D-Allose, a Trace Component in Human Serum, and Its Pharmaceutical Applicability
Article Information
Hideya Shintani1, Tomoya Shintani2,3,*, Masashi Sato4
1Department of Internal Medicine, Osaka Saiseikai Izuo Hospital, Osaka, 551-0032, Japan
2Graduate School of Agricultural Science, Ehime University, Matsuyama, 790-8577, Japan
3Department of Nutritional Representative - Supplement Adviser, The Japanese Clinical Nutrition Association, Tokyo, 153-0044, Japan
4Faculty of Agriculture, Kagawa University, Kagawa, 761-0701, Japan
*Corresponding Author: Tomoya Shintani, Graduate School of Agricultural Science, Ehime University, Matsuyama 790-8577, Japan; Department of Nutritional Representative - Supplement Adviser, The Japanese Clinical Nutrition Association, Tokyo, 153-0044, Japan
Received: 15 July 2020; Accepted: 30 July 2020; Published: 11 August 2020
Citation:
Hideya Shintani, Tomoya Shintani, Masashi Sato. D-Allose, a Trace Component in Human Serum, and Its Pharmaceutical Applicability. International Journal of Applied Biology and Pharmaceutical Technology 11 (2020): 200-213.
View / Download Pdf Share at FacebookAbstract
Abstract
Although cord blood is important in human development, its functions are not well understood. Cord blood shows high potential for use in research studies aimed at maintaining a healthy, long life, as it contains various functional components such as anticancer agents against leukemia. Recently, D-Allose, a stereoisomer of D-glucose, was found in human umbilical cord blood and sera of women. D-Allose has been shown to have anti-cancer activity, reduce reperfusion damage, and have anti-metabolic syndrome effects. Previously, Chen et al. reviewed the production and function of D-Allose but did not discuss its potential as a medical drug based on its presence in women’s serum, its safety in humans, its GMP manufacturing, its efficacy in liver injury, its influence on animal lifespan, the biological activity of its derivatives, or its applicability as a diagnostic reagent. In this review, we cite new references and describe the above points of D-Allose and its potential for pharmaceutical applications.
Keywords
D-Allose (D-All); Serum component; Medicinal plants; Reperfusion damage prevention; Anti-cancer; Anti-metabolic syndrome effects
D-Allose (D-All)articles, Serum component articles, Medicinal plants articles, Reperfusion damage prevention articles, Anti-cancer articles, Anti-metabolic syndrome effects articles
Article Details
Introduction
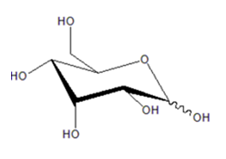
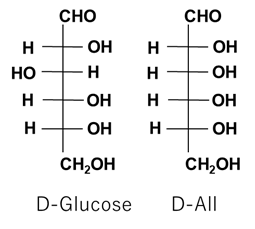
Cord blood is critical in development, but is not well understood [1,2]. Some components of maternal and cord blood have been reported to protect against oxidative toxicity in the blood [3]. Additionally, multipotent stem cells can be isolated from umbilical cord blood [4]. Cord blood shows wide potential for research on maintaining a healthy, long life because of its anti-disease and anticancer properties. Recently, D-All (Figure 1) was detected in human umbilical cord blood [5]. D-All, a stereoisomer of D-glucose, is a rare sugar present in limited quantities in nature [6]. It is considered as a "fetal sugar" because it was first found in umbilical cord blood in 2013; its levels are very low in nature [6]. The physiological role of D-All in vivo is unknown, but various physiological functions with pharmaceutical applications have been revealed. Studies of the physiological function of D-All have conventionally focused on its function as an antioxidant that suppresses oxidative stress, which is considered to be the most important function. Previously, Chen et al. reviewed the function of D-All but did not discuss its potential as a medical drug based on its presence in women’s serum, its safety in humans, the possibility of GMP manufacturing, its efficacy in liver injury, its influence on animal lifespan, the biological activity of its derivatives, or its applicability as a diagnostic reagent [7]. They focused instead on the microbial production of D-All and its general physiological activity.
The current review of recent studies discusses D-All in pharmaceutical applications. We also describe the possible involvement of D-All in the regulation of glucose metabolism and of intracellular stress in addition to the points mentioned above.
