Effect of Tetra-nortriterpenoid on the Fourth and Fifth Larval Instar of Spodoptera littoralis
Article Information
Ebrahim Eissa1, Salama Ahmed Salama2,4, Khaled Hashem Radwan3, Eman Hashem Radwan4*
1Zoology Department, Faculty of Science, South Valley University, Egypt
2Jazan University, Saudi Arabia
3Agriculture Genetic Engineer Research institute (AGERI), Cairo, Egypt
4Zoology department, Damanhour University, Egypt
*Corresponding Author: Eman Hashem Radwan, Zoology department, Damanhour University, Egypt
Received: 25 December 2019; Accepted: 05 February 2020; Published: 28 February 2020
Citation: Ebrahim Eissa, Salama Ahmed Salama, Khaled Hashem Radwan, Eman Hashem Radwan. Effect of tetra-nortriterpenoid on the fourth and fifth larval instar of Spodoptera littoralis. Journal of Bioinformatics and Systems Biology 3 (2020): 001-018.
View / Download Pdf Share at FacebookAbstract
Organic control gives a more secure choice to diminish the number of inhabitants in rural nuisance. Zanzalacht or tetra-nortriterpenoid is one of bio-pesticides containing concoction substances that have a bolstering hindrance property against Spodoptera littoralis (Lepidoptera: Noctuidae). Extraction methods of Zanzalacht; Extraction of dynamic standards has been done in Soxhlet contraption utilizing methanol as a dissolvable for 20 hours. Methanolic concentrates of leaves of chinaberry with the concentrations 0.7%, 1.0%, 1.3% were prepared. Metabolite from plants plays key protective role against the insect. The consideration currently is coordinated to the need of ecological safe bio-pesticides. Plants derived chemicals act as naturally safe option of chemical pesticides. Biological pesticides based on plant extracts specific to a target pest offer an ecologically effective solution to pest problems. They pose less risk to the environment and to human health. It was noticed that the methanolic chinaberry extract affected the normal development of the larvae of S. littoralis. The abnormalities obtained were classified into three main groups; namely the larval abnormalities, the pupal abnormalities and the abnormal moths.
Keywords
Bio-pesticide; Environment; Larvae; Spodoptera littoralis; Tetranortriterpenoids
Bio-pesticide articles, Environment articles, Larvae articles, Spodoptera littoralis articles, Tetranortriterpenoids articles
Bio-pesticide articles Bio-pesticide Research articles Bio-pesticide review articles Bio-pesticide PubMed articles Bio-pesticide PubMed Central articles Bio-pesticide 2023 articles Bio-pesticide 2024 articles Bio-pesticide Scopus articles Bio-pesticide impact factor journals Bio-pesticide Scopus journals Bio-pesticide PubMed journals Bio-pesticide medical journals Bio-pesticide free journals Bio-pesticide best journals Bio-pesticide top journals Bio-pesticide free medical journals Bio-pesticide famous journals Bio-pesticide Google Scholar indexed journals Environment articles Environment Research articles Environment review articles Environment PubMed articles Environment PubMed Central articles Environment 2023 articles Environment 2024 articles Environment Scopus articles Environment impact factor journals Environment Scopus journals Environment PubMed journals Environment medical journals Environment free journals Environment best journals Environment top journals Environment free medical journals Environment famous journals Environment Google Scholar indexed journals Larvae articles Larvae Research articles Larvae review articles Larvae PubMed articles Larvae PubMed Central articles Larvae 2023 articles Larvae 2024 articles Larvae Scopus articles Larvae impact factor journals Larvae Scopus journals Larvae PubMed journals Larvae medical journals Larvae free journals Larvae best journals Larvae top journals Larvae free medical journals Larvae famous journals Larvae Google Scholar indexed journals Spodoptera littoralis articles Spodoptera littoralis Research articles Spodoptera littoralis review articles Spodoptera littoralis PubMed articles Spodoptera littoralis PubMed Central articles Spodoptera littoralis 2023 articles Spodoptera littoralis 2024 articles Spodoptera littoralis Scopus articles Spodoptera littoralis impact factor journals Spodoptera littoralis Scopus journals Spodoptera littoralis PubMed journals Spodoptera littoralis medical journals Spodoptera littoralis free journals Spodoptera littoralis best journals Spodoptera littoralis top journals Spodoptera littoralis free medical journals Spodoptera littoralis famous journals Spodoptera littoralis Google Scholar indexed journals Tetranortriterpenoids articles Tetranortriterpenoids Research articles Tetranortriterpenoids review articles Tetranortriterpenoids PubMed articles Tetranortriterpenoids PubMed Central articles Tetranortriterpenoids 2023 articles Tetranortriterpenoids 2024 articles Tetranortriterpenoids Scopus articles Tetranortriterpenoids impact factor journals Tetranortriterpenoids Scopus journals Tetranortriterpenoids PubMed journals Tetranortriterpenoids medical journals Tetranortriterpenoids free journals Tetranortriterpenoids best journals Tetranortriterpenoids