Decorin Expression and Oncosuppression in Human Embryonic Carcinomas
Article Information
Annele Sainio1*, Mirka Pennanen1, Jukka Laine2, Riikka Lund3, Mirva Söderström2, Teijo Kuopio4, Hannu Järveläinen1,5
1Medical Biochemistry and Genetics, Institute of Biomedicine, University of Turku, Turku, Finland
2Department of Pathology, University of Turku and Turku University Hospital, Turku, Finland
3Turku Centre for Biotechnology, University of Turku, and Åbo Akademi University, Turku, Finland
4Department of Pathology, Central Finland Health Care District, and Department of Biological and Environmental Science, University of Jyväskylä, Jyväskylä, Finland
5Department of Internal Medicine, Satakunta Central Hospital, Sairaalantie 3, 28500 Pori, Finland
*Corresponding Author: Annele Sainio, Medical Biochemistry and Genetics, Institute of Biomedicine, University of Turku, Turku, Finland
Received: 13 May 2019; Accepted: 20 May 2019; Published: 05 August 2019
Citation: Annele Sainio, Mirka Pennanen, Jukka Laine, Riikka Lund, Mirva Söderström, Teijo Kuopio, Hannu Järveläinen. Decorin Expression and Oncosuppression in Human Embryonic Carcinomas. Archives of Clinical and Medical Case Reports 3 (2019): 140-152.
View / Download Pdf Share at FacebookAbstract
Human embryonic stem cells in culture can transform into malignant, cancer-like cells exhibiting lesser differentiation. After transplantation, these transformed cells can form highly malignant germ cell tumors. In humans, germ cell tumors often appear at gonadal sites, like in the testis. In this study, we examined the expression of small leucine rich proteoglycans in normal and karyotypically abnormal human embryonic stem cells using a publicly available transcriptome data. We also examined the expression of the small leucine rich proteoglycans in healthy human testis and in different human testicular non-seminoma germ cell tumors using IST Online database. Furthermore, we localized the expression of decorin, the prototype member of the small leucine rich proteoglycans, in samples representing the above testicular tissues, using in situ hybridization and immunohistochemistry. The analysis revealed that the expression of two small leucine rich proteoglycans, namely decorin and lumican, was induced in normal but not in karyotypically abnormal human embryonic stem cells during early cell differentiation. Similarly, in IST Online database the expression of these two small leucine rich proteoglycans was markedly higher in differentiated teratoma tissue than in undifferentiated embryonal carcinoma tissue. In testicular germ cell tumors decorin expression was completely lacking in the areas of undifferentiated malignant cells. The above results collectively suggest that decorin and lumican have a role in human stem cell differentiation and testicular non-seminoma germ cell tumor formation.
Keywords
Extracellular matrix, Proteoglycan, Stem cell, Teratoma, Embryonal carcinoma, In situ hybridization
Article Details
Abbreviations:
DCN-Decorin; EBs-Embryonic bodies; GAG-glycosaminoglycan; GCTs-Germ cell tumors; hESCs-Human embryonic stem cells; IHC-Immunohistochemistry; ISH-In situ hybridization; LC-Leydig cell; MC-Myoid cell; POUSF1-POU domain class 5 transcription factor 1; PG(s)-Proteoglycan(s); SLRPs-Small leucine-rich PGs; SMC-Smooth muscle cell; SSEA-Stage-spesific embryonic antigen
1. Introduction
Human embryonic stem cells (hESCs) possess unique properties, including the maintenance of pluripotency and the capability to be propagated indefinitely under certain in vitro conditions [1, 2]. However, during culturing these cells can accumulate genomic alterations and epigenomic changes that cause them to develop towards cancer-like cell types [3, 4]. After transplantation, these transformed cells can form highly malignant germ cell tumors (GCTs) [5, 6]. In humans, GCTs often appear at gonadal sites, e.g. in the testis [7], and testicular GCTs represent the most common cancer in young men [8]. Although originating from the same cellular precursor, GTCs can be divided into seminomas and non-seminomas which are categorized as distinct entities [9]. Cells of non-seminomas exhibit features of pluri- to totipotency, and are thus regarded as malignant embryonic stem cells, while seminoma forming cells have more limited potency to differentiate [10]. Very little is known about the microenvironment of non-seminoma GCTs and the role of different stromal factors in the initiation and progression of germ cell tumorigenesis [11, 12]. Extracellular proteoglycans (PGs) are key molecules in mediating signals from the ECM into the cells [13, 14, 15]. From these, the prototype of the small leucine-rich PGs (SLRPs), decorin, has gained the most interest in the regulation of cell differentiation and tumorigenesis [16-23].
