Disintegrant Properties of a Newly Derived Superdisintegrant from Lentinus tuber regium in Paracetamol Tablet Formulation
Article Information
Kenneth Chinedu Ugoeze, Nkemakolam Nwachukwu*
Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Nigeria
*Corresponding Author: Nkemakolam Nwachukwu, Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Nigeria
Received: 02 May 2020; Accepted: 26 May 2020; Published: 30 May 2020
Citation: Kenneth Chinedu Ugoeze, Nkemakolam Nwachukwu. Disintegrant Properties of a Newly Derived Superdisintegrant from Lentinus tuber regium in Paracetamol Tablet Formulation. International Journal of Applied Biology and Pharmaceutical Technology 11 (2020): 105-120.
View / Download Pdf Share at FacebookAbstract
Abstract
Background: Rapid disintegration of tablets aids early drug release. This amidst economies of production prompts the search for innovative multifunctional-excipients.
Aim: The study aims to derive new directly-compressible-superdisintegrating-diluents from Lentinus tuber regium (LTR) and evaluating them in paracetamol tablets in comparison with avicel® PH 101(AVC).
Material and Method: The pulverized LTR was coded NLTR-A. Three separate 500.00 g of NLTR-A each was modified with 3.50% w/v of sodium hypochlorite; extracting with 70.00% v/v ethanol in a Soxhlet extractor; blending with 600.00 ml of 0.50 N NaOH and treating with 200.00 ml of 0.50 N HCL. The derived powders were coded MLTR-B, MLTR-C and MLTR-D. Formulations for tablets, each weighing 300.00 mg were made, containing 41.67% w/w paracetamol, 52.33% w/w of either NLTR-A or MLTR-B or MLTR-C or MLTR-D or AVC, 0.50% w/w magnesium stearate and talc. The micromeritics of powder blends were established. Tablets were compressed at 9.81 kN and evaluated.
Results: Enhanced flowability occurred in the derived powders than in NLTR-A and AVC. Tablets exhibited minimal weight variations with hardness generally above 4.00 kgf. They disintegrated in less than 1.00 min with friability < 1.00% recorded for AVC, MLTR-B and MLTR-C and mechanical strength as AVC > MLTR-C > MLTR-B > NLTR-A > MLTR-D (p < 0.05). Each batch released 80.00% of paracetamol before 30.00 min with dissolution efficiency (%) as MLTR-B (89.73±0.02)>MLTR-D (79.50±0.02)>AVC (77.84±0.02).
Conclusion: The powders derived from LTR are applicable as directly-compressible-superdisintegrating-diluent for paracetamol tablets.
Keywords
Lentinus tuber regium; Superdisintegrant; Paracetamol; Tablet
Lentinus tuber regium articles, Superdisintegrant articles, Paracetamol articles, Tablet articles
Article Details
Introduction
The oral route of drug administration has been very popular for the usage of medicinal agents in managing diseases since it gives room for safety, permits self-medication, ease of ingestion of drug product, etc. and these enhance patient compliance [1]. Further, tablets are cheap and easy to manufacture. Among the orally administered medicinal products, the solid dosage forms, especially the tablets make up the major items of therapeutic agents that are clinically utilized to attain systemic effect, especially the modified or immediate-release tablets [2, 3]. Though there is a current increase in emphasis and attention towards the controlled release and targeted drug delivery systems within the solid dosage forms, yet, the class of solid dosage forms which when ingested, break down to discharge their active pharmaceutical ingredients (API) instantly in the gastrointestinal tract have continued to enjoy much patronage [4]. The immediate-release tablets are developed to disintegrate in a manner that its API content does not have any special rate-controlling considerations. This is necessary since the availability of the orally administered drug in the physiological system relies on the disintegration, dissolution and other bodily factors [5-7]. The tablets that are intended to disintegrate constitute the most widely used among the pharmaceutical solid dosage formulas [8-10]. The chances of absorption of the API in a medicinal product formulation, especially the solid dosage form relies on its bioavailability which on its own, depends on the solubility of the API in the gastrointestinal fluids as the drug crosses the intestines. In as much as the solubility of such API is influenced by the physical form and chemical conformation of the drug, the degree at which drugs go into solution in the physiological fluid is a function of the ease with which the tablet disintegrates. The disintegration process is specifically critical for the immediate-release dosage forms and for that reason, disintegrants are added to tablets to induce break-up when it comes in contact with the fluid [11]. A disintegrant is described as an inert substance that is added to a solid dosage formulation such as a tablet or capsule to cause their break down to achieve the release and dissolution of the API. They serve to increase the surface area of the tablet and soften the binding agent. This means that when a tablet is exposed to an aqueous media, initially, it disintegrates into granules, and then undergoes into fine particles. This enhances the rate of dissolution in the media [12, 13]. Most of the hydrophilic excipients which are insoluble in water or gastrointestinal fluids serve as good disintegrants. Currently, starch and cellulose-based materials are utilized as disintegrants. Other disintegrants are partially pregelatinized starches, microcrystalline cellulose and low-substituted hydroxypropyl cellulose. Further, chemical adjustments of starch, cellulose and povidone make it easy to create outstanding disintegrants. Precisely, croscarmellose sodium, sodium starch glycolate and crospovidone are denoted as superdisintegrants since they could be used to accomplish exceptional disintegrant activity almost instantly when they are incorporated in very low concentrations [13, 14]. Disintegrants could be distinguished by their respective mode of action [15]. The common mechanism of action includes swelling of particles, exothermic wicking reaction, particle deformation recovery, particle repulsion and heat of interaction. Swelling is often accepted as the key mechanism of tablet disintegration. When a disintegrant swells, it split up the adjoining ingredients and start up the crumbling of the tablet matrix. In wicking, the disintegrant makes it easy for liquid to be drawn into the tablet matrix and initiating a break-up. Water penetrates the tablet through the pores as well as along a hydrophilic complex by wicking of the disintegrant contained in the formulation [13, 15-18]. A disintegrant could be incorporated intragranularly, extragranularly or by both procedures especially in a wet granulation method of producing tablets [19-22].