D-Allose Found in Human Umbilical Cord Blood and Sera from Women
In 2013, it was reported that vaginal delivery following spontaneous labor leads to higher levels of D-All in umbilical cord blood sera than after elective cesarean section without labor, confirming that D-All is present in the human body [5]. This suggests that infants born via vaginal delivery can produce D-All as protection against ischemia, although this hypothesis requires further detailed investigation.
Metabolomics of celomic fluid, such as pleural effusion and abdominal dropsy, from 41 women revealed D-All in humans [8]. These results were confirmed by serum metabolomic analysis of women by another research group [9]. However, these studies did not examine the physiological significance and metabolic mechanism of D-All in the body. Previous studies [6, 8, 9] investigated specimens of only fetal or female origin. There are no reports of the presence of D-All in men, and thus, future studies involving men are warranted. Nonetheless, D-All has unique characteristics, as described in this review.
D-Allose in Medicinal Plants
No studies have demonstrated the presence of D-All in animals except in fetuses and women. However, at least three types of medicinal herbs, Halodule pinifolia, Tamarindus indica, and Crataeva nurvala, contain free D-All (Figure 2). The Indian seaweed H. pinifolia, which protects against urinary tract infections, was found to contain 3.7% D-All [10]. D-All has also been detected in the aqueous extract of T. indica pulp (2017). T. indica is a plant used in traditional medicine to treat cold, fever, stomach disorders, diarrhea, and jaundice and as a skin cleanser [11]. C. nurvala Buch-Hum is an indigenous herb extensively used in traditional medicine in South Asian countries to treat rheumatic fever, gastric irritation, and constipation [11].
GMP-Grade Manufacturing of D-Allose
D-All, a stereoisomer of D-glucose, is a C-3 epimer of glucose [13] (Figure 3). Crystalline D-All is available only in the laboratory [14], as Good Manufacturing Practice (GMP)-grade D-All has not been completely established. However, safe manufacturing at the food industrial level has nearly been achieved. D-All can be produced using the Izumoring strategy, which is a systematic method for producing all monosaccharide isomers using microbial enzymes such as L-rhamnose isomerase enzymes and/or immobilized enzymes [15]. Mass production methods developed by Izumori have provided insight into the biological properties of rare sugars [16-17]. D-All is mass produced from D-allulose, which is a structural isomer of D-All, through the action of recombinant L-rhamnose isomerase cross-linked with glutaraldehyde [18]. D-allulose is enzymatically produced from D-fructose, which is a product of glycolysis and isomerization of starch [17]. Further research, optimization, and standardization of food-grade production methods may lead to the establishment of GMP-grade production methods.
Preclinical and Clinical Safety Tests
The safety of D-All has been established to some extent in preclinical and clinical phase 1 studies. For example, the acute and sub-chronic toxicity of D-All in rats has been investigated. In an acute toxicity test, the calculated LD50 value was reported to be 20.5 g/kg. In a sub-chronic toxicity test (6 months), no abnormal values were found in the serum chemical and hematological test results. These results suggest that D-All is not toxic to rats [19].
No diarrhea or any other abnormality was observed when D-All was administered orally (8 g/kg body weight) to young rats. Urinary and fecal excretion levels of D-All during the 24 h following oral administration were 91% and 3%, respectively. The D-All content in the stomach decreased rapidly over 3 h, with only low levels found in the small intestine and cecum. These results suggest that D-All is largely absorbable from the digestive tract into the blood and is then rapidly excreted through the urine [20]. A phase 1, clinical, randomized, single-blind, crossover study was conducted in healthy subjects to evaluate D-All fermentability in the large intestine, urinary excretion, and carbohydrate energy expenditure [21].
Protection of Ischemia–Reperfusion by D-Allose
Reactive oxygen species (ROS) are very important in oxidative stress, and a small percentage of oxygen in living organisms exists as ROS [22]. ROS are produced via oxidative phosphorylation in the mitochondrial electron transfer system [23]. Under conditions that promote the generation of ROS through the action of electron transfer system inhibitors, D-glucose and D-All partially suppress the generation of mitochondrial-derived ROS and ATP [24]. This suggests that the mitochondrial respiratory chain competes with D-glucose by binding to mitochondrial respiratory chain molecules rather than by inhibiting D-glucose uptake. The molecular basis for the prevention of hypertension and inhibition of ischemic disorders at the individual level is the inhibitory effect on ROS generation in mitochondria. In rat studies, D-All was shown to attenuate brain damage and induce neuroprotection by reducing oxidative DNA damage caused by ROS [25]. D-glucose contributes to ATP synthesis to promote the production of ROS in neuronal cells. However, D-All suppresses ROS production by competing with D-glucose in the mitochondria, as previously described [26]. The mechanism underlying the therapeutic potential of D-All in brain ischemia/reperfusion injury may involve attenuation of blood-brain barrier disruption and the inflammatory response via PPARγ-dependent regulation of nuclear factor-κB [27]. Additionally, mitogen-activated protein kinase 1, a key regulator of cellular physiology and immune responses, was recently shown to have an alleviative effect on ischemia/reperfusion injury in skin flaps [28]. Because interventional methods for reducing reperfusion injury are limited, further studies of the potential of D-All are needed.