Decorin is composed of about a 42 kDa core glycoprotein with one glycosaminoglycan (GAG) side chain of either dermatan sulfate or chondroitin sulfate. Its expression was shown to be induced in normal hESCs during their early differentiation into embryonic bodies (EBs) [18, 24]. On the other hand, decorin expression has been shown to be down-regulated in malignant cells as seen in murine teratocarcinoma cells compared with healthy cells [25]. Also, the intestinal tumor formation was shown to be associated with decorin deficiency in Dcn mouse model [26]. In the present study, we first analyzed the expression of the SLRPs in normal and karyotypically abnormal hESCs during early cell differentiation in vitro, and in different human testicular non-seminoma GCTs in vivo, using publicly available databases. Next, we focused on decorin, the prototype of the SLRPs, and localized its expression in healthy human testis and in various human testicular GCTs.
2. Materials and methods
2.1 Data analysis of SLRP gene expression in normal and karyotypically abnormal hESCs
Gene expression pattern of the SLRP family members (Table 1) was analyzed in normal and culture-adapted hESCs with abnormal karyotype using the publicly available transcriptome data by Enver et al. [3]. This dataset profiles gene expression of normal (early passage) and culture-adapted (late passage) sublines of a single hESC line, reflecting the progressive adaptation of the cells caused by spontaneous differentiation in response to in vitro culture conditions. Cells were sorted for SSEA, a globoseries glycolipid antigen. Specifically, SSEA+ cells represented pluripotent stem cells, and SSEA- cells have already exited this compartment, but retain multilineage differentiation potential. The expression of POU5F1 (POU domain class 5 transcription factor 1) was used as an indicator of cellular differentiation.
|
Class I |
Class II |
Class III |
Class IV |
Class V |
|
Decorin, DCN |
Fibromodulin, FMOD |
Epiphycan, EPYC |
Chondroadherin, CHAD |
Podocan, PODN |
|
Biglycan, BGN |
Lumican, LUM |
Optican, OPTC |
Nyctalopin, NYX |
Podocan-like protein, PODNL1 |
|
Asporin, ASPN |
PRELP |
Osteoglycin, OGN |
Tsukushi, TSKU |
- |
|
ECM2 |
Keratocan, KERA |
- |
- |
- |
|
ECMX |
Osteoadherin, OMD |
- |
- |
- |
Table 1: List of the screened SLRP genes in differentiating hESCs and in IST Online Databank.
2.2 IST Online database analysis of SLRP gene expression in healthy testis and different testicular GCTs
IST Online (http://ist.medisapiens.com/) is the largest integrated and annotated human gene expression data source in the world. In this study, the database was used to compare relative gene expression of the SLRP family members (Table 1) in healthy testis tissue and different testicular non-seminoma GCTs, namely teratoma and embryonal carcinoma.
2.3 Human testicular tissue samples
Human testicular samples included 1 healthy testis tissue and 9 testicular non-seminoma GCTs (6 teratomas, 1 embryonal carcinoma, and 2 mixed GCTs). Teratomas included 1 benign mature teratoma, and 5 malignant teratomas. Healthy testis tissue was derived from orchiectomy of both testes due to prostate cancer. All samples were obtained from the archives of Turku University Hospital, Department of Pathology and Forensic Medicine, Turku, Finland. This study followed the Declaration of Helsinki, and sample collection and study protocol were approved by the National Supervisory Authority for Welfare and Health (Dnr_7324/05.01.00.06/2011). The samples were fixed at 10% neutral-buffered formalin, embedded in paraffin and cut into 5 µm consecutive sections. Sections were used for hematoxylin and eosin (HE) staining, immunohistochemistry (IHC), and in situ hybridization (ISH).