top journals Tetranortriterpenoids free medical journals Tetranortriterpenoids famous journals Tetranortriterpenoids Google Scholar indexed journals Phagocytosis articles Phagocytosis Research articles Phagocytosis review articles Phagocytosis PubMed articles Phagocytosis PubMed Central articles Phagocytosis 2023 articles Phagocytosis 2024 articles Phagocytosis Scopus articles Phagocytosis impact factor journals Phagocytosis Scopus journals Phagocytosis PubMed journals Phagocytosis medical journals Phagocytosis free journals Phagocytosis best journals Phagocytosis top journals Phagocytosis free medical journals Phagocytosis famous journals Phagocytosis Google Scholar indexed journals Botanical insecticides articles Botanical insecticides Research articles Botanical insecticides review articles Botanical insecticides PubMed articles Botanical insecticides PubMed Central articles Botanical insecticides 2023 articles Botanical insecticides 2024 articles Botanical insecticides Scopus articles Botanical insecticides impact factor journals Botanical insecticides Scopus journals Botanical insecticides PubMed journals Botanical insecticides medical journals Botanical insecticides free journals Botanical insecticides best journals Botanical insecticides top journals Botanical insecticides free medical journals Botanical insecticides famous journals Botanical insecticides Google Scholar indexed journals chemical insecticides articles chemical insecticides Research articles chemical insecticides review articles chemical insecticides PubMed articles chemical insecticides PubMed Central articles chemical insecticides 2023 articles chemical insecticides 2024 articles chemical insecticides Scopus articles chemical insecticides impact factor journals chemical insecticides Scopus journals chemical insecticides PubMed journals chemical insecticides medical journals chemical insecticides free journals chemical insecticides best journals chemical insecticides top journals chemical insecticides free medical journals chemical insecticides famous journals chemical insecticides Google Scholar indexed journals Musca domestica articles Musca domestica Research articles Musca domestica review articles Musca domestica PubMed articles Musca domestica PubMed Central articles Musca domestica 2023 articles Musca domestica 2024 articles Musca domestica Scopus articles Musca domestica impact factor journals Musca domestica Scopus journals Musca domestica PubMed journals Musca domestica medical journals Musca domestica free journals Musca domestica best journals Musca domestica top journals Musca domestica free medical journals Musca domestica famous journals Musca domestica Google Scholar indexed journals
Article Details
1. Introduction
Spodoptera littoralis (Lepidoptera: Noctuidae) is a monetarily essential polyphagous pest in Egypt causing genuine financial misfortunes in crops. A multifaceted methodology is required due to the numerous records of obstruction, creating in this insect to several groups of pesticides [1]. Bio-insecticides could be utilized as options in contrast to synthetic pesticides [2-6]. Enhancing food production, pesticides are generally utilized by agriculturists for harvest security. Fundamentally assorted synthetic substances have been structured as insecticides and herbicides to kill or suppress pests such as insects. Insecticides and herbicides may contaminate distinctive conditions through filtering and seepage [2, 6, 7]. Radwan [2] found that seed kernel of the fruit of Azadirachta indica contains standards principles which have both antifeedants and insect development directing impacts.
Expanding number of insect pests develop resistance to insecticides [2, 8]. GSTs in insects are engaged with inactivation of endogenous metabolites and detoxification of xenobiotics [2, 9, 10, 11]. It had been reported that insect could oppose numerous insecticides, such as organophosphates, carbamates and pyrethroids [6, 10, 11]. Pyrethroid and organophosphate are much of the time utilized insecticides to control hatching of Spodoptera littoralis (Lepidoptera: Noctuidae) [12]. S. littoralis is a genuine polyphagous insect pest that considerably reduces yields of various economically important crops [13]. Pest management against S. littoralis invasion has turned out to be increasingly troublesome in light of the fact that most generally utilized insecticides are incapable in controlling this pest. Different investigations revealed that S. littoralis grew high protection from insecticides such as organophosphates and pyrethroids [6, 13]. Host plant selection in phytophagous insects involves orientation; landing and at last contact assessment of potential host plants [14]. Contact chemical cues are vital for insect herbivores during the final phase of the process of host recognition where they provide vital information for the final acceptance or rejection of a plant for feeding. To survey the appropriateness of host plants, phytophagous insects use both essential and auxiliary metabolites on the plants that go about as stimulants and obstacles [15].