Anti-Cancer Effects
The inhibitory effect of D-All on cancer has been reported by many researchers. Recently, the possible anti-cancer mechanism of D-All was elucidated. Cancer dysregulates cell growth, which requires large amounts of D-glucose as a major energy source [29] and is related to the Warburg effect. Thus, overexpression of some glucose transporters (GLUTs) promotes the transport of glucose into cancer cells. D-All is also involved in thioredoxin-interacting protein (TXNIP)-mediated redox regulation [30], which has been reported to inhibit osteoclast differentiation and cancer cell proliferation [31]. Additionally, TXNIP can downregulate the overexpression of GLUT1 in many cancer cells. Thus, D-All suppresses the proliferation of many cancer cells. Moreover, D-All has been shown to prohibit cancer cells from absorbing glucose [32]. The anti-proliferative mechanism is partially attributed to p27kip1, a marker of the cell cycle transition, where D-All induces TXNIP in vitro [30], which has been confirmed in vivo [33]. Various studies have demonstrated the antitumor effect of D-All using many types of cultured cancer cells.
Interestingly, intracellular TXNIP expression is specifically and markedly enhanced in MOLT-4F cells (T-cell lymphoblastic leukemia) by D-All treatment, and increases in p27kip1, a cell cycle inhibitor, have been observed [34]. To enhance its effectiveness against leukemia, a D-All derivative with anti-proliferative activity against MOLT-4F cells has been developed [35]. In addition, D-All has been reported to suppress a variety of carcinomas, such as cervical and skin [36], ovarian [37], hepatocellular [38], pancreas [39], prostate [40-41], head, neck [42], and lung cancers [43].
Some studies have shown that combined use of D-All with other interventions is more effective than the use of other interventions alone. In particular, some studies have shown that combining D-All with other chemotherapeutic agents has synergistic anti-cancer effects, as will be described below. The antitumor efficacy of 2-deoxyglucose and D-All has been reported to be enhanced by p38 inhibition in pancreatic and ovarian cell lines [39]. D-All facilitates the efficacy of radiation by enhancing apoptosis of cancer cells by inducing TXNIP expression [44]. Combination therapy with D-All and docetaxel was recently shown to be more effective than single therapy with docetaxel [41]. A more recent study investigated the radiosensitizing and chemosensitizing potential of D-All in an in vivo model of head and neck cancer. The results suggest that D-All can enhance the antitumor effects of chemoradiotherapy while sparing normal tissues [45]. Taken together, the evidence suggests that D-All has many potential applications in clinical treatment.
Anti-Metabolic Syndrome Effects
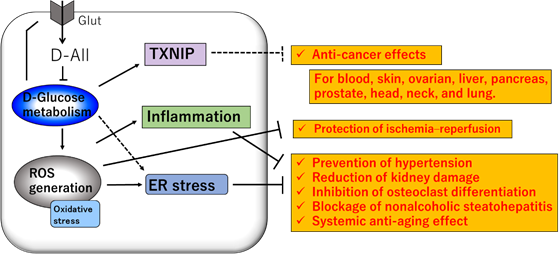
The main physiological effect of D-All is the inhibition of ROS generation by modulating carbohydrate metabolism. Unlike antioxidants with reducing power, such as vitamin C, D-All is characterized by its ability to suppress ROS generation. Recently, D-All was reported to reduce endoplasmic reticulum (ER) stress, which is related to ROS [46]. The effect of D-All on superoxide radicals and ER stress was examined in cultured human coronary artery endothelial cells [47]. ER stress was measured with an ER stress-sensitive secreted protein and by determining the phosphorylation levels of key proteins in the unfolded protein response, namely CHOP47, eIF2α, and JNK1. D-glucose increased ER stress, whereas treatment with D-All reduced superoxide radicals and ER stress in vitro.
