Compound Heterozygous ITGB3 Pathogenic Variants In A Patient With Glanzmann Thrombasthenia
Article Information
Antonia Lenz1#, Doris Boeckelmann1#*, Martina Bührlen2, Kathrin Dengler3, Jürgen Kunz4, Barbara Zieger1
#Authors contributed equally to this work
1Department of Pediatrics and Adolescent Medicine, Division of Pediatric Hematology and Oncology, Medical Center - University of Freiburg, Faculty of Medicine, Germany
2Department of Pediatrics and Adolescent Medicine, Klinikum Bremen Mitte, Bremen, Germany
3medical laboratory Bremen, coagulation and hematology, Haferwende, Bremen, Germany
4LADR-MVZ Bremen, Germany
*Corresponding Author: Dr. Doris Boeckelmann, Department of Pediatrics and Adolescent Medicine, Division of Pediatric Hematology and Oncology, Medical Center - University of Freiburg, Faculty of Medicine, Germany
Received: 14 July 2020; Accepted: 31 July 2020; Published: 09 September 2020
Citation: Antonia Lenz, Doris Boeckelmann, Martina Bührlen, Kathrin Dengler, Jürgen Kunz, Barbara Zieger. Compound Heterozygous ITGB3 Pathogenic Variants In A Patient With Glanzmann Thrombasthenia. Archives of Clinical and Medical Case Reports 4 (2020): 813-819.
View / Download Pdf Share at FacebookAbstract
Glanzmann Thrombasthenia is a rare platelet function disorder which is characterized by decreased expression and/or dysfunction of the platelet receptor αIIbβ3 (GPIIb/IIIa). The disease is due to alterations in ITGA2B or ITGB3 the genes encoding for the receptor subunits αIIb (GPIIb) and β3 (GPIIIa) and mostly inherited autosomal recessively. We report about a one-year old girl presenting with petechiae and hematomas shortly after birth. Platelet light transmission aggregometry was impaired after stimulation with ADP, arachidonic acid and collagen. Stimulation with ristocetin reached normal values, but showed an undulating course. Flow cytometry revealed severely decreased expression of CD41 (αIIbβ3). Molecular genetic analysis of the candidate genes and family genotyping identified two compound heterozygous variants in Exon 10 of ITGB3: c.1552C>T (p.Gln518*) and c.1639T>G (p.Cys547Gly). According to the guidelines of ACMG the variants were classified as pathogenic (Class 5). The nonsense variant c.1552C>T has not been reported before.
Keywords
Inherited platelet disorder; Glanzmann Thrombasthenia; ITGB3; Bleeding symptoms
Inherited platelet disorder articles, Glanzmann Thrombasthenia articles, ITGB3 articles, Bleeding symptoms articles
Inherited platelet disorder articles Inherited platelet disorder Research articles Inherited platelet disorder review articles Inherited platelet disorder PubMed articles Inherited platelet disorder PubMed Central articles Inherited platelet disorder 2023 articles Inherited platelet disorder 2024 articles Inherited platelet disorder Scopus articles Inherited platelet disorder impact factor journals Inherited platelet disorder Scopus journals Inherited platelet disorder PubMed journals Inherited platelet disorder medical journals Inherited platelet disorder free journals Inherited platelet disorder best journals Inherited platelet disorder top journals Inherited platelet disorder free medical journals Inherited platelet disorder famous journals Inherited platelet disorder Google Scholar indexed journals Glanzmann Thrombasthenia articles Glanzmann Thrombasthenia Research articles Glanzmann Thrombasthenia review articles Glanzmann Thrombasthenia PubMed articles Glanzmann Thrombasthenia PubMed Central articles Glanzmann Thrombasthenia 2023 articles Glanzmann Thrombasthenia 2024 articles Glanzmann Thrombasthenia Scopus articles Glanzmann Thrombasthenia impact factor journals Glanzmann Thrombasthenia Scopus journals Glanzmann Thrombasthenia PubMed journals Glanzmann Thrombasthenia medical journals Glanzmann Thrombasthenia free journals Glanzmann Thrombasthenia best journals Glanzmann Thrombasthenia top journals Glanzmann Thrombasthenia free medical journals Glanzmann Thrombasthenia famous journals Glanzmann Thrombasthenia Google Scholar indexed journals Thrombasthenia articles Thrombasthenia Research articles Thrombasthenia review articles Thrombasthenia PubMed articles Thrombasthenia PubMed Central articles Thrombasthenia 2023 articles Thrombasthenia 2024 articles Thrombasthenia Scopus articles Thrombasthenia impact factor journals Thrombasthenia Scopus journals Thrombasthenia PubMed journals Thrombasthenia medical journals Thrombasthenia free journals Thrombasthenia best journals Thrombasthenia top journals Thrombasthenia free medical journals Thrombasthenia famous journals Thrombasthenia Google Scholar indexed journals ITGB3 articles ITGB3 Research articles ITGB3 review articles ITGB3 