Double Trouble Unmasked: Femoral Pseudoaneurysm and Deep Venous Thromboses Following Coronary Angiography
Article Information
Mariam Jamil1**, Khurram Arshad1**, Antoine Egbe1*, Yazan Alamro1, Essa Kadiri1, Shafiq Qaiser2
1Corewell Health Dearborn Internal Medicine, Dearborn, MI, USA
2Department of Cardiology, Corewell Health Dearborn Internal Medicine, Dearborn, MI, USA
*Corresponding authors: Antoine Egbe, Corewell Health Dearborn Internal Medicine, MI 48126, USA.
**Author Note: Maryam Jamil and Khurram Arshad are equal contributors and share the co-first authorship
Received: 02 February 2024; Accepted: 14 February 2024; Published: 29 April 2024
Citation: Antoine Egbe, Mariam Jamil, Khurram Arshad, Yazan Alamro, Essa Kadiri1, Shafiq Qaiser. Double Trouble Unmasked: Femoral Pseudoaneurysm and Deep Venous Thromboses Following Coronary Angiography. Cardiology and Cardiovascular Medicine. 8 (2024): 154-158.
View / Download Pdf Share at FacebookAbstract
Iatrogenic pseudoaneurysms, while infrequent, represent a formidable complication of interventional cardiac catheterization, particularly in cases involving femoral artery access. This report details a unique case involving a 73-year-old male, receiving warfarin for a mechanical aortic valve, who developed a substantial right groin hematoma attributed to an iatrogenic pseudoaneurysm post-cardiac catheterization, subsequently resulting in concomitant deep venous thromboses (DVTs) in the right leg. The pathophysiology of DVTs following left heart catheterization typically involves the formation of a sizable pseudoaneurysm, exerting pressure on adjacent veins, leading to blood pooling and subsequent clot formation. Alternatively, the development of large hematomas in the thigh region may cause compression of adjacent veins. This case posed a clinical dilemma, necessitating a meticulous assessment of the risk-benefit profile associated with reversing the patient’s anticoagulation. The patient, managed with warfarin for a mechanical aortic valve, presented with deep venous thrombosis alongside a suspected actively extravasating pseudoaneurysm resulting in a groin hematoma. A secondary challenge involved determining the optimal approach for managing the pseudoaneurysm, requiring collaboration with a multidisciplinary team comprising vascular surgery, cardiology, and interventional radiology.
In conclusion, despite the infrequent occurrence of major complications following left heart catheterizations, their potential life or limb-threatening nature, coupled with the rising volume of cardiac catheterizations, underscores the critical importance of early recognition of potential complications. Vigilance regarding secondary complications, such as the concurrent deep venous thromboses presented in our case, is imperative for comprehensive patient care. This case adds to the growing body of knowledge guiding clinical practitioners in managing intricate scenarios associated with interventional cardiac procedures.
Keywords
Pseudoaneurysm; Deep venous thromboses; Coronary artery bypass grafting
Pseudoaneurysm articles; Deep venous thromboses articles; Coronary artery bypass grafting articles
Article Details
1. Introduction
Pseudoaneurysm or false aneurysm occurs when blood leaks from the vessel and collects in surrounding tissue forming a hematoma mostly secondary to blood vessel wall injury. Although the incidence of vascular complications after diagnostic catheterization is getting low, reflecting refinements in angiographic technique and catheter construction we still see several complications with interventional catheterization [1]. Pseudoaneurysm formation is one of the common vascular complications we see at the point access site, especially if the femoral artery(0.2%-3%) [2] is used as an access point compared to the radial artery(0.05%) [3]. Once pseudoaneurysm forms its complications and progression could be assessed based on the size, mechanism of injury, duration, patient comorbidities as well as neck diameter. Potential complications include but are not limited to rapture, embolization, expansion, and extravasation with arterial/venous wall compression leading to limb ischemia or DVT [4]. We present a rare case of DVT formation in our patient most likely secondary to pseudoaneurysm, a compressive complication mentioned above.