Along with reductions in ROS and ER stress or suppression of inflammation, D-All showed the following effects: prevention of hypertension, reduction of kidney damage, inhibition of osteoclast differentiation, and anti-aging effects (Figure 4).
D-All has been reported to suppress the generation of reactive oxygen-producing enzymes and increases in blood pressure [48], because ROS generated from the vascular endothelium contribute to increased blood pressure. A significant decrease in both systolic and diastolic blood pressure was observed in salt-sensitive hypertensive rats raised on a saline diet after administration of 2 g/kg body weight/day of D-All. D-All has a protective effect against ischemic organ damage, such as cerebral infarction and myocardial infarction, which are caused by large amounts of ROS. In addition, D-All is not only a functional food that can inhibit cancer cell and osteoblast proliferation, but also a pharmaceutical and quasi-drug with increasing potential for development.
Cisplatin is a potent antineoplastic agent widely used to treat various forms of cancer. However, its therapeutic use is limited because of its dose-dependent nephrotoxicity. Inflammatory mechanisms may play an important role in the pathogenesis of cisplatin-induced nephrotoxicity, which D-All has been shown to ameliorate in mice [49]. As renal impairment caused by metabolic syndrome is also known to be induced by inflammation, it would be interesting to assess the effect of D-All on renal impairment in future studies.
Anti-aging effects mediated by reduction in D-glucose metabolism have been reported in experiments using nematodes [50]. In a nematode life extension study, Caenorhabditis elegans wild-type N2 and daf-16 (nematode homolog of FOXO1, a transcription factor downstream of the insulin signaling pathway) mutants were used. The mean life span of N2 nematodes treated with D-All (28 mM) was prolonged by approximately 24%. In contrast, no lifespan extension was observed in the daf-16 mutant. These results suggest that D-All-induced prolonged lifespan is mediated by the insulin signaling pathway.
In general, the rate of bone resorption by osteoclasts and bone formation by osteoblasts is balanced to maintain bone strength. In osteoporosis, there is excessive bone breakdown by osteoclasts, and the osteoblasts cannot keep up with bone formation. D-All may prevent osteoporosis by inhibiting osteoclast differentiation in vitro [30].
Nonalcoholic steatohepatitis (NASH) is characterized by excess lipid accumulation and inflammation in hepatocytes. A study aiming to provide insights into the preventive effects of D-All on the onset of NASH suggested that D-All prevents NASH by blocking hepatic lipid accumulation and progressive inflammation. [51]. More recently, the improvement effect of D-All was also reported in a rat model of liver injury, which involved the use of a toxic substance that overproduces active oxygen [52].
Applications in Medicinal and Functional Foods
Based on its high solubility, it could be presumed that D-All can be used as a syrup, similar to D-glucose. D-All is a zero-caloric sweetener with a sweetness 80% of that of sucrose [53, 54]. D-All, as a sucrose substitute, is an ideal food additive. Moreover, as it is a reducing sugar with an aldehyde group, it can be recruited in the Maillard reaction, which is a chemical reaction between amino acids and reducing sugars that results in improvements in color and taste [55]. In addition, since D-All is a monosaccharide, it exerts an osmotic pressure, which could prevent microbial contamination; this pressure is expected to be higher than that of sugar. Thus, D-All is a promising candidate for applications in medicinal and functional foods.
Applications in Medical Drugs or Medical Reagents
Applications in medical drugs or medical reagents, in addition to foods, are expected. D-All, as an immunosuppressant, could increase allograft survival [56]. Some studies have found that D-All has a cryoprotective effect on cell survival [57]. D-All could also reduce tissue injury [58] and be used during organ transplantation to decrease tissue damage via its anti-oxidative effects [59].
The antioxidant functions of D-All could make it a therapeutic option for various diseases resulting from oxidative stress. D-All has the potential for clinical application as a pharmaceutical agent, such as in the clinical therapy or prevention of cancer, stroke [60], and hypertension, because of its remarkable physiological functions discussed so far.
D-All has potential therapeutic applications in diseases caused by nematode parasitism. Its effectiveness in these diseases may be due to species differences in the metabolic effects of D-All. The effect of metronidazole on trichomonad parasites was valid for prohibiting the parasite from developing drug resistance [61]. D-All can repress the growth of the nematode Caenorhabditis elegans [62], and the nematocidal activity of newly obtained D-All derivatives against C. elegans larvae has also been reported [63]. Further studies on its applications to ward off parasitic nematodes are required.