The uncoated tablets intended for rapid disintegration and release of the API could be produced by wet granulation (WG), dry granulation (DG) or direct compression (DC) methods. Tablets are designed to house the API besides the excipients such as diluents or fillers, binders, disintegrants, anti-adherents, glidants, lubricants, etc. which play key roles in the tablet formulation [9, 10]. The use of the DC technique is currently widespread because its mode of application is simple and achievable in a less significant time frame. It is an efficient process that is very suitable for the manufacture of heat or moisture-sensitive drugs. Its utilization enhances a cautious choice of an excipient which should demonstrate equitable flowability and compressibility [23, 24]. With the extended application of the DC technology in tablet manufacture, the need for excipients with superior and multifunctional applications becomes vital. Looking at these challenges, there is a great need to explore novel directly compressible excipients with improved flowability and compressibility that could yield tablets with enhanced mechanical strength and yet superdisintegrating. Such novel excipients could be developed cost-effectively by particle engineering. This facilitates the modification of the properties of excipients regarded as “generally recognized as safe” (GRAS) to bring about particles that could retain ideal properties for application as directly compressible diluents [25-28].
The current research is focused on the application of a novel direct compressible diluent with added superdisintegrating ability (developed by modifying the physical properties of a species of edible mushroom, Lentinus tuber regium) in the direct compression of paracetamol tablet. Paracetamol is poorly water-soluble. The knowledge of its poor flowability and poor compressibility features are widespread, making the manufacture of its tablets almost exclusively by wet granulation, an economically disadvantageous technique when compared to the direct compression [29]. The emerging of a directly compressible excipient suitable for paracetamol, especially one with additional superdisintegrating ability will be an added economic value to the direct compression technology. The processing and the properties of a novel directly compressible superdisintegrating powder got by series of modification of the native Lentinus tuber regium powder has been documented [30]. Lentinus tuber regium (LTR) is a species of edible mushroom (Basidiomycete) that grows mostly in the wild especially in tropical and subtropical regions of the world. It has been grown in the laboratory with much success and its nutritional, medicinal values and application as a disintegrant in tablet formulation have been acknowledged [31-33]. Several pharmaceutical excipients have been developed from Lentinus tuber regium through co-processing and have been applied in various drug formulations [34-38].
Materials and methods
Materials
Sclerotia of Lentinus tuber regium (Port Harcourt, Nigeria) sodium hypochlorite (Multipros, Lagos, Nigeria), ethanol, n-hexane (JHD, Guangzhou, China), paracetamol powder (Nagpur, Maharashtra, India), magnesium stearate, (Organix, Mumbai, India) and talc (Fisher Scientific, Loughborough, England).
Methods
Collection, Identification and Processing of Samples
The sample of the sclerotia of Lentinus tuber regium used was procured from the Mile 3 main market, Port Harcourt, Nigeria. It was identified and allotted a specimen code OG-ACC-001-UPH-C-057 and deposited in the University of Port Harcourt reference herbarium. The processing and the properties of the modified Lentinus tuber regium (LTR) powders have been reported by Ugoeze et al (2019) [30]. The sclerotia of the LTR was powdered to 250.00 μm and labelled as the native Lentinus tuber regium (NLTR-A). A 500.00 g of NLTR-A was immersed in 3.50% w/v sodium hypochlorite and agitated for 30.00 min. The paste was splashed in turn with distilled water till it was neutral to litmus. The wet mass was dried at 60.00°C for 2.00 h, powdered to 250.00 μm and labelled as the modified Lentinus tuber regium powder (MLTR-B). Additional 500.00 g of NLTR-A was extracted with a 70.00% ethanol in a Soxhlet extractor and dried at 60.00oC for 2.00 h. It was pulverized to 250.00 μm and named as the modified Lentinus tuber regium powder (MLTR-C). Extra 500.00 g of NLTR-A was plunged in 600.00 ml of 0.50 N sodium hydroxide in a 1.00 L beaker and stirred continually for 30.00 min. The ensuing product was washed with distilled water until it was neutral to litmus. Excess liquid was removed and it was agitated in 200.00 ml of 0.50 N hydrochloric acid for 30.00 min in a water bath at 100.00°C. The mass was washed in purified water repeatedly until it was neutral. It was dried to constant weight at 60.00°C, powdered to 250.00 μm and labelled as the modified Lentinus tuber regium powder (MLTR-D).
