than to a real metabolic cellular effect.
Intriguingly, depending on the condition of incubation, the two extracts had the totally opposite effects on COX-2/PGE2. In the basal state, both the HPE and the FUE increased the COX-2 relative expression and the release of extracellular PGE2, thus showing a slight pro-inflammatory effect, which could be significant and necessary to the resolution of the first stages of acute inflammation. When incubated with LPS, PGE2 secretion was dampened by the extracts. With respect to the HPE, several studies have suggested that this dampening may be assigned mainly to verbascoside and 8-O-p-coumaroylharpagide [21,22]. Different compounds are thought to be responsible for the inhibitory activity of the FUE, mainly quercetin and salicylic acid [23,24]. Accordingly it can be said that, in the arachidonic acid pathway, different molecules led to the same results.
In addition to the lipid mediators and the antioxidant capabilities, we also investigated the potential cytokine modulation driven by our extracts. In contrast with our precedent results on COX-2/PGE2, in which both the FUE and the HPE had similar outcomes, the PBMC cytokine profiles revealed to be quite different. Besides testing our extracts on cells in the basal state, we also evaluated PBMCs stimulated with PHA, a T cell mitogen, in order to compare and understand their impact in a context of antigen-like stimulation condition vs at a physiological state [25,26].
First, in the basal state, the HPE had little effect on cytokine secretion globally, with the exception of IL-21 and IL-23, which were drastically decreased. IL-21 and IL-23 are typical features of Th17 lymphocytes and could help maintaining the pool of Th17 cells [27]. What is more, it has been suggested that Th17 response plays an important role in multiple autoimmune disease, such as rheumatoid arthritis [28] or psoriasis [29] for example. Given that Harpagophytum is employed more and more as a complement in the treatment of rheumatoid arthritis, the effects of our extract support the reason behind its efficiency within this pathology [30-32]. However, in PHA stimulated PBMCs, the HPE failed to prevent the increase of IL-21 significantly nor, more importantly, the increase of IL-23 secretion. Thus, the impact of the HPE on the Th17 lymphocyte subset would need further investigation in a context of chronic inflammation.
The results of the FUE in the basal state differed from that of the HPE. The FUE strongly increased the secretion of pro-inflammatory cytokines, which is consistent with our hypothesis and previous observations, those being that the FUE is pro-inflammatory in the basal state. Besides, although the FUE increased the production of IL-4 (Th2 group) in parallel to the pro-inflammatory cytokines [33], the overall equilibrium between Th1 and Th2 was unbalanced by the extract in favor of Th1. This is in keeping with what has been observed by Park et al., who found that quercetin, one of the constituent of meadowsweet [34], favored the Th1 over the Th2 profile. In addition, the FUE enhanced the secretion of MIP-1α and MIP-1β, which are two CC-type chemokines involved in the recruitment of mononuclear cells and considered as pro-inflammatory via their CCR1 and CCR5 receptors [16,35].
In a context of excessive inflammation, even if the HPE impaired the overall cytokine secretion profile, the effect was significant for only three cytokines, due to their strong individual variability. Surprisingly, the HPE strongly increased the production of TNFα and IL-8. It also increased IFNγ which is thought to act as a protecting agent during rheumatoid arthritis [28]. Therefore, this result may also confirm the interest of using Harpagophytum procumbens for this pathology. Nonetheless, it is important to remember that IFNγ is a major effector of the Th1 response, inhibiting the development of a Th2 or Th17 response [36]. As a consequence, it remains difficult to predict the global impact of these cytokines.
As regards the FUE extract, a wide panel of cytokines was impacted by the extract in stimulated conditions. In particular, it increased the secretion of IL-23 and TNFα and decreased IL-21 and IL-12p70. The elevated concentration of TNFα seems to be in contradiction with previous studies of this plant. For example, Drummond et al., when studying either meadowsweet extract or quercetin alone, found that they inhibited the secretion of TNFα [37]. However, findings of elevated concentrations are in accordance with the study of Liao and Lin, who found that quercetin enhanced inflammation in mice macrophages via the increase of TNFα, IL1β and IL6 [38].