2. Case Presentation
A 73-year-old male, with a pertinent medical history of a mechanical aortic valve replacement and ongoing warfarin therapy, obstructive coronary artery disease status post coronary artery bypass grafting (CABG), hypertension, and hyperlipidemia was admitted to our emergency department presenting with complaints of right groin pain and swelling. The patient had undergone cardiac catheterization six days prior, prompted by complaints of chest pain and a positive stress test. Access was gained through the right femoral artery, and no intervention was deemed necessary as coronary angiography indicated a patent left internal mammary artery (LIMA) to the left anterior descending (LAD) graft and normal native coronary arteries.
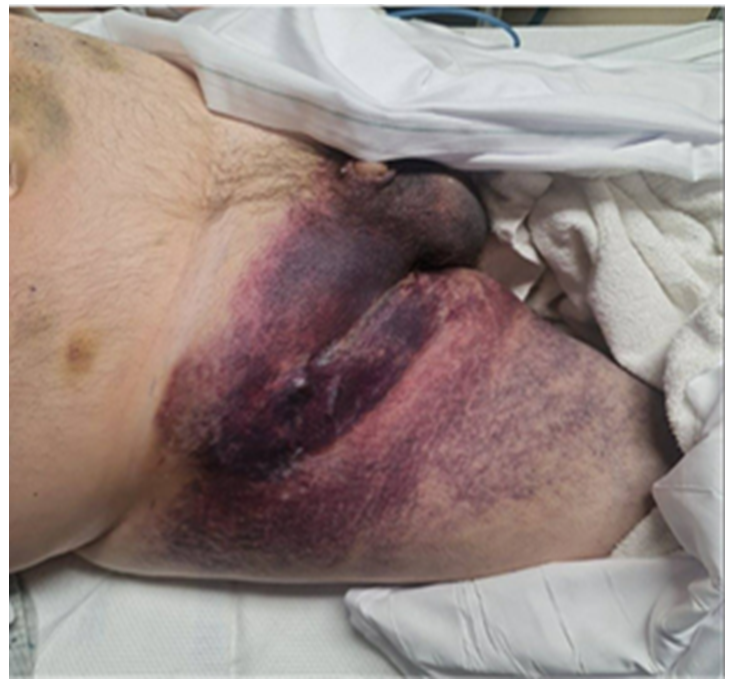
Three days following the procedure, the patient experienced mild groin discomfort three days that evolved into swelling and purplish discoloration of the overlying skin in the right inguinal region. Over the next 24-48 hours, the swelling worsened and extended to encompass the scrotum and the right anterior thigh, accompanied by severe escalating right groin pain. Upon review of systems, the patient reported mild lightheadedness while standing and difficulties with urination due to scrotal swelling. There were no concurrent manifestations of right leg weakness or sensory disturbances. The patient reported resumption of his warfarin therapy on the day of the cardiac catheterization upon discharge, coupled with twice-daily enoxaparin injections and a daily aspirin, up until his presentation at the emergency department. The patient arrived in a hemodynamically stable condition. Physical examination revealed a tense, non-pulsatile, and tender right groin hematoma, accompanied by overlying superficial purplish skin discoloration involving the right pubic area, right scrotum, and the right anterior thigh (Figure 1). No palpable thrill or audible bruit over the hematoma was appreciated. Additionally, there was noticeable swelling of the right lower extremity compared to the left. On neurovascular examination of the right lower extremity, he had strong peripheral pulses with no noticeable motor or sensory deficits. Pertinent labs included a stable hemoglobin level of 10.1mg/dl and an INR of 2.6 given his use of anticoagulants for his mechanical aortic valve.