The cotton leaf worm is oppressed, in Egypt to dense treatments with insecticides and resistance to those insecticides has been evolved. The utilization of characteristic items promptly accessible in nature could lessen the requirement for imported pesticides. Family Meliaceae represents one of the most critical plants. It contains the neem tree Azedarachta indica and Melia azedarach (Zanzalacht). Azadirachtin weakens the advancement of insects, tetra-nortriterpenoid and azadirachtin are natural growth inhibitor [2, 6].The Egyptian cotton leaf worm, Spodoptera littoralis (Lepidoptera: Noctuidae) is a poly-phagous herbivore, it segregates between host plants of various qualities. The choice of an appropriate host plant in S. littoralis is appeared to be guided by unstable prompts. A standout amongst the most favorable circumstances of insecticides are the capacity to sanitize the objective nuisance and to cause disfigurements in its gonads, so the present investigation meant to assess the impact of chinaberry tree separate on the regenerative limit of insects with respect to fecundity and fertility. The point of the present examination is to research the impact of leaves concentrate of Zanzalacht tree on the cotton leaf worm; S. littoralis.
Human requests a high quality and adequate amount of sustenance from rural creation. Agricultural pests are an obstacle to food production worldwide and they have turned out to be progressively impervious to an assortment of bug sprays [16, 17]. Most chemical insecticides are extremely dangerous, and their utilization isn’t prescribed by environmentalists because an increase in the doses potentially harms non-target organisms. Botanical insecticides are viewed as a potential alternative to controlling in the pest population. Botanical insecticides, which are derived from natural substances extracted from plants, are typically alright for the environment [2, 6, 17, 18]. Their applications leave no concoction deposits that can hurt non-target life forms and the earth [2, 19].
The obstruction of Spodoptera to different substance needs to be monitored because these pests are quickly spreading [20]. It is very earnest to control these irritation populaces utilizing elective techniques, especially plant-based bio-pesticides. The insusceptible protection system in bugs goes about as an obstruction to diseases when presented to remote specialists. Insects have both cellular and humoral immune defenses; both stimulate each other to exert their activity [2, 6, 15]. Phagocytosis becomes one of the parameters in the cell resistance mechanism. A trial of the adequacy of phagocytosis from insect larvae to determine changes in the immune response that occur in the body of the insect needs to be done when exposed by a toxic substance. Lacey et al. [21], Sigh and Kaur [22] assessed the viability of an organic operator amid its application or presentation, particularly in deciding the extent of bug mortality. The deadly centralization of a bio-pesticide for bug control impacts the time required by a population to become resistant to it, leading to an eventual resurgence of the pest’s population once it has acquired resistance. Most bio-pesticides are viewed as harmful to the biological community whenever connected over a generally lengthy time span period. When utilizing a characteristic compound as a bio-pesticide, the concentrations are very important parameters when trying to deter pest resistance [2, 6]).
2. Material and Methods
littoralis (Egyptian cotton leaf worm) eggs were raised to pupation at 25ºC and 70% RH. Larvae were nourished castor bean leaves (Ricinus communis) and adults were fed 10% sugar solution and provided with leaves of Nerium oleander for oviposition [23]. The S. littoralis utilized in the experiments started from a laboratory culture started in 2017, Egypt. All formative stages were kept at 25 C, 70% relative humidity and at a light: dark cycle of 16: 8h. Fourth and fifth instar larvae appearing dietary admission and quick weight increment were utilized for all tests. The quantity of eggs laid/female, percent of egg bring forth and mortality of hatchlings and pupae were recorded. Three reproduces per treatment were performed. Measurable examination Data were dissected utilizing investigation of difference (ANOVA) noteworthiness at (P<0.05) [24]. The colony of S. littoralis had been raised in the laboratory were kept in perforated plastic cups, each 8cm in diameters and 8 cm in deep. Each cup was covered with another one, the bottom of which containing a sufficient amount of the artificial diet as described by Hegazy [25]; 500 gm kidney beans, 50 gm medical dried yeast, 60 gm agar, 9.5 gm methyl-phydroxy benzoate, 16 gm L-ascorbic acid, 1.1 gm formalin and 293 ml water. Egg batches were collected daily then transferred to the perforated cups until hatching took place.