Lastly, in the presence of PHA both of our extracts enhanced IL-8 secretion. IL-8, which is a CXC chemokine, is known as a potent attractor of monocytes as well as neutrophils [39]. In addition, we observed that the extracts sped up the differentiation of THP-1 monocytes into macrophages. Thus, the extracts might speed up leukocyte chemotaxis, especially monocytes and facilitate their activation toward macrophages. At the same time, their capacity to trigger the secretion of pro-inflammatory cytokines could then indicate a capacity to favor the induction of adaptive immunity and host protection. Especially for the FUE, this capacity might also be supported by the fact that it enhanced the PBMC secretion of chemokines MIP-1α and β in the basal state.
5. Conclusion
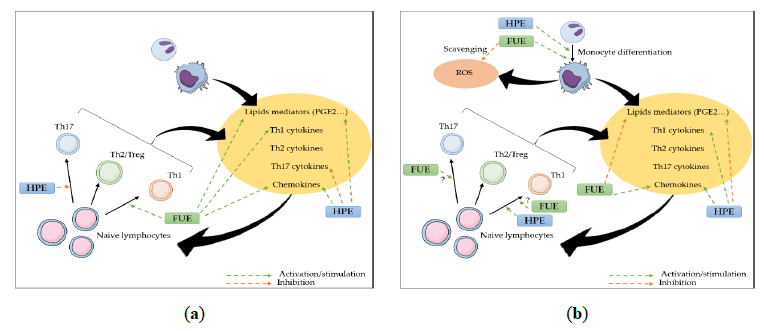
In summary, this study suggests that our extracts do indeed have an anti-inflammatory effect, but that their impact on the immune response seem to be of a rather immune-stimulant nature. Indeed, though both plants are known as anti-inflammatory herbs, apart from their similar anti-inflammatory effect on COX-2/PGE2, both could improve neutrophil and monocyte recruitment, as well as monocytes/macrophages and Th1, and presumably Th17, activation (Figure 6).
Author Contributions: Conceptualization, Edwige Ranouille, Albert Tourette, Pierre Chalard and Florence Caldefie-Chezet; Data curation, Juliette Cholet; Formal analysis, Juliette Cholet and Maël Gainche; Funding acquisition, Pierre Chalard and Florence Caldefie-Chezet; Investigation, Juliette Cholet, Maël Gainche, Clémence Ogéron, Alexandre Berry and Florence Caldefie-Chezet; Methodology, Juliette Cholet, Marjolaine Vareille-Delarbre, Caroline Decombat and Maël Gainche; Project administration, Pierre Chalard and Florence Caldefie-Chezet; Resources, Maël Gainche, Clémence Ogéron, Alexandre Berry and Florence Caldefie-Chezet; Supervision, Marjolaine Vareille-Delarbre, Caroline Decombat, Pierre Chalard and Florence Caldefie-Chezet; Validation, Caroline Decombat and Florence Caldefie-Chezet; Visualization, Juliette Cholet and Maël Gainche; Writing – original draft, Juliette Cholet, Maël Gainche and François Senejoux; Writing – review & editing, Juliette Cholet, Marjolaine Vareille-Delarbre, Caroline Decombat, Alexandre Berry, Laetitia Delort, Isabelle Ripoche, Marion Vermerie, Didier Fraisse, Catherine Felgines, Edwige Ranouille, Jean-Yves Berthon, Yves Troin, François Senejoux, Pierre Chalard and Florence Caldefie-Chezet.
Funding
This work was co-supported by the European Union through the European Funds for Regional Development (FRI cluster 2 Plantinauv).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Katani? J, Boroja T, Mihailovi? V, Nikles S, Pan SP. In vitro and in vivo assessment of meadowsweet (Filipendula ulmaria) as anti-inflammatory agent. J. Ethnopharmacol 193 (2016): 627–636.
- Mncwangi N, Chen W, Vermaak I, Viljoen AM, Gericke N. Devil’s Claw—A review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J Ethnopharmacol 143 (2012): 755–771.