PubMed articles ITGB3 PubMed Central articles ITGB3 2023 articles ITGB3 2024 articles ITGB3 Scopus articles ITGB3 impact factor journals ITGB3 Scopus journals ITGB3 PubMed journals ITGB3 medical journals ITGB3 free journals ITGB3 best journals ITGB3 top journals ITGB3 free medical journals ITGB3 famous journals ITGB3 Google Scholar indexed journals Bleeding symptoms articles Bleeding symptoms Research articles Bleeding symptoms review articles Bleeding symptoms PubMed articles Bleeding symptoms PubMed Central articles Bleeding symptoms 2023 articles Bleeding symptoms 2024 articles Bleeding symptoms Scopus articles Bleeding symptoms impact factor journals Bleeding symptoms Scopus journals Bleeding symptoms PubMed journals Bleeding symptoms medical journals Bleeding symptoms free journals Bleeding symptoms best journals Bleeding symptoms top journals Bleeding symptoms free medical journals Bleeding symptoms famous journals Bleeding symptoms Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals aggregometry articles aggregometry Research articles aggregometry review articles aggregometry PubMed articles aggregometry PubMed Central articles aggregometry 2023 articles aggregometry 2024 articles aggregometry Scopus articles aggregometry impact factor journals aggregometry Scopus journals aggregometry PubMed journals aggregometry medical journals aggregometry free journals aggregometry best journals aggregometry top journals aggregometry free medical journals aggregometry famous journals aggregometry Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals radiograph articles radiograph Research articles radiograph review articles radiograph PubMed articles radiograph PubMed Central articles radiograph 2023 articles radiograph 2024 articles radiograph Scopus articles radiograph impact factor journals radiograph Scopus journals radiograph PubMed journals radiograph medical journals radiograph free journals radiograph best journals radiograph top journals radiograph free medical journals radiograph famous journals radiograph Google Scholar indexed journals Electrocardiography articles Electrocardiography Research articles Electrocardiography review articles Electrocardiography PubMed articles Electrocardiography PubMed Central articles Electrocardiography 2023 articles Electrocardiography 2024 articles Electrocardiography Scopus articles Electrocardiography impact factor journals Electrocardiography Scopus journals Electrocardiography PubMed journals Electrocardiography medical journals Electrocardiography free journals Electrocardiography best journals Electrocardiography top journals Electrocardiography free medical journals Electrocardiography famous journals Electrocardiography Google Scholar indexed journals
Article Details
1. Introduction
Glanzmann Thrombasthenia (GT) is a well characterized platelet disease with mostly autosomal recessive inheritance (OMIM #273800). The key feature of GT is severely impaired or absent platelet aggregation in response to multiple platelet agonists except for ristocetin and a reduction/absence of integrin αIIbβ3 surface expression or dysfunction of the receptor [1]. The integrin αIIbβ3 is the main platelet fibrinogen receptor and consists of 2 subunits: αIIb (ITGA2B; 30 exons) and β3 (ITGB3; 15 exons). The encoding genes are less than 200 kb apart on chromosome 17q21 [2-4]. There are three classical types of GT. In type I, αIIbβ3 expression is very low (<5%), whereas in GT type II αIIbβ3 expression is slightly reduced (5-20%) and in variant-type GT αIIbβ3 is dysfunctional and may be due to a defect in genes involved in integrin activation [1, 5, 6]. The subunits of αIIb and β3 consolidate as a non-covalent heterodimer [7]. The subunit αIIb has four major extracellular domains known as β-propeller, thigh, calf-1 and calf-2. The subunit β3 comprises of 8 domains: four EGF (epidermal growth factor) domains, the domains bI, hybrid, PSI (plexin/semaphoring/integrin) and the β-tail [8-14]. Variants causing GT can affect biosynthesis as well as structure and function of αIIbβ3 complex [15]. To date the public version of Human Genome Mutation Database (HGMD) comprises 204 pathogenic variants in ITGA2B and 149 in ITGB3 [access 06/2020]. Affected patients are suffering from moderate to severe hemorrhagic syndrome. Symptoms can appear rapidly after birth including gastrointestinal bleeding, petechiae, hematomas and mucocutaneous bleeding (i.e. epistaxis) [16]. There are several other defects in the primary hemostasis which may present with similar symptoms (i.e. other platelet receptor defects). Therefore, comprehensive diagnostics are important to identify the cause of bleeding diathesis.