Formulation of Paracetamol Tablets
Formulations of tablets, each intended to weigh 300.00 mg and containing paracetamol 41.67% w/w (125.00 mg), 52.33% w/w (172.00 mg) of either NLTR-A or MLTR-B or MLTR-C or MLTR-D or avicel® PH 101 (AVC) (used as direct compressible diluent/superdisintegrant), 0.50% w/w (1.50 mg) of magnesium stearate and talc as a lubricant and glidant respectively to be added before the compression of tablets. Avicel is used as a standard for comparison.
Assessment of the Micromeritic Properties of Powder Blends
Micromeritic studies were carried out to evaluate the batches of blends of powders containing paracetamol. Three replicate readings were taken. The mean value of data and standard deviations were calculated as appropriate.
Bulk, Tapped and Particle Densities
A 15.00 g of each batch of the formulations was employed in the determination of bulk and tapped densities using the Stampfvolumeter (STAV 2003JEF, Germany). The bulk and tapped densities were calculated using equations 1 and 2.
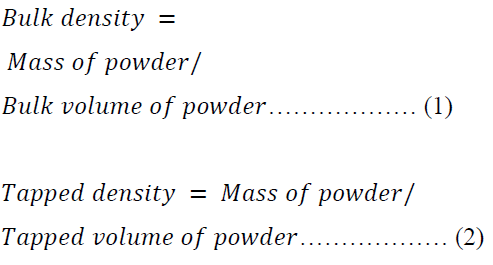
The particle density was determined by solvent displacement method using n-hexane [39]. The weight of an empty 25.00 ml pycnometer was noted as W. When it was filled with n-hexane, the new weight was coded as W1. The weight of the n-hexane was obtained from the variation between W1 and W. The weight of a 0.50 g of powder was labelled as W3 which was poured into the pycnometer filled with n-hexane. The excess displaced fluid was dabbed and the new weight of the density bottle and its contents was noted as W4.
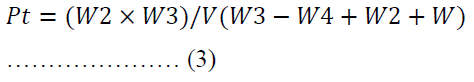
Where V is the volume of the pycnometer.
Angle of Repose
The angle of repose of the powder blend containing NLTR-A was determined by using a modification of the method of Jones and Pilpel [40]. A 20.00 g of sample was poured into a cylindrical paper roll fixed on top of a flat base whose diameter is known and is the same as the internal diameter of the cylinder. The cylinder was slowly pulled out vertically to have the formation of a cone-shaped heap of powder on the flat base. The height of the heap of powder was estimated. The dynamic method was adopted in the evaluation of the angle of repose of the powders containing MLTR-B, MLTR-C, MLTR-D and AVC. A clean glass funnel was clamped to a retort stand such that the tip of the funnel gave a gap of 2.40 cm to the base. The powders were, in turn, poured into the funnel until the heap of powder formed touched the tip of the funnel which stopped further discharge of the powder from the funnel orifice. The diameter of the circumference of the heap of powder was estimated. The angle of repose was calculated using equation 4.
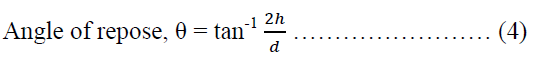
Where θ is the angle of repose, h is the height of the heap of powder, d is the diameter of the heap of powder.
Calculation of the Hausner’s Ratio, Carr’s Index and Porosity
The Hausner’s ratio and the Carr’s index (CI) [41, 42] was calculated using equations 5 and 6 respectively.

Porosity was calculated using equation 7.
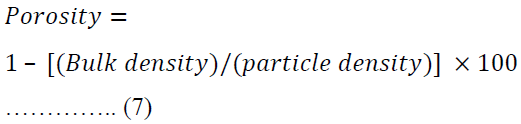
Compression of Paracetamol Tablets
The lubricant and glidant were added appropriately to the respective batches of the formulations of paracetamol and compressed at 9.81 kN for 25.00 s in a single station manual hydraulic tablet press, Model C, (Carver Inc., Wisconsin, USA) fitted with a set of flat-faced punch and die of 10.00 mm in diameter.
Evaluation of paracetamol tablets
The batches of paracetamol tablets were evaluated 24.00 h after compression to allow the tablets to recover from the stress undergone during compression. The physical form of the tablets such as texture, wholesomeness, shape and colour were evaluated. The British Pharmacopoeia methods [43] were used to evaluate the uniformity of weight, hardness, friability and disintegration time. Readings were carried out in triplicates. The mean and standard deviations were calculated as appropriate.
Uniformity of Weight
This was conducted by weighing twenty tablets individually on an analytical electronic balance (Ohaus, China).
Disintegration Time
The disintegration time of 6 tablets was determined using a tablet disintegration apparatus (Erweka, ZT 122, Germany) in 900.00 ml of 0.10 N HCl maintained at 37.0 0± 1.00°C.
Hardness and Thickness of Tablets
The hardness of a total of ten tablets per batch was evaluated singly using a diametrical digital tablet hardness tester (Veego, India) which automatically displayed the tablet hardness and its thickness.
Friability
The friability of ten tablets per batch was evaluated in a tablet friabilator (Erweka TAR 220, Germany) set at 25.00 rpm for 4.00 min. The friability (F) was calculated in percentage using equation 8.