- Andersen ML, Santos EH, Seabra M. de LV, da Silva AA, Tufik S. Evaluation of acute and chronic treatments with Harpagophytum procumbens on Freund’s adjuvant-induced arthritis in rats. J Ethnopharmacol 91 (2004): 325–330.
- Jang MH, Lim S, Han SM, Park HJ, Shin I, et al. Harpagophytum procumbens Suppresses Lipopolysaccharide-Stimulated Expressions of Cyclooxygenase-2 and Inducible Nitric Oxide Synthase in Fibroblast Cell Line L929. J Pharmacol Sci 93 (2003): 367–371.
- Katani? J, Pferschy-Wenzig EM, Mihailovi? V, Boroja T, Pan SP, et al. Phytochemical analysis and anti-inflammatory effects of Filipendula vulgaris Moench extracts. Food and chemical toxicology 122 (2018): 151-162.
- Mahomed, Ismail M., and John AO Ojewole. Analgesic, antiinflammatory and antidiabetic properties of Harpagophytum procumbens DC (Pedaliaceae) secondary root aqueous extract. Phytotherapy research 18 (2004): 982-989.
- Samard?i? S, Arsenijevi? J, Bo?i? D, Milenkovi? M, Teševi? V, et al. Antioxidant, anti-inflammatory and gastroprotective activity of Filipendula ulmaria (L.) Maxim. and Filipendula vulgaris Moench. Journal of ethnopharmacology 213 (2018): 132-137.
- Drummond EM, Harbourne N, Marete E, Jacquier JC, O'Riordan D, et al. An in vivo study examining the antiinflammatory effects of chamomile, meadowsweet, and willow bark in a novel functional beverage. Journal of dietary supplements 10 (2013): 370-380.
- Fiebich BL, Heinrich M, Hiller KO, Kammerer N. Inhibition of TNF-α synthesis in LPS-stimulated primary human monocytes by Harpagophytum extract SteiHap 69. Phytomedicine 8 (2001): 28-30.
- Gandhi GR, Neta MT, Sathiyabama RG, Quintans JD, e Silva AM, et al. Flavonoids as Th1/Th2 cytokines immunomodulators: A systematic review of studies on animal models. Phytomedicine 44 (2018): 74-84.
- Balakrishnan N, Panda AB, Raj NR, Shrivastava A, Prathani R. The evaluation of nitric oxide scavenging activity of Acalypha indica Linn root. Asian Journal of Research in Chemistry 2 (2009): 148-150.
- Dubost N, Ou B, Beelman R. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem 105 (2007): 727–735.
- Ordonez A, Gomez J, Vattuone M, Isla M. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem 97 (2006): 452–458.
- Gillespie KM, Chae JM, Ainsworth EA. Rapid measurement of total antioxidant capacity in plants. Nat Protoc 2 (2007): 867–870.
- Meda NR, Fraisse D, Gnoula C, Vivier M, Felgines C, et al. Characterization of antioxidants from Detarium microcarpum Guill. et Perr. leaves using HPLC-DAD coupled with pre-column DPPH assay. Eur Food Res Technol 243 (2017): 1659–1666.
- Wang J, Tian Y, Phillips KLE, Chiverton N, Haddock G, et al. Tumor necrosis factor α- and interleukin-1β-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum 65 (2013): 832–842.
- Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid Redox Signal 20 (2014): 1126–1167.
- Pukalskien? M, Slapšyt? G, Dedonyt? V, Lazutka JR, Mierauskien? J, et al. Genotoxicity and antioxidant activity of five Agrimonia and Filipendula species plant extracts evaluated by comet and micronucleus assays in human lymphocytes and Ames Salmonella /microsome test. Food Chem Toxicol 113 (2018): 303–313.
- Moilanen J, Karonen M, Tähtinen P, Jacquet R, Quideau S, et al. Biological activity of ellagitannins: Effects as anti-oxidants, pro-oxidants and metal chelators. Phytochemistry 125 (2016): 65–72.
- Omololu PA, Rocha JBT, Kade IJ. Attachment of rhamnosyl glucoside on quercetin confers potent iron-chelating ability on its antioxidant properties. Exp Toxicol Pathol 63 (2011): 249–255.