Here, we report compound heterozygous pathogenic variants in ITGB3 causing Glanzmann disease, to our knowledge one of them is novel and has not been reported before.
2. Patient, Materials and Methods
The index patient is a one-year old Caucasian girl presenting with petechiae and hematomas distributed over the whole body already several hours postpartum. The girl also experienced postnatal cytomegalic virus infection and has two small muscular ventricular septal (VSD) defects, which are not relevant considering hemodynamics. After the baby started crawling even larger hematomas developed. Dentition caused prolonged bleeding for a day and was stopped using tranexamic acid. Sometimes mild bleeding symptoms were observed after scratching. Post-puncture bleeding symptoms occurred for 2 hours after blood sampling. Blood analyses revealed that the platelet count was increased (502 G/l). Normal values were found for red and white blood cell count and blood smear. Global coagulation tests (aPTT, INR) and von Willebrand parameters were normal. The girl is the first child of non-consanguine parents. The mother is easily prone to hematomas after insignificant trauma. During delivery the mother suffered major blood loss due to placenta accreta and received two red blood cell concentrates. There was no bleeding during and after a nose operation. No bleeding diathesis is reported for the father.
2.1 Platelet Functional Analyses
2.1.1 Platelet count and platelet aggregometry: Platelet count was measured using an automated cell counter (Sysmex XN Series, Norderstedt, Germany). Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were prepared by centrifugation as described before [17]. Platelet counts in PRP were adjusted to a concentration of 250 G/L with PPP. Platelet aggregometry analyses were performed after stimulation with the platelet agonists: collagen (0,19 mg/mL; Mölab, Germany), adenosine diphosphate (ADP; 20 μmoL/L; Mölab, Germany), epinephrine (100 µM Mölab, Germany), and ristocetin (0.6 und 1.5 mg/mL; Mölab, Germany) using APACT 4004 (Labitec, Ahrensburg, Germany).
2.1.2 Flow cytometry: Flow cytometry analysis (Navios, Beckman Coulter, Krefeld, Germany) of CD41, CD42b, CD62P (Beckman Coulter, Krefeld, Germany) and platelet-bound fibrinogen (Dako, Carpinteria, CA) was performed according to standard protocol of LADR GmbH medical care unit (Bremen, Germany).
2.2 Molecular genetic analysis
Written Informed consent was given for all family members. Genomic DNA from EDTA blood was isolated using QIAamp® DNA Blood Mini Kit (Qiagen, Hilden, Germany). For the index patient all exons of the genes ITGA2B and ITGB3 were amplified by PCR with intronic primers. The purification products were sequenced directly. Analysis was performed by sequence assembly and alignment software Codon Code® Aligner and variant analyzing software Alamut® visual. Variants were classified according to the guidelines of the ACMG (American College of Medical Genetics and Genomics). Family genotyping was done for the two pathogenic variants in ITGB3.
3. Results
Platelet aggregometry analysis revealed severely impaired aggregation after stimulation with ADP, adrenaline, collagen and arachidonic acid. Agglutination after stimulation with ristocetin reached normal values (Table 1). Flow cytometry analysis showed reduced basal expression of CD41 (GPIIb/IIIa) compared to healthy control (Figure 1) whereas expression of CD42 (GPIb) was normal. Also CD62 expression was normal after activation with TRAP and ADP. Molecular genetic analysis of the index patient identified two heterozygous variants in exon 10 of ITGB3 (Table 2). One variant (c.1552C>T) is resulting in a premature Stop Codon at amino acid 518 (p.Gln518*; position without signal peptide: p.Gln492*). The variant is neither listed in population data bases (dbSNP, gnomAD, ESP) nor in disease databases (HGMD public version, Sinai Central, ClinVar).
The second heterozygous variant c.1639T>G leads to an exchange of the highly conserved (up to C. elegans) cysteine at position 547 by the physiochemical different glycine (p.Cys547Gly; position without signal peptide: Cys521Gly). This non-synonymous coding variant is found in dbSNP as a not validated entry (rs902952044) and in gnomAD with low allele frequency of 0.0032% (amount of counted allele frequencies: 1/ amount of all alleles: 30984). The variant is listed in HGMD public version. In silico prediction is concordant pathogenic in PolyPhen2, Mutation Taster and SIFT. Family genotyping of Exon 10 identified heterozygous c.1639T>G (p.Cys547Gly) as paternal variant, whereas, c.1552C>T (p.Gln518*) was found maternally. Family genotyping confirmed compound heterozygosity in index patient (Figure 2).