2.4 Immunohistochemistry
Three different ready-to-use mouse monoclonal antibodies from Ventana Medical Systems/Roche Diagnostics were used: CD30 (Ber-2), CkPan (AE1/AE3/PCK26), and PLAP (NB10) with BenchMark XT immunostainer and ultraVIEW Universal DAB Detection Kit (Ventana/Roche, Tucson, Arizona, USA). The IHC analyses for decorin were performed with a rabbit polyclonal antibody (H-80, Santa Cruz Inc., Santa Cruz, CA, dilution 1:400) as previously described. IHC analyses were performed in the Department of Pathology, and Medical Biochemistry and Genetics. All tissue sections were scanned with Pannoramic Digital Slide scanner (The Pannoramic 250 Flash, 3DHISTECH Ltd., Hungary). Digital images were viewed with Pannoramic Viewer (3DHISTECH).
2.5 In situ hybridization for decorin
ISH for decorin was performed on all tissue samples by probing the samples with human decorin antisense and sense single-stranded RNA riboprobes as previously described in detail. ISH analyses were performed in the Department Medical Biochemistry and Genetics. Similarly to the IHC images, the Pannoramic Digital Slide scanner (3DHISTECH) and Pannoramic Viewer (3DHISTECH) were used to view the images.
3. Results
3.1 Expression of SLRPs in normal and karyotypically abnormal hESCs during early cell differentiation
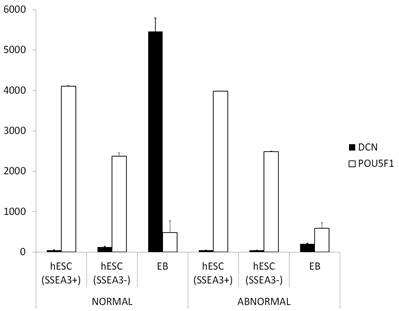
Gene expression pattern of the SLRPs in normal hESCs, compared with culture-adapted hESCs with karyotypic abnormalities, during early cell differentiation was analyzed using the publicly available transcriptome data by Enver et al. [3]. The analysis revealed that in normal hESCs, the expression of two SLRPs, namely decorin (Figure 1) and lumican (data not shown), was markedly increased during cell differentiation into EBs. In contrast, in abnormal hESCs, no similar increase in either decorin or lumican expression was detected during the formation of early EBs.
Figure 1: The expression of decorin during early differentiation of hESCs. Analysis was performed using the publicly available transcriptome data by Enver et al. [3]. In the figure there are average normalized gene expression levels of decorin (DCN) and pluripotent stem cell marker POU5F1 in karyotypically NORMAL or ABNORMAL undifferentiated hESCc (SSEA3+), in early spontaneously differentiated hESCs (SSEA3-) and in late embryonic body (EB). The measurements are from three biological replicates and capped bars on the top of the columns indicate standard deviations of the results. POUSF1, POU domain class 5 transcription factor 1; SSEA, stage-spesific embryonic antigen.
3.2 Relative gene expression of SLRPs in healthy human testis and in different human testicular non-seminoma GCTs
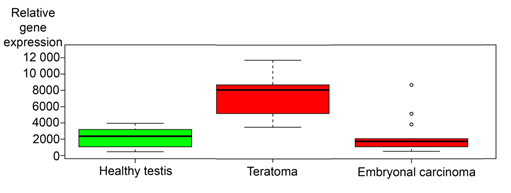
Next the relative gene expressions of the SLRPs (See material and methods, Table 1) were analyzed using IST Online databank (http://ist.medisapiens.com/). The analysis revealed that the relative expression of most SLRPs, was higher in teratoma samples than in embryonal carcinoma (data not shown). Representative box plot image of the gene expression of the prototype member of the SLRPs, decorin, is shown in Figure 2. As an exception to other SLRPs, the expression of nyctalopin, opticin and podocan-like protein 1, was on similar level in teratoma tissue compared to embryonal carcinoma (data not shown). Gene expression of decorin in healthy testis is included in Figure 2 to indicate that decorin is expressed also in differentiated human testicular tissue.