2.1 Extraction methods
Extraction of the dynamic standards has been done in Soxhlet contraption utilizing methanol as a dissolvable for 20 hours [26]. The concentrates were dried at 60 C [27]. Dried extracts were presented in tightly corked 20 ml vails and stored in a refrigerator until used. Methanolic concentrates of leaves of chinaberry with the concentrations 0.7%, 1.0%, 1.3% were prepared. The previously mentioned concentrations were incorporated in the artificial diet of S. littoralis. Three groups each consisting of sixty of 4th and fifth instar larvae of S. littoralis was exposed to treated artificial diet. Larval, pupation and adult emergence were recorded. All the pupae and adults acquired were gathered and checked for irregularities. Sixty larvae were taken in every test and there were three replicate. Statistical analysis Data were subjected to students' T-test and least significance difference (LSD) test [28].
3. Results and Discussion
Impacts of leaves methanolic extract of chinaberry on the formative phases of Spodoptera littoralis; Preliminary experiments had shown that the concentrations of 4% of methanolic chinaberry leaves extracts caused 100% mortality of all larval stages within two days after treatment. The larvae of S. littoralis had been subjected to three lower concentrations; 0.7, 1, 1.3 of methanolic chinaberry leaves extracts (Tables 1, 2). Two parameters were taken into account in these experiments; the beginning of each larval instar and the length of the inter-larval between two successive ecdysis; mortality during each instar was utilized as a third parameter. Newly molted fourth and fifth instar larvae of S littoralis had been derived into groups each group consist of sixty larvae fed on artificial diet containing 0.7, 1, 1.3% of the methanolic chinaberry leaves extracts for 24hrs., 48hrs., 72hrs for each concentration. The control groups were fed on the artificial diet only.
3.1 Effects of methanolic extracts of chinaberry, Melia azedarach on the developmental stages of S. littoralis
The application of methanolic chinaberry extracts to various developmental stages of the cotton leaf worm S. littoralis revealed that the fourth instar of S. littoralis was the most susceptible stage for the chinaberry extract treatment. The fifth and sixth instars showed less response to the chinaberry extract treatment as demonstrated in the fourth, fifth and sixth instars of S. littoralis (Table 1, 2) and (Figures 1-19). The effect of the methanolic chinaberry extract on the larval instars of S. littoralis was not absolute since it depended upon the age of the larval instar, the concentration of the extraction and the duration of the treatment-period on the instar. The larval pupal intermediates were found to retain the head and thorax of the larval form while possessing the abdomen of the pupal form. Some larval pupal intermediates did retain the larval thoracic legs. The pupal-larval intermediates possessed the appearance of the pupae. These forms retained the larval head and the larval cuticle. They remained as such without further development until they died. Similar findings were obtained by Radwan, [2] and Radwan et al. [6], in her work on the house fly Musaca domestica. The degrees of abnormalities of abnormal pupae were classified into; comprised pupae, comprised pupae with crumpled thorax, and comprised pupae with normal thorax, all the abnormal pupae had never produced adult forms and died within days. Moths emerging from apparently normal pupae often had deformed wings and occasionally failed to emerge from the pupal cases. Malformed pupae were obtained by Radwan, [2] and Radwan et al. [6] working on the adult stage of the house fly Musca domestica after treatment with different concentrations of chinaberry leaf extracts.
Table 1: The development of forth larval instar Spodoptera littoralis in relation to the treatment of chinaberry leaves.
Table 2: The development of fifth larval instar Spodoptera littoralis in relation to the treatment of chinaberry leaves.
Figure 1: The mean no. of fourth instar larvae of S littoralis.
Figure 2: The mean mortality % of S littoralis.
Figure 3:The mean no. of fifth normal larvae of S littolaris.
Figure 4: The meanmortality % of S littolaris.