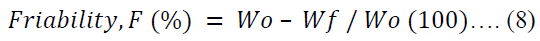
Where Wo is the initial weight of tablets before the test and Wf is the final weight of tablets after the test.
Tensile strength and hardness-friability-ratio
The tensile strength [44] of each batch of the tablets was calculated using equation 9.
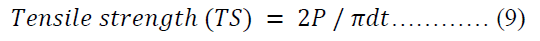
Where P is the hardness of the tablet, d is the diameter of the tablet, t is the thickness of the tablet.
The tablet hardness-friability ratio (HFR) of each batch of tablets was calculated from their respective values of hardness and friability.
Standardization of Paracetamol
A 100.00 mg of pure paracetamol powder was dissolved with a sufficient quantity of 95.00% v/v ethanol and made up to 100.00 ml using phosphate buffer pH 5.80 [43] and the same was employed to obtain serial fold dilutions of the stock solution to obtain the concentrations of 1.00, 2.00, 4.00, 6.00, 8.00 and 10.00 mg % of paracetamol solutions. The 2.00 mg % concentration was scanned in an ultraviolet/visible (UV/Vis) spectrophotometer (Jenway 6405, England) to determine the wavelength of maximum absorption (λmax) for the standard paracetamol. Using the λmax of 244.00 nm got, the absorption of the serially diluted solutions of paracetamol were determined. The standard calibration curve was fitted.
Drug Release Studies
The rotating paddle method (apparatus 2) was adopted in the dissolution rate studies in a dissolution apparatus (Erweka DT 600, Germany) [43, 45] with the paddle speed of 50.0 rotations per minute (rpm) in 900.0 ml of phosphate buffer (pH 5.80) maintained at 37.00 ± 0.50°C for 30.00 min. A 5.00 ml sample was withdrawn at predetermined intervals of 2.00, 5.00, 10.00, 15.00, 20.00, 25.00 and 30.00 min. The withdrawn samples were replaced with the same volume of phosphate buffer maintained at the same temperature. The absorbance of each sampled solution was read in a spectrophotometer at a wavelength of 244.00 nm.
Total Drug Content
The mean of twenty tablets per batch weighed together and crushed was calculated. A portion of the crushed tablets equivalent to the mean weight was weighed into a 100.00 ml volumetric flask, dispersed with phosphate buffer (pH 5.80), made up to 100.00 ml and filtered. Dilutions of the filtrate were then scanned in the spectrophotometer at 244.00 nm [43].
Statistical Evaluation
The figures were presented as a mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed followed by Fisher’s Least Significant Difference (LSD) post hoc test to determine the level of significance.
Results
Micromeritic Properties
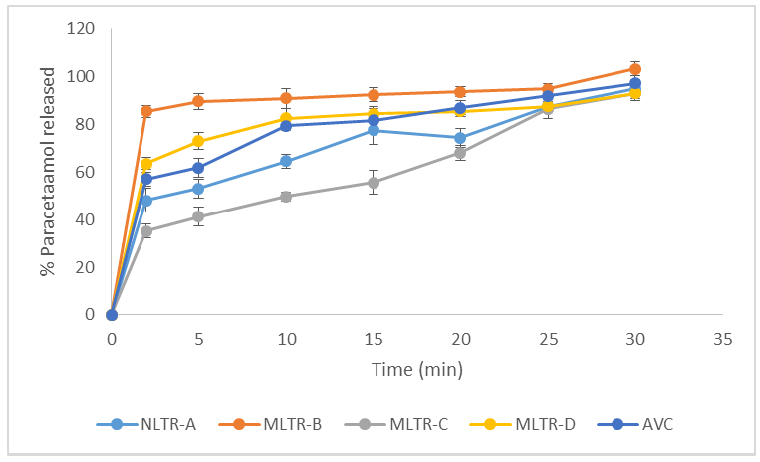
The results of some flow properties of the blend of powders containing paracetamol are shown in Table 1. The physical parameters of paracetamol tablets are shown in Table 2. Glossy, flat-faced round and white tablets without damages were obtained. The dissolution profile of the batches of tablets is presented in Figure 1.