Figure 2: Relative gene expression of decorin in healthy human testis, teratomas and embryonal carcinomas. Analyses are based on IST Online database. Note that decorin expression is at the highest in teratoma tissue samples. The continuous lines in the box plot images represent the median expression level of decorin in different tissue samples and capped bars in the box blot images indicate standard deviations of the results included in the databank.
3.3 Localization of decorin expression in healthy human testis and in different human testicular non-seminoma GCTs
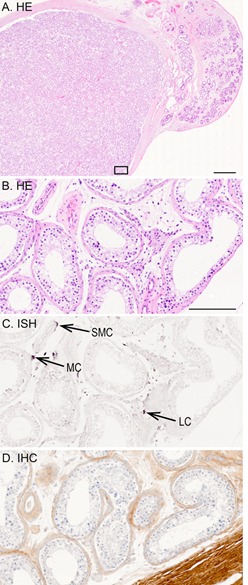
Decorin localization in different testicular tissues was performed using ISH and IHC. According to ISH for decorin, in healthy adult human testis, decorin mRNA expression was detected in spindle-like peritubular myoid cells surrounding seminiferous tubules, and in Leydig cells residing in the interstitium between the tubules (Figure 3). Also, individual smooth muscle cells in the vascular wall of small arteries were positive for decorin mRNA expression. In contrast, germ cells as well as Sertoli cells inside the tubules were negative for decorin mRNA. This was also true for vascular endothelial cells within the testis. The results of the IHC analysis for decorin were similar to those of ISH mentioned above. In healthy testis, positive decorin immunoreactivity was detected in peritubular myoid cells, Leydig cells and the wall of small arteries, while germ cells and Sertoli cells were negative for decorin (Figure 3 C-D).
Figure 3: A representative tissue sample of a healthy adult human testis. (A) HE staining of testis and epididymis. Images B-D are magnified views from the marked testis area in image A; (B) HE staining; (C) ISH for decorin; (D) IHC for decorin. Positive DIG-reaction in ISH can be seen in purple and positive immunoreactivity in brown. Arrows point to decorin positive peritubular myoid cell (MC), Leydig cell (LC) residing in the interstitium between the tubules, and smooth muscle cell (SMC) in the vascular wall of a small artery. Scale bar in image A=2 mm, and in B-D=200 µm.
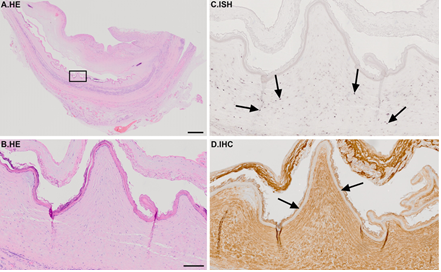
ISH further revealed that in the tissue sample representing mature differentiated teratoma, decorin mRNA was widely detected in stromal cells inside the teratoma tissue (Figure 4). However, epithelial cells, fat and glial tissues as well as endothelial cells were negative for decorin mRNA. Regarding the IHC, positive immunoreactivity for decorin was seen widely within the tumor area (Figure 4D).
Figure 4: Representative tissue sample of a benign embryonal testicular teratoma. (A) HE staining. Images B-D are magnified views from the marked area in image A; (B) HE staining; (C) ISH for decorin; (D) IHC for decorin. Positive DIG-reaction in ISH can be seen in purple and positive immunoreactivity in brown, respectively. Arrows in image C indicate decorin expressing stromal cells. Arrows in image D point to decorin negative epithelial layer. Note that decorin expression and immunoreactivity are widely detected in the teratoma sample. Scale bar in A=2 mm and B-D=200 µm.