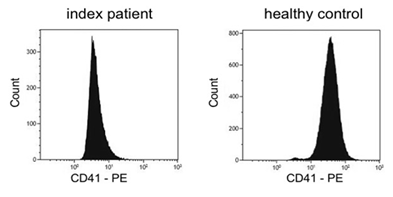
Figure 1: Flow cytometric quantification of platelet surface αIIbβ3 (CD41). Reduced expression of CD41 (Arbitrary unit: 340) in patient’s platelets compared to healthy control (Arbitrary unit: 780).
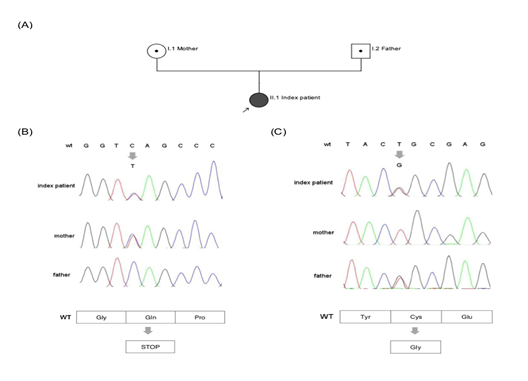
Figure 2: A Pedigree: Parents (I.1 mother and I.2 father) are carrier of heterozygous variants in exon 10; index patient (II.1) is affected compound heterozygous; arrow marks index patient. B Chromatogram (ITGB3, Exon10) shows heterozygous c.1552C>T in index patient and mother, resulting in a premature Stop Codon and wildtype sequence for father (Gln). C Chromatogram (ITGB3, Exon10) shows heterozygous c.1639 T>G in index patient and father and wildtype sequence for mother. Amino acid change is from Cys to Gly.
|
Inductor |
Max. aggregation/agglutination |
Normal control |
|
ADP (20µmol) |
3% |
60-90% |
|
Adrenaline (100 µM). |
9% |
60-90% |
|
Collagen (0,19 mg/mL) |
45% |
60-90% |
|
Arachidonic acid (0,5 mg/mL) |
3% |
60-90% |
|
Ristocetin (0,6 mg/mL) |
2% |
<20% |
Table 1: Platelet aggregometry analysis.
|
Specification |
Variant 1 |
Variant 2 |
|
Nucleotide |
c.1552C>T |
c.1639T>G |
|
Amino acid |
p.Gln518* |
p.Cys547Gly |
|
Allele frequency |
- |
gnomAD: ALL 0.0032% |
|
Variant type |
Nonsense |
Missense |
|
In silico prediction |
- |
Pathogenic in PolyPhen2, Mutation Taster, SIFT |
|
Occurrence in HGMD public |
- |
Yes (Buitrago et al., 2015) |
|
Domain |
β3 |
β3 |
|
Class according to ACMG |
5 (pathogenic, serious consequences) |
5 (pathogenic) |
|
Family genotyping |
||
|
Index patient |
Heterozygous |
Heterozygous |
|
Mother |
Heterozygous |
WT |
|
Father |
WT |
Heterozygous |
WT, wild type; HGMD access 06/2020
Table 2: Characteristics of the two variants identified in ITGB3 (NM_000212).
5. Discussion
In this study we describe the early onset of Glanzmann Thrombasthenia due to compound heterozygous variants in exon 10 of ITGB3. To our knowledge the nonsense variant has not been reported so far. The nonsense variant c.1552C>T (p.Gln518*) interrupts the reading frame by a premature Stop codon (normal protein length 788 aa). The mRNA produced might be a target for nonsense mediated decay. Due to loss of protein function we classified the variant as pathogenic (Class 5). The second allele carries a heterozygous non-synonymous coding variant c.1639T>G, (p.Cys547Gly). In silico prediction was concordant pathogenic in 3 programs. According to UniProt (https://www.uniprot.org/) the cysteines at position 547 and 534 form a disulfide bond. The amino acid exchange from cysteine to glycine is likely causing substantial impairment of the tertiary structure due to the loss of the disulfide bond (534-547). The importance of cysteine at position p.547 is supported by the described variant c.1641C>G resulting in exchange from cysteine to Tryptophan (p.Cys547Trp) in a Glanzmann Thrombasthenia patient [18]. Kannan et al. used molecular modeling and showed that Cys547 is located in the EGF-3 domain of β3, which has intense contact with the calf-1 domain of αIIb. They presumed that the loss of Cys547 may cause conformational changes which lead to retention of the aberrant αIIbβ3 complex [18]. Furthermore, in HEK293 cells expressing the Cys547Gly variant a profound reduction in β3 expression was found [19]. Buitrago and colleagues anticipated that Cys547Gly would decrease receptor expression due to the loss of the disulfide bonding. According to these findings we classified the variant as Class 5 (pathogenic).