Table 1: Some flow properties of the blend of powders containing paracetamol
|
Parameters |
NLTR-A |
MLTR-B |
MLTR-C |
MLTR-D |
AVC |
|
Bulk density (g/mL) |
0.36 ± 0.00 |
0.41 ± 0.01 |
0.49 ± 0.00 |
0.58 ± 0.00 |
0.41 ± 0.00 |
|
Tapped density (g/mL) |
0.50 ± 0.01 |
0.45 ± 0.01 |
0.53 ± 0.03 |
0.62 ± 0.20 |
0.53 ± 0.00 |
|
Particle density (g/mL) |
1.10 ± 0.45 |
1.52 ± 0.22 |
1.66 ± 0.10 |
1.77 ± 0.03 |
1.19 ± 0.24 |
|
Flow rate(g/s) |
No Flow |
8.40 ± 0.00 |
7.34 ± 0.00 |
10.15 ± 0.05 |
No Flow |
|
Angle of repose (o) |
53.16 ± 0.02 |
27.54 ± 0.01 |
28.21 ± 0.01 |
27.80± 0.02 |
49.16 ± 0.02 |
|
Carr’s index (%) |
28.00 ± 0.17 |
8.88 ± 0.19 |
9.25 ± 0.01 |
6.45 ± 0.05 |
24.29 ± 0.22 |
|
Hausner’s ratio |
1.37 ± 0.05 |
1.09 ± 0.53 |
1.20 ± 0.30 |
1.07 ± 0.10 |
1.32 ± 0.20 |
|
Porosity (%) |
73.95 ± 0.10 |
77.30 ± 1.01 |
64.74 ± 0.20 |
69.89 ± 0.15 |
77.34 ± 0.55 |
Table 2: Physical parameters of paracetamol tablets
|
Parameters |
NLTR-A |
MLTR-B |
MLTR-C |
MLTR-D |
AVC |
|
Total drug content (%) |
101.99±1.00 |
102.34±1.02 |
100.79±0.95 |
98.65±0.55 |
100.05±1.05 |
|
Mean tablet weight (mg) |
290.10±0.36 |
300.07±0.09 |
295.00±0.10 |
301.23±0.25 |
301.40±0.00 |
|
Disintegration time (min) |
0.20 ± 1.13 |
0.12 ± 0.57 |
0.14 ± 1.52 |
0.18 ± 0.15 |
3.58 ± 0.25 |
|
Hardness(kgf) |
4.38±0.04 |
4.92±0.03 |
5.30±0.02 |
4.49±0.35 |
6.93±0.05 |
|
Friability (%) |
1.38 ± 0.15 |
0.50 ± 0.01 |
0.20 ± 0.01 |
1.49± 0.01 |
0.15 ± 0.01 |
|
Tensile strength (kg/m2) |
7.31± 0.02 |
8.72±0.17 |
9.56± 0.05 |
6.65±0.13 |
10.82±0.03 |
|
HFR |
3.18±0.02 |
9.82±0.02 |
26.49±0.15 |
3.02±0.02 |
46.11±0.10 |
Discussion
An increase in the bulk densities of the modified forms of the LTR observed was consistent (p < 0.05) though, with similar values recorded for MLTR-B and AVC (p > 0.05). Similar results were observed in the tapped densities for MLTR-C and AVC (p > 0.05) (Table 1). The contrast of the individual bulk and tapped densities of NLTR-A, MLTR-B, MLTR-C and MLTR-D indicated no significant dissimilarities (p > 0.05). A variation in the bulk and tapped densities was only noted in AVC (p < 0.05). These reports signify that the modified LTR powders have minor interparticulate interaction leading to enhanced flowability. Contrary to this is the high interparticulate interactions existing in the NLTR-A and AVC with its attendant poor flowability. These findings are confirmable with the inability of the NLTR-A and AVC to flow freely from the funnel contrary to the enhanced flow rate recorded in the modified powders in the order, MLTR-D > MLTR-B > MLTR-C (p < 0.05). Largely, the results of the bulk and tapped densities, flow rate, angle of repose, Carr’s index and Hausner’s ratio indicated that the corresponding methods of modification used in deriving MLTR-A, MLTR-B, MLTR-C and MLTR-D brought about a great reduction in interparticulate interactions, resulting in improvement of the flow properties of the modified powders of the LTR in association with the NLTR and AVC [41- 43, 46 - 49]. In general, the degree of flowability of blends of the directly compressible powders containing paracetamol could be presented as MLTR-D > MLTR-B > MLTR-C > AVC > NLTR-A.
Evaluation of the Properties of the Tablets
Tablet weight
The tablets showed minimal weight variation across the batches (p < 0.05) except in MLTR-D and AVC where similar weights were recorded (p > 0.05) with the coefficient of variations being in the range of 0.03-0.30 % which is lower than the Pharmacopoeia limits of ± 5.00 % for tablets weighing ≥ 250.00 mg [43].
Tablet Disintegration Time
The batches of tablets containing the NLTR-A and the modified LTR disintegrated in less than 1.00 min while the batch containing AVC disintegrated a little above 3.00 min (Table 2). These values were much < 15.00 min which is the upper limit stipulated in the British Pharmacopoeia for uncoated tablets for immediate release [43]. The early disintegration of the tablets containing the modified LTR gave room for the early dissolution of paracetamol, boosting its bioavailability and quick onset of action since its aqueous solubility is not very excellent. These results were achievable since the modification processes adopted resulted in powders with more hydrophilic property [30]. The assessment of tablet disintegration serves as a useful tool in the checking and regulating of batch-to-batch discrepancies in distinct tablets in the course of production, though, sometimes it may not point to the certainty of the bioavailability of the API [50].
Tablet Hardness
The hardness of the batches of tablets was generally above 4.00 kgf with the batch containing AVC maintaining the highest value (Table 2). A minimum hardness of 4.00 kgf is considered alright for uncoated oral tablets for immediate release, though, such tablets are expected to have a breaking force of 4.00-10.00 kgf [51]. The hardness of a tablet could be related to further tablet characteristics like the density and porosity. The value of tablet hardness largely appreciates through ordinary storage, its value depending on its shape, chemical composition, the type of binder and its concentration used in its formulation, as well as the level of compression pressure, applied [52].