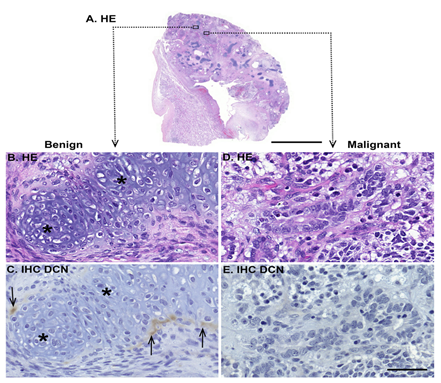
Figure 5: Representative tissue sample of a malignant testicular teratoma. (A) HE staining. Images B-E are magnified views from the marked area in image A., and they show examples of benign (B-C) and malignant areas (D-E) inside the tumor tissue. (B) and (D) are HE staining; (C) and (E) are IHC for decorin. Positive immunoreactivity can be seen in brown. Note that decorin immunoreactivity is detected only in specific areas of differentiated, benign tumor, in this case surrounding the cartilage tissue. Undifferentiated teratoma tissue does not express decorin. Asterisks in images B-C indicate differentiated cartilage tissue, and arrows in image C point out individual decorin expressing benign cells surrounding the cartilage. Scale bar in image A=5 mm, in B-E=50 µm.
In malignant teratomas, decorin mRNA was present only in specific areas of benign tumor, e.g. in the surrounding islands of differentiated cartilage tissue (Figure 5B-C). Decorin expression was not detected in the areas of undifferentiated malignant tumor tissue exhibiting blastic and mitotic cellular phenotypes, with necrosis (Figure 5D-E). Similarly, decorin immunoreactivity was detected only in areas of differentiated benign tumor, e.g. cartilage tissue, whereas undifferentiated teratoma tissue did not express decorin (Figure 5C and E).
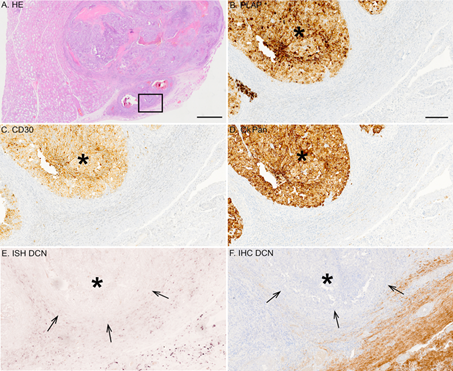
Similarly, in tissue samples representing embryonal carcinoma (Figure 6) and mixed GCT (data not shown) decorin mRNA was completely lacking in the malignant tissue areas. Identical to the ISH, malignant cells were devoid of decorin immunoreactivity in both embryonal carcinoma (Figure 6F) and mixed GCTs (data not shown).
Figure 6: Representative tissue sample of a malignant testicular embryonal carcinoma. (A) HE staining; (B) IHC for PLAP; (C) IHC for CD 30; (D) IHC for CkPan; (E) ISH for decorin; (F) IHC for decorin. Positive DIG-reaction in ISH can be seen in purple and positive immunoreactivity in brown, respectively. Arrows in images E and F point to the border of the carcinoma tissue and asterisks mark the area of the carcinoma. Note that malignant cells are totally negative for decorin expression. Scale bar in A 2 mm and B-F=200 µm.
4. Discussion
Our data analysis revealed that the expression of decorin and lumican was associated with the differentiation of normal hESCs, i.e., the expression level of these two SLRPs increased significantly during the development of late EBs. No similar increase in their expression was seen during the development of EBs in karyotypically abnormal hESC cultures. Previously, both decorin and lumican have been identified among the gene products expressed in differentiated, normal hESCs [18, 24], but the expression profile of these PGs in karyotypically abnormal hESCs remained unknown. During normal physiological cell differentiation, decorin expression was suggested to be positively involved in various processes including gonad differentiation [27], myogenesis [28, 29], chondrogenic differentiation [30], and intestinal cell maturation [26]. Regarding lumican, its expression was shown to be related to the differentiation of myofibroblasts [31] and fibrocytes [32].