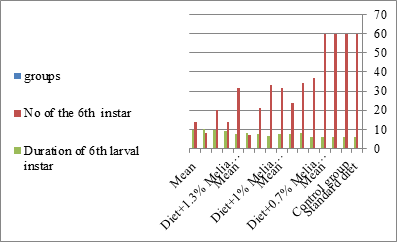
Figure 5: The mean no. of sixth instar larvae and duration of larval instar S littolaris.
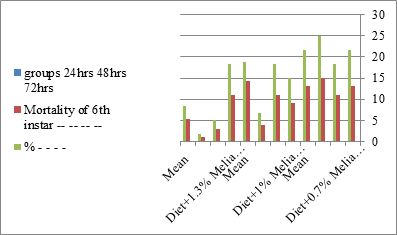
Figure 6:The mean % mortality of sixth instar larvae of S. littolaris.
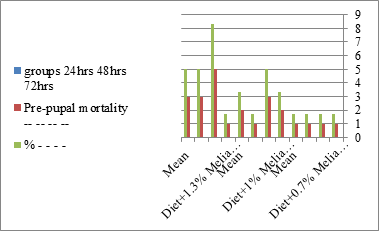
Figure 7:The mean % of pre pupal mortality of S. littoralis.
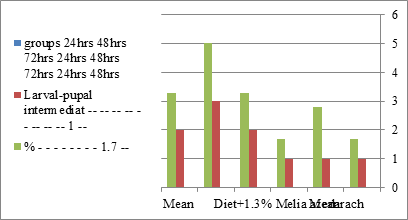
Figure 8:The mean % of larval pupal intermediate of S. littolaris.
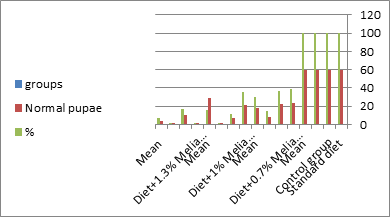
Figure 9: The mean no. of normal pupae% of S. littolaris.
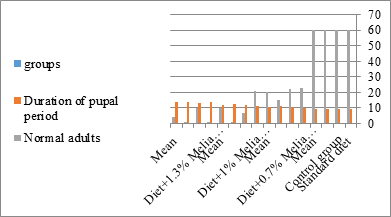
Figure 10:The mean duration period of S. littolaris.
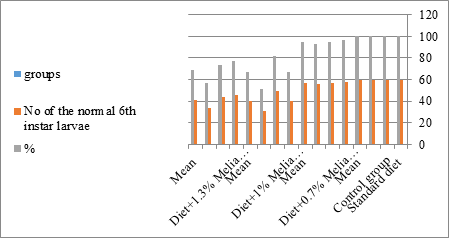
Figure 11: The mean no. of normal sixth instar larvae of S. littolaris.
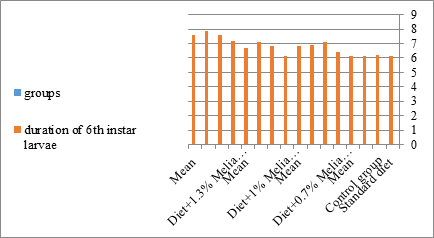
Figure 12:The mean duration period of sixth instar larvae of S. littolaris.
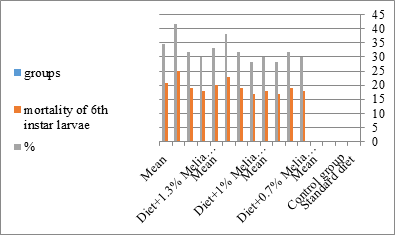
Figure 13:The mean% of the mortality of the sixth instar larvae of S. littolaris.
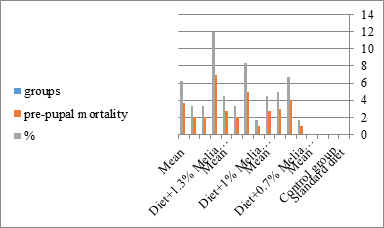
Figure 14: The mean% of the pre pupal mortality of S. littolaris.
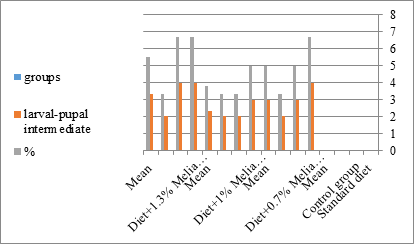
Figure 15:The mean % of larval pupal intermediate of S. littolaris.