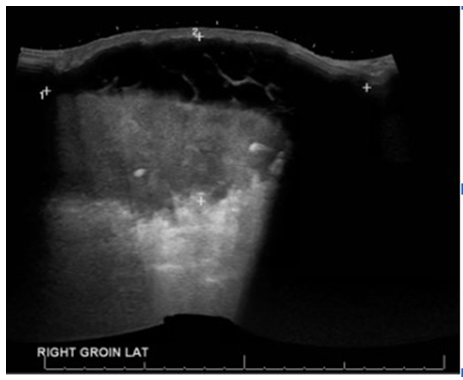
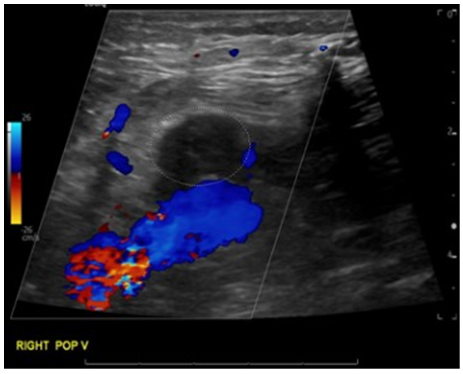
A sonographic evaluation of the right groin was conducted, and it revealed large septated fluid collections in the right groin, measuring up to 16 × 0.6 cm laterally and 20.7 × 9.9 cm medially, extending into the scrotum (Figure 2). Doppler imaging indicated no blood flow within these fluid collections. Interestingly, an ultrasound duplex of the patient’s right lower limb, prompted by the noticeable swelling of his right lower leg compared to the left, identified concurrent venous thrombosis involving the right superficial femoral and popliteal veins (Figure 3).
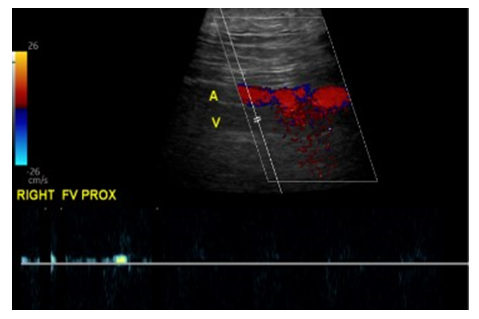
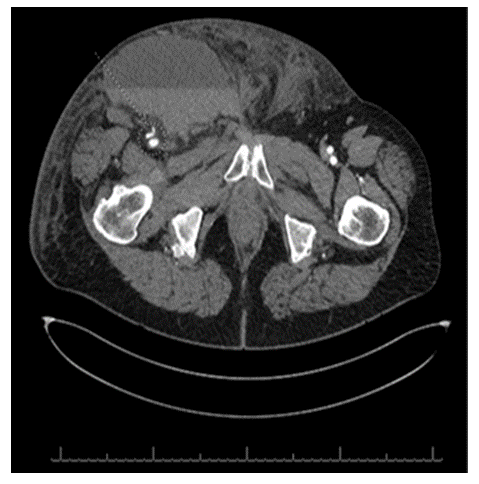
A CT angiography of the abdomen and pelvis with lower extremity runoff was conducted and it revealed a focal pseudoaneurysm involving the distal common femoral artery measuring approximately 1.3 cm across (Figure 4). Additionally, a large superficial and anterior fluid collection was observed in the right inguinal region, communicating into the right perineum. (Figure 5). The collection exhibited a fluid hematocrit level, indicative of an acute or subacute hematoma. No internal opacification with contrast was noted, however, the possibility of communication of this fluid collection with the aforementioned pseudoaneurysm could not have been ruled out based on our CTA imaging results.
In response to the identified risk of an expanding hematoma potentially communicating with a pseudoaneurysm (PSA), the patient's anticoagulants and antiplatelet medications were kept on hold. Recognizing the delicate balance needed in the setting of a mechanical aortic valve (AV), an expanding hematoma, and concurrent complication of an ipsilateral deep vein thrombosis (DVT), a decision was made to reverse his international normalized ratio (INR) with two units of fresh frozen plasma. A multidisciplinary team, including Interventional Radiology, Vascular Surgery, and Cardiology, was convened. Following a thorough review of the imaging, the Interventional Radiology team determined that the PSA would not be amenable to a thrombin injection due to the large overlying hematoma, and therefore the vascular surgery team scheduled him for a surgical hematoma evacuation and possible pseudoaneurysm repair.
During the procedure, successful drainage of the hematoma was achieved. However, the identification of the pseudoaneurysm (PSA) presented challenges due to excessive blood staining, suboptimal tissue planes, and localized tissue destruction resulting from the hematoma. In the absence of active bleeding from the artery or surrounding tissues, a 10 French Jackson-Pratt (JP) drain was placed without any additional intervention or repair of the PSA. The patient tolerated the procedure well, with no apparent complications. He was discharged subsequently on his previous anticoagulation regimen.