The physical properties of D-All are also being investigated to determine their application in clinical diagnosis. To improve noninvasive positron emission tomography investigations, the behavior of labeled D-All derivatives in organs was examined [64]. In a basic assessment of its stability in such application, high-performance liquid chromatography analysis showed no decomposition of the compound even after up to 6 h in rabbit blood plasma [64]. The diagnostic properties of novel fluorine-18-labeled D-All were also tested [65]. Further research on D-All will lead to a promising future for applications of D-All and its derivatives as medical drugs or medical reagents.
Discussion and Conclusion
There are many reports on the oxidative stress-reducing effect of D-All [7]. Higher amounts of D-All have been detected in fetal umbilical cord in spontaneously delivered fetuses than in fetuses born via cesarean section. These results suggest that D-All may reduce oxidative stress at birth in humans, although further studies need to be conducted to determine its functions in detail.
Inhibition of cancer cell proliferation could be the most important physiological effect of D-All. In general, cancer mortality has been found to be lower in women than in men [66], but the reason for this is unknown. The fact that D-All and its anticancer and anti-metabolic syndrome effects have been reported only in the blood of women and not of men may support this epidemiological study.
The World Health Organization recommends restricting consumption of sugars to no more than 10% of daily caloric intake, with a proposal to lower this level to 5% or less for optimal health [67]. Naturally occurring d-All is an attractive alternative sweetener. The results of animal studies have indicated a utilizable energy value of approximately zero, and safety tests for future functional sweeteners have already been completed.
A systematic review of the anticancer effect of TXNIP inducer was conducted to summarize the potential of thioredoxin system inhibitors for cancer treatment [68]. Because D-All is a trace component of serum and circulates throughout the body in the bloodstream, it may be effective against systemic cancers. The effect of D-All on the thioredoxin system may be very promising for clinical cancer therapy in the future. However, while the research available on the anti-cancer effect of D-All is being examined, discussions on the mechanism of action and relevance of involvement of D-All in cancer are rare and are not included in this review. The studies and evidence presented in this review, such as those based on in vitro experiments, were in preliminary stages, and the conclusions derived are speculative. Further research is necessary to more accurately describe mechanisms and applications of D-All in cancer treatment.
D-All, a stereoisomer of D-glucose, is a rare sugar present in limited quantities in nature. D-All has been detected at low levels in human cord blood and in medicinal herbs. However, it is challenging to study D-All owing to its low abundance. D-All could be produced by the Izumoring strategy, which is a systematic method for the production of all monosaccharide isomers using microbial enzymes. The mass production methods developed by Izumori have provided insights into its biological properties. The safety of D-All has been established in rats and in clinical studies, and its utilizable energy value is approximately zero. Studies have demonstrated various functions of D-All, such as anti-tumor activity, anti-hypertension effects, and brain protection from ischemic injury (Figure 5). These functions mainly result from the suppression of ROS generation via competition for D-glucose utilization. Based on its beneficial health effects, D-All is expected to be a potent anti-metabolic syndrome drug and pharmaceutical precursor. These beneficial properties make it a suitable candidate for medicinal foods or functional foods.
Funding
This study was partially supported by the Sasakawa Scientific Research Grant from The Japan Science Society [2018-6021].
Acknowledgments
We thank Ken Izumori (Kagawa University, Kagawa, Japan), Masaaki Tokuda (Kagawa University, Kagawa, Japan), and the late Kazuhiro Okuma (Matsutani Chemical Industry Co., Ltd., Hyogo, Japan) for their valuable advice.
Conflicts of Interest
The authors declare no conflicts of interest. Tomoya Shintani is an employee of Matsutani Chemical Industry Co., Ltd. (Hyogo, Japan), which manufactures and sells sugar and carbohydrate products. However, the company provided no financial support for this study. This manuscript was written as joint research while Tomoya Shintani was at Ehime University.
References
- Hassink SG, Spear ML, De Lancey E, et al. Placental Leptin: An important new growth factor in intrauterine and neonatal development? Pediatric Research 41 (1997): 232-232.
- Bermúdez L, García-Vicent, C, López J, et al. Assessment of ten trace elements in umbilical cord blood and maternal blood: association with birth weight. Journal of Translational Medicine 13 (2015): 291.