The expression of the SLRPs were analyzed using IST Online database (http://ist.medisapiens.com/) where the relative gene expression of each molecule was compared between differentiated teratoma tissue and undifferentiated carcinoma tissue of testicular origin. The results showed that the relative gene expression of most SLRPs was markedly upregulated in teratoma samples, compared with embryonal carcinomas. Gonadal teratomas such as pre-pubertal testicular teratomas are considered to be derived from benign, non-transformed germ cells, the so-called primordial germ cells [33]. However, when teratomas develop in the post-pubertal testis they are usually derived from both normal germ cells and carcinoma in situ cells [34]. Nevertheless, pre-pubertal teratomas can be morphologically similar to post-pubertal ones [7]. This emphasizes the importance of identifying different molecular and cellular factors in the pericellular matrix of GCTs. In the present study, we have shown that the expression of specific ECM macromolecules, particularly decorin, is involved in germ cell derived tumorigenesis.
Additionally, we localized decorin mRNA and molecule in different human testicular GCT tissue samples using ISH and IHC, and found that its expression takes place only in the normal connective tissue, and is completely lacking in the areas of malignant cells. Previously, decorin expression in human testis has been located in the dispersed interstitial fibroblastic cells, and in the perivascular cells of small arteries and capillaries, whereas germ cells and Sertoli cells have been shown to be negative for decorin expression [35, 36]. According to the Human Protein Atlas web portal (http://www.proteinatlas.org), decorin immunostaining in healthy testis has also been shown to be expressed in low levels in Leydig cells [37-38]. These results are in accordance with our present study. Interestingly, Leydig cells have been shown to originate from both vascular smooth muscle cells and pericytes [39-40], as well as from testicular myoid cells [41]. In our study, individual cells representing all the aforementioned cell types were found to be decorin positive. Thus, decorin may be involved in the differentiation of Leydig cells.
Because benign teratoma represents a differentiated tumor in comparison to undifferentiated malignant teratomas and embryonal carcinomas, the observed lack of decorin in malignant tumors may in part be involved in the progression of tumorigenesis in human GCTs via multiple actions. Decorin is able, among other functions, to evoke cell cycle arrest, apoptosis, and antiangiogenic programs [22, 42, 43, 44, 45]. Furthermore, in breast carcinoma cells, decorin has been demonstrated to induce mitophagy [46] which is known to play an important role in the maintenance of cellular homeostasis. Defects in mitophagy promote tumorigenesis [47, 48]. We have previously shown that cancer cells of epithelial or mesenchymal origin are not able to express decorin [49, 50, 51]. To the best of the authors’ knowledge, decorin expression has not been explored before in human GCTs.
In summary, the SLRPs are differentially expressed in hESCs during their early differentiation in vitro, and in various testicular GCTs ex vivo. In particular, decorin expression is induced in normal, but not in karyotypically abnormal hESCs during their differentiation in culture. In testicular non-seminoma GCTs, decorin expression is completely lacking in the areas of malignant cells. Our results suggest that the SLRPs, especially decorin, play a role in the differentiation process of embryonal cells, and in the development of testicular GCTs in human.
Funding
This work was supported by the Research Funds of Turku University and University Hospital, Satakunta Central Hospital and Cancer Foundation of Southwestern Finland.
Acknowledgements
The excellent technical assistance of Jaakko Liippo and Sinikka Collanus is highly appreciated.
Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Reubinoff BE, Pera MF, Fong CY, et al. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nature biotechnology 18 (2000): 399-404.
- Mitalipova MM, Rao RR, Hoyer DM, et al. Preserving the genetic integrity of human embryonic stem cells. Nature biotechnology 23 (2005): 19-20.
- Enver T, Soneji S, Joshi C, et al. Cellular differentiation hierarchies in normal and culture-adapted human embryonic stem cells. Human molecular genetics 14 (2005): 3129-3140.
- Lund RJ, Närvä E and Lahesmaa R. Genetic and epigenetic stability of human pluripotent stem cells. Nature reviews. Genetics 13 (2012): 732-744.
- Blum B and Benvenisty N. The tumorigenicity of human embryonic stem cells. Advances in Cancer Research 100 (2008): 133-158.
- Ben-david U and Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nature reviews. Cancer 11 (2011): 268-277.
- Ulbright TM. Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 12 (2005): 61-79.