Tablet Friability, Tensile Strength And Hardness-Friability-Ratio (HFR)
The friability of the tablets (Table 2) across the batches varied significantly within the NLTR-A, MLTR-B, MLTR-C, MLTR-D and AVC (p < 0.05). This parameter depends on the moisture content of the compressed granules as well as the tablet shape [53]. The evaluation of friability is correlated to tablet hardness and is considered as a measure the capability of the tablet to resist the abrasions or fragmentations encountered during packaging and product handling [54]. For uncoated tablets, the British Pharmacopoeia [43] stipulated values less than 1.00 % especially for tablets prepared by wet granulation but it has been documented that the tablets produced by direct compression could display values of friability above 1.00 % [55-56]. Considering the limits specified in the British Pharmacopoeia [43], the batches of tablets containing MLTR-B, MLTR-C and AVC exhibited higher mechanical strength with AVC presenting the highest ability to resist tablet breakage when considered along those of NLTR-A and MLTR-D.
Besides friability, other measures adopted in the assessment of the mechanical strength of a tablet are the tablet tensile strength and the hardness-friability-ratio (HFR). The tensile strength is an evaluation of the strength of the bond holding the compressed powder together [44]. The arrangement of the hardness and friability, a word noted as the hardness-friability ratio (HFR) is an estimation of the mechanical strength of tablets, being a ratio that compares the strength of the tablet to its weakness and it has been documented that the higher the HFR values, the stronger the tablet [57-58]. Considering the mechanical strength of the batches of the tablets and using the results got from tensile strength and HFR, the batches of tablets prepared with MLTR-B, MLTR-C and AVC have greater mechanical strength than those containing NLTR-A and MLTR-D and it could be rated as AVC > MLTR-C > MLTR-B > NLTR-A > MLTR-D (p < 0.05).
Total Drug Content And Drug Release Studies
Considering the total drug content test, the British Pharmacopoeia [43] specifies that the formulation batch conforms to the test if each different content is between 85.00 % and 115.00 % of the average content. It also stipulates that the product fails to meet the terms with the test if more than one individual content is outside these limits or if one individual content is outside the limits of 75.00 % to 125.00 % of the average content. The results got across the batches of tablets ranged between 98.65±0.55 – 102.34±1.02% and are within the pharmacopoeia stipulation for total drug content.
Figure 1 shows the dissolution profile of the paracetamol tablets. Generally, each batch released a higher percentage of paracetamol in a very short time, then the release pattern showed a gradual increase as time progressed. Maximal release of paracetamol was recorded within 30.00 min in each batch. A 50.00 % release of the drug (T50) was recorded in the following times (min): MLTR-B (1.25) < MLTR-D (1.50) < AVC (2.00) < NLTR-A (4.00) < MLTR-C (12.25). On the other hand, 80.00 % of the paracetamol content (T80) was released in following times (min): MLTR-B (2.00) < MLTR-D (9.25) < AVC (15.75) < NLTR-A (23.50) < MLTR-C (24.25). Each batch released up to 90.00 % of its content before 30.00 min. The maximal release of paracetamol in each batch of tablets studied was achieved in a very short time frame if viewed along with the stipulation of the British Pharmacopoeia for paracetamol tablet formulated for conventional release, not less than 80.00 % of the drug content should be released within 30.00 min. Further, the dissolution efficiency (%) of the respective batches of the tablets were recorded as MLTR-B (89.73±0.02) > MLTR-D (79.50±0.02) > AVC (77.84±0.02) > NLTR-A (69.72±0.02) > MLTR-C (59.35±0.02). With these the order of efficiency of drug release could be presented as MLTR-B > MLTR-D > AVC > NLTR-A > MLTR-C. From these results, the highest release of paracetamol could be achieved by using MLTR-B as a multifunctional direct compressible bulking agent and superdisintegrant in comparison with AVC and other modified forms of LTR as shown above. The dissolution efficiency points to the degree of bioavailability of paracetamol achievable if any of the powders are used in the formulation of its tablets, especially as a direct compressible superdisintegrating diluent.
Conclusion
The application of the modified powders, MLTR-B, MLTR-C and MLTR-D derived from the native Lentinus tuber regium (NLTR-A) as a direct compressible superdisintegrating diluent in paracetamol tablet formulation in comparison with avicel PH 101(AVC) yielded high-quality tablets with mechanical strength rated as AVC > MLTR-C > MLTR-B > NLTR-A > MLTR-D and dissolution efficiency of paracetamol graded as MLTR-B (89.73±0.02) > MLTR-D (79.50±0.02) > AVC (77.84±0.02) > NLTR-A (69.72±0.02) > MLTR-C (59.35±0.02), showing that avicel PH 101 yielded stronger tablets than the modified powders which, though, gave room for enhanced bioavailability of paracetamol, especially with MLTR-B and MLTR-D. Therefore, the novel directly compressible superdisintegrating diluent derived from the edible native Lentinus tuber regium could serve as a direct compressible superdisintegrating diluent for paracetamol tablet formulation.
Funding
There was no support from any funding body towards this work.
Conflict of Interest
The authors do not have any conflicts of interest in this work.
References
- Quodbach J, Kleinebudde P. A critical review on tablet disintegration. Pharmaceutical Development and Technology 21 (2016): 763-774.
- Sastry SV, Nyshadham JR, Fix JA. Recent technological advances in oral drug delivery–a review. Pharmaceutical Science & Technology today 3 (2000): 138-145.