- Yoshioka T, Kawada K, Shimada T, et al. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. American Journal of Obstetrics and Gynecology 135 (1979): 372-376.
- Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 103 (2004): 1669-1675.
- Izumori K. Production and application of functional monosaccharides including rare sugar. Oleoscience 13 (2013): 421-422.
- Hashimoto F, Nishiumi S, Miyake O, et al. Metabolomics analysis of umbilical cord blood clarifies changes in saccharides associated with delivery method. Early Human Development 89 (2013): 315-320.
- Chen Z, Chen J, Zhang W, et al. Recent research on the physiological functions, applications, and biotechnological production of D-allose. Appl Microbiol Biotechnol 102 (2018): 4269-4278.
- Virgiliou C, Valianou L, Witting M, et al. Metabolic profile of human coelomic fluid. Bioanalysis 9 (2017): 37-51.
- Zhang Z, Hong Y, Chen M, et al. Serum metabolomics reveals metabolic profiling for women with hyperandrogenism and insulin resistance in polycystic ovary syndrome. Metabolomics 16 (2020): 20.
- Kannan RR, Arumugam R, Anantharaman P. Chemical composition and antibacterial activity of Indian seagrasses against urinary tract pathogens. Food Chemistry 135 (2012): 2470-2473.
- Doughari J. Antimicrobial activity of Tamarindus indica Linn. Tropical Journal of Pharmaceutical Research 5 (2006): 597-603.
- Moniruzzaman M, Mannan MA, Hossen Khan MF, et al. The leaves of Crataeva nurvala Buch-Ham. modulate locomotor and anxiety behaviors possibly through GABAergic system. BMC Complementary and Alternative Medicine 18 (2018): 283.
- Kozakai T, Fukada K, Kuwatori R, et al. Aqueous phase behavior of the rare monosaccharide D-allose and X-ray crystallographic analysis of D-allose dihydrate. Bulletin of the Chemical Society of Japan 88 (2015): 465-470.
- Ishii T, Senoo T, Kozakai T, et al. Crystal structure of β-d,l-allose. Acta crystallographica. Section E, Crystallographic communications 71 (2015): o139.
- Izumori K. Bioproduction strategies for rare hexose sugars. Die Naturwissenschaften 89 (2002): 120-124.
- Granström TB, Takata G, Tokuda M, et al. Izumoring: a novel and complete strategy for bioproduction of rare sugars. Journal of Bioscience and Bioengineering 97 (2004): 89-94.
- Shintani T. Food industrial production of monosaccharides using microbial, enzymatic, and chemical methods. Fermentation 5 (2019): 47.
- Bhuiyan SH, Itami Y, Rokui Y, et al. D-Allose production from D-psicose using immobilized L-rhamnose isomerase. Journal of Fermentation and Bioengineering 85 (1998): 539-541.
- Iga Y, Nakamichi K, Shirai Y, et al. Acute and sub-chronic toxicity of D-allose in rats. Bioscience, Biotechnology, and Biochemistry 74 (2010): 1476-1478.
- Iga Y, Matsuo T. D-allose metabolism in rats. Journal of Japanese Society of Nutrition and Food Science 63 (2010): 17-19.
- Kitagawa M, Tanaka M, Yoshikawa Y, et al. Evaluation of absorption and fermentability of D-mannose, D-sorbose, and D-Allose in humans. Luminacoids Res 22 (2018): 75–82.
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Current Biology 24 (2014): R453-R462.
- Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxidants & Redox Signaling 11 (2009): 1373-1414.
- Murata A, Sekiya K, Watanabe Y, et al. A novel inhibitory effect of D-allose on production of reactive oxygen species from neutrophils. Journal of Bioscience and Bioengineering 96 (2003): 89-91.
- Nakamura T, Tanaka S, Hirooka K, et al. Anti-oxidative effects of d-allose, a rare sugar, on ischemia-reperfusion damage following focal cerebral ischemia in rat. Neuroscience Letters 487 (2011): 103-106.
- Ishihara Y, Katayama K, Sakabe M, et al. Antioxidant properties of rare sugar D-allose: Effects on mitochondrial reactive oxygen species production in Neuro2A cells. Journal of Bioscience and Bioengineering 112 (2011): 638-642.