- Litchfield K, Summersgill B, Yost S, et al. Whole-exome sequencing reveals the mutational spectrum of testicular germ cell tumours. Nature communications 6 (2015): 5973.
- Oosterhuis JW and Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nature reviews. Cancer 5 (2005): 210-222.
- Nettersheim D and Schorle H. The plasticity of germ cell cancers and its dependence on the cellular microenvironment. Journal of Cellular and Molecular Medicine 21 (2017): 1463-1467.
- Diez-torre A, Silvan U, De wever O, et al. Germinal tumor invasion and the role of the testicular stroma. The International journal of developmental biology 48 (2004): 545-557.
- Diez-torre A, Silvan U, Diaz-nunez M, et al. The role of microenvironment in testicular germ cell tumors. Cancer biology and therapy 10 (2010): 529-536.
- Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annual Review of Biochemistry 67 (1998): 609-652.
- Iozzo RV and Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix biology: journal of the International Society for Matrix Biology 42 (2015): 11-55.
- Merline R, Schaefer RM and Schaefer L. The matricellular functions of small leucine-rich proteoglycans (SLRPs). Journal of cell communication and signaling 3 (2009): 323-335.
- Chen XD, Fisher LW, Robey PG, et al. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 18 (2004): 948-958.
- Gasimli L, Stansfield HE, Nairn AV, et al. Structural remodeling of proteoglycans upon retinoic acid-induced differentiation of NCCIT cells. Glycoconjugate journal 30 (2013): 497-510.
- Gasimli L, Hickey AM, Yang B, et al. Changes in glycosaminoglycan structure on differentiation of human embryonic stem cells towards mesoderm and endoderm lineages. Biochimica et biophysica acta 1840 (2014): 1993-2003.
- Järvinen TA and Prince S. Decorin: A Growth Factor Antagonist for Tumor Growth Inhibition. BioMed research international (2015): 654765.
- Ma HI, Hueng DY, Shui HA, et al. Intratumoral decorin gene delivery by AAV vector inhibits brain glioblastomas and prolongs survival of animals by inducing cell differentiation. International journal of molecular sciences 15 (2014): 4393-4414.
- Melchior-becker A, Dai G, Ding Z, et al. Deficiency of biglycan causes cardiac fibroblasts to differentiate into a myofibroblast phenotype. The Journal of biological chemistry 286 (2011): 17365-17375.
- Neill T, Schaefer L and Iozzo RV. Oncosuppressive functions of decorin. Molecular and cellular oncology, 2 (2015): 975645.
- Schaefer L, Tredup C, Gubbiotti MA, et al. Proteoglycan neofunctions: regulation of inflammation and autophagy in cancer biology. The FEBS journal 284 (2017): 10-26.
- Yang AX, Mejido J, Luo Y, et al. Development of a focused microarray to assess human embryonic stem cell differentiation. Stem cells and development 14 (2005): 270-284.
- Heffron CC, Gallagher MF, Guenther S, et al. Global mRNA analysis to determine a transcriptome profile of cancer stemness in a mouse model. Anticancer Research 27 (2007): 1319-1324.
- Bi X, Tong C, Dockendorff A, et al. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis 29 (2008): 1435-1440.
- Miqueloto CA and Zorn TM. Characterization and distribution of hyaluronan and the proteoglycans decorin, biglycan and perlecan in the developing embryonic mouse gonad. Journal of anatomy 211 (2007): 16-25.
- Brandan E, Cabello-verrugio C and Vial C. Novel regulatory mechanisms for the proteoglycans decorin and biglycan during muscle formation and muscular dystrophy. Matrix biology: journal of the International Society for Matrix Biology 27 (2008): 700-708.
- Li Y, Li J, Zhu J, et al. Decorin gene transfer promotes muscle cell differentiation and muscle regeneration. Molecular therapy: the journal of the American Society of Gene Therapy 15 (2007): 1616-1622.
- Twomey JD, Thakore PI, Hartman DA, et al. Roles of type VI collagen and decorin in human mesenchymal stem cell biophysics during chondrogenic differentiation. European cells and materials 27 (2014): 237-250.