- Mahboob MB, Riaz T, Jamshaid M, et al. Oral films: A comprehensive review. International Current Pharmaceutical Journal 5 (2016): 111-117.
- Sharma D, Singh M, Kumar D, et al. Formulation development and evaluation of fast disintegrating tablet of cetirizine hydrochloride: a novel drug delivery for pediatrics and geriatrics. Journal of Pharmaceutics (2014).
- Sharma S, Singh G, Gupta GD. Formulation design and optimization of mouth dissolving tablets of domperidone using sublimation technique. An International Journal of Pharmaceutical Sciences 1 (2010).
- Jadhav SB, Mali AD, Rajeghadage SH, et al. Formulation and evaluation of immediate release tablets of Imipramine hydrochloride. Int J Biomed Adv Res 5 (2014): 559-560.
- Patel N, Natarajan R, Rajendran NN, et al. Formulation and evaluation of immediate release bilayer tablets of telmisartan and hydrochlorothiazide. Int J Pharm Sci Nanotech 4 (2011): 1477-1482.
- Yassin S, Goodwin DJ, Anderson A, et al. The disintegration process in microcrystalline cellulose based tablets, part 1: influence of temperature, porosity and superdisintegrants. Journal of Pharmaceutical Sciences 104 (2015): 3440-3450.
- Sharma N, Pahuja S, Sharma N. Immediate release tablets: a review. Int J Pharm Sci & Res 10(2019): 3607-3618.
- Dave RH. Overview of pharmaceutical excipients used in tablets and capsules. Drug Topics 24 (2008): 1-3.
- Markl D, Zeitler JA. A review of disintegration mechanisms and measurement techniques. Pharmaceutical Research 34 (2017): 890-917.
- Adjei FK, Osei YA, Kuntworbe N, et al. Evaluation of the disintegrant properties of native starches of five new cassava varieties in paracetamol tablet formulations. Journal of Pharmaceutics (2017).
- Desai PM, Liew CV, Heng PW. Review of disintegrants and the disintegration phenomena. Journal of Pharmaceutical Sciences 105 (2016): 2545-2555.
- Rowe RC, Sheskey PJ, Weller PJ (eds). Handbook of Pharmaceutical Excipients, 4th, American Pharmaceutical Association, Washington D.C. (2003).
- Onuki Y, Kosugi A, Hamaguchi M, et al. A comparative study of disintegration actions of various disintegrants using Kohonen's self-organizing maps. Journal of Drug Delivery Science and Technology 43 (2018): 141-148.
- Aulton ME, Taylor KM. Aulton's pharmaceutics: The Design and Manufacture of Medicines (2013).
- El-Barghouthi M, Eftaiha AA, Rashid I, et al. A novel superdisintegrating agent made from physically modified chitosan with silicon dioxide. Drug Development and Industrial Pharmacy (2008).
- Gandhi L, Akhtar S. Comparative study on effect of natural and synthetic superdisintegrants in the formulation of orodispersible tablets. Journal of Drug Delivery and Therapeutics 9 (2019): 507-513.
- Sheen PC, Kim SI. Comparative study of disintegrating agents in tiaramide hydrochloride tablets. Drug Development and Industrial Pharmacy 15 (1989): 401-414.
- Sekulovic D, Birmancevic M. The investigation of the disintegration time of tablets with metamizol and propyphenazone prepared by the method of direct compression. Pharmazie 41 (1986).
- Sekulovic D, Tufegdzic N, Birmancevic M. The investigation of the influence of Explotab® on the disintegrations of tablets. Pharmazie 41 (1986): 153-154.
- Johnson JR, Wang LH, Gordon MS, et al. Effect of formulation solubility and hygroscopicity on disintegrant efficiency in tablets prepared by wet granulation, in terms of dissolution. Journal of Pharmaceutical Sciences 80 (1991): 469-471.
- Liesbeth M. Direct compression versus granulation. Pharmaceutical Technology Europe 23 (2011).
- Tousey MD. The manufacturing process: Tablet and capsule manufacturing. Solid dose Experts Techceuticals Training 15 (2015): 1-2.
- Mînea LA, Mehta R, Kallam M et al. Evaluation and characteristics of a new direct compression performance excipient. Pharmaceutical Technology 35 (2011).
- Russell R. Synthetic excipients challenge all-natural organics: Offer advantages/challenges to developers and formulators. Pharmaceutical Technology (2003) 28 (2004): 38-50.
- Reimerdes D. The near future of tablet excipients. Manufacturing Chemist 64 (1993): 14-15.
- Reimerdes D, Aufmuth KP. Tabletting with co-processed lactose-cellulose excipient. Manufacturing chemist 63 (1992): 21.
- Martinello T, Kaneko TM, Velasco MV, et al. Optimization of poorly compactable drug tablets manufactured by direct compression using the mixture experimental design. International Journal of Pharmaceutics 322 (2006): 87-95.
- Ugoeze KC, Nwachukwu N, Anyino PC. The Effect of Modification Methods on the Properties of Lentinus Tuber Regium Powders. Journal of Pharmaceutical Technology, Research and Management 7 (2019): 23-30.