3. Discussion
The incidence of pseudoaneurysms is on the rise with the increasing rates of peripheral interventional procedures. The incidence rate of PSAs after diagnostic catheterization is believed to range between 0.2% to 8% [5]. Catheterizations are not the only cause of PSAs. Pseudoaneurysms can also occur after trauma, mycotic infections, or at the anastomotic site of a native artery and a synthetic graft [6]. In the case of our patient, the pseudoaneurysm was secondary to his recent left heart catheterization with femoral access. Several factors are known to predispose patients to the formation of PSAs after interventional procedures. These factors include; anti-platelet agents like aspirin and clopidogrel, anticoagulation, female gender, large sheath size, length of procedure, low or high puncture sites, hemodialysis, peripheral arterial disease, simultaneous artery and vein catheterization, hypertension, obesity, and poor postprocedural compression [7,8]. Our patient was obese, had hypertension, and was on anticoagulation before his left heart catheterization. PSAs typically present as a pulsatile mass with the presence of a systolic bruit. They can be associated with a hematoma, critical limb ischemia, claudication, venous thrombosis, or neuropathy. The mainstay method of diagnosing a PSA is a duplex arterial ultrasound. In the case of our patient, he had a large pulsatile mass in his groin with associated hematomas. Color flow imaging is important because it helps us differentiate between pseudoaneurysms and hematomas [9]. Color flow imaging of PSAs will demonstrate flow within the hypoechoic mass while hematomas will have no flow within the hypoechoic mass. In the case of our patient, he had numerous hematomas. These hematomas were compressing on nearby structures, leading to the deep venous thrombosis seen in the superficial femoral and popliteal veins. He also had extensive bruising on the right groin associated with significant pain and scrotal edema/swelling. The scrotal edema/swelling and urinary obstructive symptoms he presented with her an atypical presentation for patients with femoral arterial pseudoaneurysms. Until the 1990s, surgical management was the only known way of managing PSAs. Since then many other treatment modalities have been tried and found to be efficacious. They include ultrasound-guided thrombin injection, ultrasound-guided compression therapy, percutaneous injection of fibrin adhesive, or blind compression using Femostop compression devices. Ultrasound-guided compression has a success rate of about 75% to 98% [10]. Interestingly the success rate drops to about 30-73% for those on anticoagulation [10]. Steinkamp et al. [10] 2000 demonstrated the efficacy of ultrasound-guided compression in 98 patients who developed PSA post peripheral/cardiac interventional procedures. In 88 of 98 patients, they were able to obliterate the PSA on the first attempt while an additional 10 of these patients needed a second trial for PSA obliteration to occur. In total 96 out of 98 patients (98%) had their PSAs obliterated. These results are similar to the findings found by Eisenberg et al. [11] back in 1999. They used ultrasound-guided compression on 283 patients who had PSAs. It was successful in 72.1% of patients on the first attempt and it was successful in 74.4% of patients on the second attempt. 70% of patients who were on anticoagulation at the time of the compression attempt failed to have their PSA obliterated. Another commonly used treatment modality is ultrasound-guided thrombin injection. This involves the injection of thrombin into the PSA sac. Thrombin converts fibrinogen to fibrin. The presence of fibrin in the PSA sac leads to the formation of clots thereby obliterating the PSA. This modality has a success rate of about 90%. Its possible complications include downstream thrombosis if the thrombin injection leaks into the arterial/venous system, local infection, and anaphylaxis [12]. Tomasz et al. [13] reported a 94.8% success rate in 135 patients that were treated with UGTI. On a second attempt, the success rate increased to 98.5%. Similarly, Wendy et al. [14] found a 97.4% (129/133) success rate in the usage of UGTI for those with iatrogenic femoral arterial pseudoaneurysms. Surgery remains one of the treatment modalities for PSAs. It is however associated with more complications, higher costs, longer hospital stays, bleeding, and perioperative myocardial infarction. Surgery is reserved for infected PSAs, rapidly expanding PSAs, PSAs with compressive symptoms like critical limb ischemia, and neuropathy, and PSAs where other treatment modalities have been unsuccessful [15]. In the case of our patient since the size of his pseudoaneurysm was less than 2cm, hence a conservative approach was undertaken. Also, we believed the pseudoaneurysm was not the culprit at the root cause of the DVT but rather the hematomas were, so the plan was to drain the hematomas and relieve the lower extremity veins of this obstruction. The takeaway point from this article is femoral arterial pseudoaneurysms are becoming common. They are diagnosed with a thorough physical exam that usually demonstrates groin swelling, bruising, and a pulsatile mass. A duplex arterial ultrasound is the diagnostic imaging of choice. It is necessary to also perform a duplex venous ultrasound since significantly large PSAs or hematomas can compress nearby veins and lead to deep venous thrombosis. Conservative management is appropriate for non-expanding PSAs with a size of less than 2cm.