- Jester JV, Brown D, Pappa A, et al. Myofibroblast differentiation modulates keratocyte crystallin protein expression, concentration, and cellular light scattering. Investigative ophthalmology and visual science 53 (2012): 770-778.
- Pilling D, Vakil V, Cox N, et al. TNF-alpha-stimulated fibroblasts secrete lumican to promote fibrocyte differentiation. Proceedings of the National Academy of Sciences of the United States of America 112 (2015): 11929-11934.
- Mosbech CH, Rechnitzer C, Brok JS, et al. Recent advances in understanding the etiology and pathogenesis of pediatric germ cell tumors. Journal of pediatric hematology/oncology 36 (2014): 263-270.
- Jørgensen N, Müller J, Giwercman A, et al. DNA content and expression of tumour markers in germ cells adjacent to germ cell tumours in childhood: probably a different origin for infantile and adolescent germ cell tumours. The Journal of pathology 176 (1995): 269-278.
- Adam M, Schwarzer JU, Kohn FM, et al. Mast cell tryptase stimulates production of decorin by human testicular peritubular cells: possible role of decorin in male infertility by interfering with growth factor signaling. Human reproduction (Oxford, England) 26 (2011): 2613-2625.
- Ungefroren H, Ergun S, Krull NB, et al. Expression of the small proteoglycans biglycan and decorin in the adult human testis. Biology of reproduction 52 (1995): 1095-1105.
- Uhlén M, Björling E, Agaton C, et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Molecular and cellular proteomics: MCP 4 (2005): 1920-1932.
- Uhlén M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based Human Protein Atlas. Nature biotechnology 28 (2010): 1248-1250.
- Davidoff MS, Middendorff R, Enikolopov G, et al. Progenitor cells of the testosterone-producing Leydig cells revealed. The Journal of cell biology 167 (2004): 935-944.
- Davidoff MS, Middendorff R, Muller D, et al. The neuroendocrine Leydig cells and their stem cell progenitors, the pericytes. Advances in Anatomy, Embryology, and Cell Biology 205 (2009): 1-107.
- Landreh L, Spinnler K, Schubert K, et al. Human testicular peritubular cells host putative stem Leydig cells with steroidogenic capacity. The Journal of clinical endocrinology and metabolism 99 (2014): 1227-1235.
- Grant DS, Yenisey C, Rose RW, et al. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene 21 (2002): 4765-4777.
- Järveläinen H, Sainio A and Wight TN. Pivotal role for decorin in angiogenesis. Matrix biology: journal of the International Society for Matrix Biology 43 (2015): 15-26.
- Neill T, Painter H, Buraschi S, et al. Decorin antagonizes the angiogenic network: concurrent inhibition of Met, hypoxia inducible factor 1alpha, vascular endothelial growth factor A, and induction of thrombospondin-1 and TIMP3. The Journal of biological chemistry 287 (2012): 5492-5506.
- Neill T, Schaefer L and Iozzo RV. Decorin: a guardian from the matrix. The American journal of pathology 181 (2012): 380-387.
- Neill T, Torres A, Buraschi S, et al. Decorin induces mitophagy in breast carcinoma cells via peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha) and mitostatin. The Journal of biological chemistry 289 (2014): 4952-4968.
- Chourasia AH, Tracy K, Frankenberger C, et al. Mitophagy defects arising from BNip3 loss promote mammary tumor progression to metastasis. EMBO reports 16 (2015): 1145-1163.
- Springer MZ and Macleod KF. In Brief: Mitophagy: mechanisms and role in human disease. The Journal of pathology 240 (2016): 253-255.
- Böström P, Sainio A, Kakko T, et al. Localization of decorin gene expression in normal human breast tissue and in benign and malignant tumors of the human breast. Histochemistry and cell biology 139 (2013): 161-171.
- Sainio A, Nyman M, Lund R, et al. Lack of decorin expression by human bladder cancer cells offers new tools in the therapy of urothelial malignancies. PloS one 8 (2013): 76190.
- Salomäki HH, Sainio AO, Söderström M, et al. Differential expression of decorin by human malignant and benign vascular tumors. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society 56 (2008): 639-646.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