- Okhuoya JA, Etugo JE. Studies of the cultivation of Pleurotus tuberregium (FR) Sing. an edible mushroom. Bioresource Technology 44 (1993): 1-3.
- Okjuoya JA. Cultivation of Pleurotus tuber-regium (Fr) Sing on various farm wastes. InProceedings of the Oklahoma Academy of Science 71 (1991): 1-3.
- Iwuagwu MA, Onyekweli AO. Preliminary investigation into the use of Pleurotus tuber-regium powder as a tablet disintegrant. Tropical Journal of Pharmaceutical Research 1 (2002): 29-37.
- Ugoeze KC, Nwaokenye C, Ibezim CN. Studies on the disintegrant and drug release rate enhancing properties of admixtures of corn starch BP and Lentinus tuber-regium powders in wet granulated paracetamol tablet. African Journal of Pharmaceutical Research and Development 5 (2013): 83-90.
- Ugoeze KC, Nwachukwu N, Ibezim CN. Containing Lentinus tuber regium Based Co-processed Filler-Binder.
- Ugoeze KC, Okpara C. Characterization of a novel coprocessed powder of Lentinus tuber regium and polyvinylpyrollidone (Povilent). International Research Journal of Pharmaceutical and Applied Sciences 5 (2015): 1-7.
- Ugoeze KC, Nkoro VO. The physico-technical properties of a multicomponent Lentinus tuber regium based co-processed excipient (Fizlent). American Journal of Pharmacy and Pharmacology 2 (2015): 13-20.
- Ugoeze KC, Nkoro VO, Nwachukwu N. Application of a Multicomponent Lentinus tuber regium Based Co-Processed Excipient (Fizlent) as a Novel Directly-Compressible Filler-Binder-Superdisintegrant in Ibuprofen Tablet Formulation. American Journal of Biomedical Science and Engineering 1 (2015): 45-50.
- Odeku OA, Awe OO, Popoola B, Odeniyi MA, Itiola OA. Compression and mechanical properties of tablet formulations: Containing corn, sweet potato, and cocoyam starches as binders. Pharmaceutical Technology (2003) 29 (2005): 82-90.
- Jones TM, Pilpel N. The flow properties of granular magnesia. Journal of Pharmacy and Pharmacology 18 (1966): 81-93.
- Hausner HH. Friction conditions in a mass of metal powder. Polytechnic Inst. of Brooklyn. Univ. of California, Los Angeles; (1967).
- Carr RL. Classifying flow properties of solids. Chem. Eng. 1 (1965): 69-72.
- British Pharmacopoeia. Her Majesty Stationary Office, University Press, Cambridge (2012).
- Fell JT, Newton JM. Determination of tablet strength by the diametral-compression test. Journal of Pharmaceutical Sciences 59 (1970): 688-691.
- Pharmacopeial Forum. United States Pharmacopeial Convention, Rockville 31 (2005): 909. Available at http://www.uspnf.com/pharmacopeial-forum. (Retrieved on 20th April 2020).
- The United States Pharmacopoeia, U.S.P/ NF. The United States Pharmacopeial Convention, Rockville, (2009).
- Well J. Pharmaceutical preformulation: the physicochemical properties of drug substances. In: Aulton ME (ed.). The science of dosage form design, 2nd ed. Churchill Livingstone, Toronto (2003): 113–138.
- Neumann BS. Advances in pharmaceutical sciences.
- Pilpel N. The flow properties of magnesia. Journal of Pharmacy and Pharmacology 16 (1964): 705-716.
- Zhao N, Augsburger LL. The influence of granulation on super disintegrant performance. Pharmaceutical Development and Technology 11 (2006): 47-53.
- Promode RS. An overview on bilayered tablet technology. Int. J. Pharm. Bio. Sci. 5 (2014): 113 – 128.
- Martins E, Christiana I, Olobayo K. Effect of Grewia gum on the mechanical properties of Paracetamol tablet formulations. African Journal of Pharmacy and Pharmacology 2 (2008): 1-6.
- Chowhan ZT. Role of binders in moisture?induced hardness increase in compressed tablets and its effect on in vitro disintegration and dissolution. Journal of Pharmaceutical Sciences 69 (1980): 1-4.
- Rudnic E, Schwartz JB. Oral solid dosage forms. In: Remington’s Pharmaceutical Sciences, 18th Gennaro AR (ed). Mack Publishing Company, Easton, Pennsylvania, U.S.A, (1990): 1633-1665.
- Wells JT, Laugridge JR. Dicalcium phosphate dihydrate - microcrystalline cellulose systems in direct compression tableting. Int. J. Pharm. Tech. Prod. Mfr. 2 (1981): 1-8.
- Ofoefule SI. Tablet dosage forms 111. Ofoefule SI (ed), Textbook of Pharmaceutical Technology and Industrial Pharmacy. Samakin (Nig.) Enterprises, Lagos, Nigeria (2002): 58-66.
- Odeku OA, Itiola OA. Evaluation of the effects of khaya gum on the mechanical and release properties of paracetamol tablets. Drug Development and Industrial Pharmacy 29 (2003): 311-320.
- Odeku OA. Assessment of Albizia zygia gum as binding agent in tablet formulations. Acta Pharmaceutica 55 (2005): 263-276.


 Impact Factor: * 3.0
Impact Factor: * 3.0 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