- Huang T, Gao D, Hei Y, et al. D-allose protects the blood brain barrier through PPARγ-mediated anti-inflammatory pathway in the mice model of ischemia reperfusion injury. Brain Research 1642 (2016): 478-486.
- Ju J, Hou R. D-allose alleviates ischemia/reperfusion (I/R) injury in skin flap via MKP-1. Molecular Medicine 26 (2020): 21.
- Jose C, Bellance N, Rossignol R. Choosing between glycolysis and oxidative phosphorylation: a tumor's dilemma?. Biochimica et Biophysica Acta 1807 (2011): 552-561.
- Yamaguchi F, Takata M, Kamitori K, et al. Rare sugar D-allose induces specific up-regulation of TXNIP and subsequent G1 cell cycle arrest in hepatocellular carcinoma cells by stabilization of p27kip1. International Journal of Oncology 32 (2008): 377-385.
- Yamada K, Noguchi C, Kamitori K, et al. Rare sugar D-allose strongly induces thioredoxin-interacting protein and inhibits osteoclast differentiation in Raw264 cells. Nutrition Research 32 (2012): 116-123.
- Noguchi C, Kamitori K, Hossain A, et al. D-Allose Inhibits Cancer Cell Growth by Reducing GLUT1 Expression. The Tohoku Journal of Experimental Medicine 238 (2016): 131-141.
- Hoshikawa H, Mori T, Mori N. In vitro and in vivo effects of D-allose: up-regulation of thioredoxin-interacting protein in head and neck cancer cells. The Annals of Otology Rhinology and Laryngology 119 (2010): 567-571.
- Hirata Y, Saito M, Tsukamoto I, et al. Analysis of the inhibitory mechanism of D-allose on MOLT-4F leukemia cell proliferation. Journal of Bioscience and Bioengineering 107 (2009): 562-568.
- Ishiyama H, Yanagita RC, Takemoto K, et al. Development of a d-allose-6-phosphate derivative with anti-proliferative activity against a human leukemia MOLT-4F cell line. Carbohydrate Research 487 (2020): 107859.
- Sui L, Dong Y, Watanabe Y, et al. The inhibitory effect and possible mechanisms of D-allose on cancer cell proliferation. International Journal of Oncology 27 (2005): 907-912.
- Sui L, Nomura R, Dong Y, et al. Cryoprotective effects of D-allose on mammalian cells. Cryobiology 55 (2007): 87-92.
- Yokohira M, Hosokawa K, Yamakawa K, et al. Potential inhibitory effects of D-allose, a rare sugar, on liver preneoplastic lesion development in F344 rat medium-term bioassay. Journal of Bioscience and Bioengineering 105 (2008): 545-553.
- Malm SW, Hanke NT, Gill A, et al. The anti-tumor efficacy of 2-deoxyglucose and D-allose are enhanced with p38 inhibition in pancreatic and ovarian cell lines. Journal of Experimental & Clinical Cancer Research 34 (2015): 31.
- Jeong RU, Lim S, Kim MO, et al. Effect of D-allose on prostate cancer cell lines: phospholipid profiling by nanoflow liquid chromatography-tandem mass spectrometry. Analytical and Bioanalytical Chemistry 401 (2011): 689-698.
- Naha N, Lee HY, Jo MJ, et al. Rare sugar D-allose induces programmed cell death in hormone refractory prostate cancer cells. Apoptosis : an International Journal on Programmed Cell Death 13 (2008): 1121-1134.
- Indo K, Hoshikawa H, Kamitori K, et al. Effects of D-allose in combination with docetaxel in human head and neck cancer cells. International Journal of Oncology 45 (2014): 2044-2050.
- Kanaji N, Kamitori K, Hossain A, et al. Additive antitumour effect of D-allose in combination with cisplatin in non-small cell lung cancer cells. Oncology Reports 39 (2018): 1292-1298.
- Hoshikawa H, Indo K, Mori T, et al. Enhancement of the radiation effects by D-allose in head and neck cancer cells. Cancer Letters 306 (2011): 60-66.
- Hoshikawa H, Kamitori K, Indo K, et al. Combined treatment with D-allose docetaxel and radiation inhibits the tumor growth in an in vivo model of head and neck cancer. Oncology Letters 15 (2018): 3422-3428.
- Shintani T, Ohkuma K, Sakoguchi H, et al. Rare sugars D-Psicose and D-Allose as calorie restriction mimetic. Journal of the Brewing Society of Japan 108 (2013): 565-574.