- Abdelouahab N, Heard C. Effect of the Major Glycosides of Harpagophytum procumbens (Devil’s Claw) on Epidermal Cyclooxygenase-2 (COX-2) in Vitro. J Nat Prod 71 (2008): 746–749.
- Huang THW, Tran VH, Duke RK, Tan S, Chrubasik S. Harpagoside suppresses lipopolysaccharide-induced iNOS and COX-2 expression through inhibition of NF-κB activation. J Ethnopharmacol 104 (2006): 149–155.
- Harbourne N, Marete E, Jacquier JC, O’Riordan D. Effect of drying methods on the phenolic constituents of meadowsweet (Filipendula ulmaria) and willow (Salix alba). LWT - Food Sci Technol 42 (2009): 1468–1473.
- Tordiman C, Coge F, Andre N, Rique H, Spedding M, et al. Characterisation of cyclooxygenase 1 and 2 expression in mouse resident peritoneal macrophages in vitro; interactions of non steroidal anti-inflammatory drugs with COX2. Biochim. Biophys. Acta BBA - Lipids Lipid Metab 1256 (2009): 249–256.
- Carvalho EVMM, Oliveira WF, Coelho LCBB, Correia MTS. Lectins as mitosis stimulating factors: Briefly reviewed. Life Sci 207 (2018): 152–157.
- Song C, Luchtman D, Kang Z, Tam EM, Yatham LN, et al. Enhanced inflammatory and T-helper-1 type responses but suppressed lymphocyte proliferation in patients with seasonal affective disorder and treated by light therapy. J Affect Disord 185 (2015): 90–96.
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu. Rev. Immunol 27 (2019): 485–517.
- Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: A two-year prospective study (the DAMAGE study cohort). Arthritis Rheum 54 (2006): 1122–1131.
- Menon B, Gullick NJ, Walter GJ, Rajasekhar M, Garrood T, et al. Interleukin-17+CD8+ T Cells Are Enriched in the Joints of Patients With Psoriatic Arthritis and Correlate With Disease Activity and Joint Damage Progression: IL-17+CD8+ T Cell Enrichment in the PsA Joint. Arthritis Rheumatol 66 (2014): 1272–1281.
- Denner SS. A Review of the Efficacy and Safety of Devil?s Claw for Pain Associated With Degenerative Musculoskeletal Diseases, Rheumatoid, and Osteoarthritis: Holist. Nurs Pract 21 (2007): 203–207.
- Dudics S, Langan D, Meka R, Venkatesha S, Berman B, et al. Natural Products for the Treatment of Autoimmune Arthritis: Their Mechanisms of Action, Targeted Delivery, and Interplay with the Host Microbiome. Int J Mol Sci 19 (2018): 2508.
- Yarnell E. Herbs for Rheumatoid Arthritis. Altern Complement Ther 23 (2017): 149–156.
- Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 74 (2015): 5–17.
- Park H, Lee CM, Jung ID, Lee JS, Jeong Y, et al. Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int Immunopharmacol 9 (2009): 261–267.
- Surmi BK, Hasty AH. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vascul Pharmacol 52 (2010): 27–36.
- Shachar I, Karin N. The dual roles of inflammatory cytokines and chemokines in the regulation of autoimmune diseases and their clinical implications. J. Leukoc Biol 93 (2013): 51–61.
- Drummond EM, Harbourne N, Marete E, Martyn D, Jacquier J, et al. Inhibition of Proinflammatory Biomarkers in THP1 Macrophages by Polyphenols Derived From Chamomile, Meadowsweet and Willow bark: ANTIINFLAMMATORY POLYPHENOLS FROM HERBS. Phytother Res 27 (2013): 588–594.
- Liao YR, Lin JY. Quercetin, but Not Its Metabolite Quercetin-3-glucuronide, Exerts Prophylactic Immunostimulatory Activity and Therapeutic Antiinflammatory Effects on Lipopolysaccharide-Treated Mouse Peritoneal Macrophages Ex Vivo. J. Agric. Food Chem 62 (2014): 2872–2880.
- Laing K. Chemokines. Dev Comp Immunol 28 (2004): 443–460.

 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 