4. Conclusion
Femoral pseudoaneurysms are a rare but possible complication of coronary angiography that physicians have to be aware of. We have to be familiar with the possible complications of femoral pseudoaneurysms including the formation of an ipsilateral deep venous thrombosis in the affected lower extremity. Hematomas are a common complication of coronary angiography. Iatrogenic hematomas can also lead to deep venous thrombosis depending on the size of the hematomas.
References
- Messina LM, Brothers TE, Wakefield TW, et al. Clinical characteristics and surgical management of vascular complications in patients undergoing cardiac catheterization: interventional versus diagnostic procedures. J Vasc Surg 13 (1991): 593-600.
- Poonai N, Lim R, Lynch T. Pseudoaneurysm formation following a traumatic wrist laceration. Cand Jr Emerg Med 13 (2015): 48-52.
- Ranganath A, Hanumanthaiah D. Radial artery pseudo aneurysm after percutaneous cannulation using the Seldinger technique.. Indian J Anaesth 55 (2011): 274-276.
- Tulla K, Kowalski A, Qaja E. Femoral Artery Pseudoaneurysm. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Madia C. Management trends for post-catheterization femoral artery pseudoaneurysms. JAAPA 32 (2019): 15-18.
- Webber GW, Jang J, Gustavson S, et al. Contemporary management of post-catheterization pseudoaneurysms. Circulation 115 (2007): 2666-74.
- Sarkadi H, Csore J, Veres DS, et al. Incidence of and predisposing factors for pseudoaneurysm formation in a high-volume cardiovascular center. PLoS One 16 (2021): e0256317.
- Ahmad F, Turner SA, Torrie P, et al. Iatrogenic femoral artery pseudoaneurysms--a review of current methods of diagnosis and treatment. Clin Radiol 63 (2008): 1310-6.
- Schneider C, Malisius R, Küchler R, et al. A prospective study on ultrasound-guided percutaneous thrombin injection for the treatment of iatrogenic post-catheterization femoral pseudoaneurysms. Int J Cardiol 131 (2009): 356-61.
- Steinkamp HJ, Werk M, Felix R. Treatment of postinterventional pseudoaneurysms by ultrasound-guided compression. Invest Radiol 35 (2000): 186-92.
- Eisenberg L, Paulson EK, Kliewer MA, et al. Sonographically guided compression repair of pseudoaneurysms: further experience from a single institution. AJR Am J Roentgenol 173 (1999): 1567-73.
- Stolt M, Braun-Dullaeus R, Herold J. Do not underestimate the femoral pseudoaneurysm. Vasa 47 (2018): 177-185.
- Jargiello T, Sobstyl J, Swiatlowski L, et al. Ultrasound-guided thrombin injection in the management of pseudoaneurysm after percutaneous arterial access. J Ultrason 18 (2018): 85-89.
- Ehieli WL, Bozdogan E, Janas G, et al. Imaging-guided percutaneous thrombin injection for the treatment of iatrogenic femoral artery pseudoaneurysms. Abdom Radiol (NY) 44 (2019): 1120-1126.
- Stone PA, Campbell JE, AbuRahma AF. Femoral pseudoaneurysms after percutaneous access. Journal of Vascular Surgery 60 (2014): 1359-1366.


 Impact Factor: * 3.5
Impact Factor: * 3.5 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 14.80%
Acceptance Rate: 14.80%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 