- Mooradian AD, Haas MJ, Onstead-Haas L, et al. Naturally occurring rare sugars are free radical scavengers and can ameliorate endoplasmic reticulum stress. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition (2019): 1-11.
- Kimura S, Zhang GX, Nishiyama A, et al. D-allose, an all-cis aldo-hexose, suppresses development of salt-induced hypertension in Dahl rats. Journal of Hypertension 23 (2005): 1887-1894.
- Miyawaki Y, Ueki M, Ueno M, et al. D-allose ameliorates cisplatin-induced nephrotoxicity in mice. The Tohoku Journal of Experimental Medicine 228 (2012): 215-221.
- Shintani T, Sakoguchi H, Yoshihara A, et al. D-Allose, a stereoisomer of D-glucose, extends the lifespan of Caenorhabditis elegans via sirtuin and insulin signaling. Journal of Applied Glycoscience 66 (2019): 139-142.
- Yamamoto R, Iida A, Tanikawa K, et al. Dietary D-allose ameliorates hepatic inflammation in mice with non-alcoholic steatohepatitis. Food Science and Technology Research 23 (2017): 319-327.
- Suna S, Tokuda M. Effects of D-allose and D-allulose on DEHP toxicities in rats. International Journal of Pharma Sciences and Research 11 (2020) 21-26.
- Mooradian AD, Smith M, Tokuda M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clinical Nutrition ESPEN 18 (2017): 1-8.
- Iida T, Okuma K. Properties of rare sugars (D-psicose, D-allose, D-tagatose) and their use. Oreoscience 13 (2013): 435- 440.
- Sun Y, Hayakawa S, Puangmanee S, et al. Chemical properties and antioxidative activity of glycated α-lactalbumin with a rare sugar, D-allose, by Maillard reaction. Food Chem 95 (2006): 509–517.
- Hossain M, Wakabayashi H, Goda F, et al. Effect of the immunosuppressants FK506 and D-allose on allogenic orthotopic liver transplantation in rats. Transplant Proc 32 (2000): 2021–2023.
- Sui L, Nomura R, Dong Y, et al. Cryoprotective effects of D-allose on mammalian cells. Cryobiology 55 (2007): 87–92.
- Tanaka S, Sakamoto H. Effects of D-allose on the endocytic activity of dendritic cells and the subsequent stimulation of T cells. Cell Immunol 271 (2011): 141–146.
- Kashiwagi H, Asano E, Noguchi C, et al. Beneficial effect of D-allose for isolated islet culture prior to islet transplantation. J Hepatobiliary Pancreat Sci 23 (2016): 37–42.
- Gao D, Kawai N, Nakamura T, et al. Anti-inflammatory effect of D-allose in cerebral ischemia/reperfusion injury in rats. Neurol Med Chir (Tokyo) 53 (2013): 365–374.
- Harada M, Kondo E, Hayashi H, et al. D-Allose and D-psicose reinforce the action of metronidazole on trichomonad. Parasitol Res 110 (2012): 1565–1567.
- Sakoguchi H, Yoshihara A, Izumori K, et al. Screening of biologically active monosaccharides: growth inhibitory effects of D-allose, D-talose, and L-idose against the nematode Caenorhabditis elegans. Biosci Biotechnol Biochem 80 (2016): 1058–1061.
- Sakoguchi H, Shintani T, Ishiyama H, et al. Nematocidal activity of 6- O-octanoyl- And 6- O-octyl-d-allose against larvae of Caenorhabditis elegans. Biosci Biotechnol Biochem 83 (2019): 2194-2197.
- Toyohara J, Yamamoto H, Tago T. Searching for Diagnostic Properties of Novel fluorine-18-labeled D-allose. Ann Nucl Med 33 (2019): 855-865.
- Yamamoto H, Wada K, Toyohara J, et al. Radiosynthesis of 18 F-labeled D-Allose Carbohydrate Research 486 (2019): 107827.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer Journal of Cliniians 6 (2018): 394-424.
- Mooradian AD. In search for an alternative to sugar to reduce obesity. International Journal for Vitamin and Nutrition Research 89 (2019): 113-117.
- Jia JJ, Geng WS, Wang ZQ, et al. The role of thioredoxin system in cancer: strategy for cancer therapy. Cancer Chemother Pharmacol 84 (2019): 453-470.



 Impact Factor: * 3.0
Impact Factor: * 3.0 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