5. Conclusions
Biochemical and molecular genetic analysis led to early diagnosis for this patient with Glanzmann Thrombasthenia. Early diagnosis helped to treat bleeding symptoms more effective and improves surgical management. Identifying the disease-causing variants led to a better understanding of molecular genetic mechanisms affecting the αIIbβ3 integrin receptor. In addition, the results will help regarding the genetic counseling for the parents.
Acknowledgements
We thank Eileen Lerner for excellent technical assistance.
Conflicts of Interest
We have no conflict of interest to report.
Author Contributions
A.L. and D.B. contributed equally to the study design, performed molecular genetic analyses and data interpretation and wrote the manuscript. M.B. took care of the patient and collected blood samples. K.D. performed platelet aggregometry. J.K. performed flow cytometry analysis. B.Z. contributed to the study design, supervision of the molecular genetic analyses and manuscript editing. All authors critically reviewed the manuscript, with approval of the final draft.
References
- George JN, Caen JP, Nurden Glanzmann's thrombasthenia: the spectrum of clinical disease. Blood 75 (1990): 1383-1395.
- Bray PF, et al. Physical linkage of the genes for platelet membrane glycoproteins IIb and IIIa. Proc Natl Acad Sci U S A 85 (1988): 8683-8687.
- Heidenreich R, et al. Organization of the gene for platelet glycoprotein IIb. Biochemistry 29 (1990): 1232-1244.
- Wilhide CC, et al. The human integrin beta3 gene is 63 kb and contains a 5'-UTR sequence regulating expression. Blood 1997. 90(10): 3951-61.
- Caen JP, Congenital Bleeding Disorders with Long Bleeding Time and Normal Platelet Count* I. Glanzmann’s Thrombasthenia (Report of Fifteen Patients). Amercian Journal of Medicine (1966).
- Nurden AT, et al. Glanzmann thrombasthenia: a review of ITGA2B and ITGB3 defects with emphasis on variants phenotypic variability and mouse models. Blood 118 (2011): 5996-6005.
- Bray PF, et al. Biogenesis of the platelet receptor for fibrinogen: evidence for separate precursors for glycoproteins IIb and IIIa. Proc Natl Acad Sci USA 83 (1986): 1480-1484.
- Adair BD, Yeager M. Three-dimensional model of the human platelet integrin alpha IIbbeta 3 based on electron cryomicroscopy and x-ray crystallography. Proc Natl Acad Sci US 99 (2002): 14059-14064.
- Carrell NA, et al. Structure of human platelet membrane glycoproteins IIb and IIIa as determined by electron microscopy. J Biol Chem 260 (1985): 1743-1749.
- Xiao T, et al. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature 432 (2004): 59-67.
- Xiong JP, et al. Crystal structure of the complete integrin alphaVbeta3 ectodomain plus an alpha/beta transmembrane fragment. J Cell Biol 186 (2009): 589-600.
- Xiong JP, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science 294 (2001): 339-345.
- Ye F, et al. Recreation of the terminal events in physiological integrin activation. J Cell Biol 188 (2010): 157-173.
- Zhu J, et al. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell 32 (2008): 849-861.
- O'Toole TE, et al. Efficient surface expression of platelet GPIIb-IIIa requires both subunits. Blood 74 (1989): 14-18.
- Nurden AT, Glanzmann thrombasthenia. Orphanet J Rare Dis 1 (2006): 10.
- Lahav J, et al. Sustained integrin ligation involves extracellular free sulfhydryls and enzymatically catalyzed disulfide exchange. Blood 100 (2002): 2472-2478.
- Kannan M, et al. Molecular defects in ITGA2B and ITGB3 genes in patients with Glanzmann thrombasthenia. J Thromb Haemost 7 (2009): 1878-1885.
- Buitrago L, et al. alphaIIbbeta3 variants defined by next-generation sequencing: predicting variants likely to cause Glanzmann thrombasthenia. Proc Natl Acad Sci USA 112 (2015): E1898-E1907.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