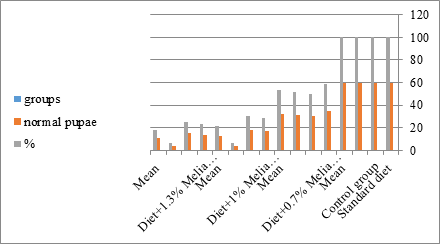
Figure 16: The mean % of the normal pupae of S. littolaris.
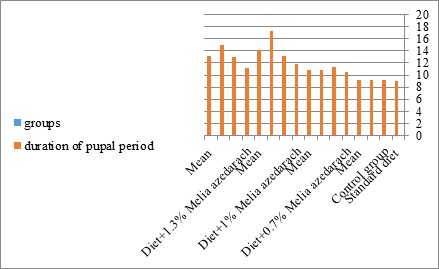
Figure 17: The mean duration of pupal period of S. littoralis.
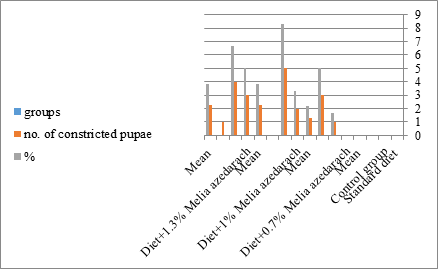
Figure 18: The mean of %of constricted pupae of S. littolaris.
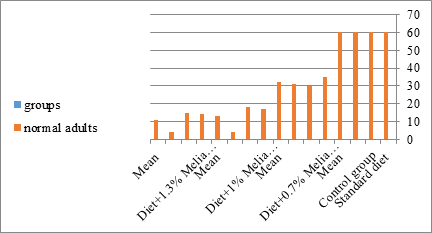
Figure 19: The mean of normal adults of S. littolaris.
3.2 Impact of chinaberry, Melia azedarach leaves extract
Newly molted fourth instar larvae of S. littoralis had been divided into 12 groups, each group comprising sixty larvae fed on an artificial diet containing 0.7, 1, 1.3%, respectively of ethanolic chinaberry leaves extract for 24 hrs., 48hrs., 72hrs for each concentration. The control groups were fed on an artificial diet only. It is shown in Table 1 and Figures 1-11, that the metamorphosis in all larval groups that exposed to Melia azedarach leaves extracts was retarted. The mean duration of the fourth instar was prolonged with the increase in the concentration of Melia azedarach. Leaves extract. The mean larval pupal duration ranged from 3.1 days in the control groups to 3.9, 3.9, 4.1 days in concentrations 0.7, 1, 1.3% groups; respectively. It was noted that 4.1 days was the maximum fourth larval instar duration resulting from 1.3% treatment for 72 hrs. group.
The mortality percentage increased with the increase of the treatment period in each concentration. The mortality was 31.7, 50, 53.3 for 0.7, 1, 1.3%; respectively. The mean duration of the fifth larval instar resulting from treated fourth larval instars were 4.5, 5.3, 5.3 days for 0.7, 1, 1.3% groups; respectively, compared with 3.2 days for the control groups. It was obvious that the duration averages of the fifth larval instar were varied from the control in increasing manner reaching a maximum of 5.3 days in 1% treatment for 72 hrs., group and in 1.3 treatment for 24hrs., as well. The mortality percentages of the fifth larval instar were 31.7, 38.3, 41.7% for the concentrations of 0.7, 1, 1.3% groups; respectively. The mortality of fifth larval instar resulting from treated fourth larval instar with the different extract concentrations reached 41.7 for 1.3% treatment in 72hrs, group. The duration of the sixth larval instar resulting from the treated larval instar with the three mentioned concentrations of M. azedarach leaves extract was also increased with the increase of the concentrations. The larval duration varied from 6.2 days in the control group instead of 7.9, 7.9, 10 days for 0.3, 1, 1.3% group; respectively. The mean duration of the sixth larval instar reached a maximum of 10 days in 1.3% treatment for 72 hrs. The sixth larval instar mortality percentages were 25, 18.3, 18.3% for 0.3, 1, 1.3% groups; respectively. As shown in Table 1, the pre-pupal mortality percentages were 1.7, 3.3, 8.3 for 0.3, 1, 1.3%; respectively and no dead pre-pupae in the control were observed. The mean duration of the pupal period was increased with the increase of the concentration from 9.2 days in the control groups to 11, 12.2, 14 days for 0.3, 1, 1.3% groups; respectively. The mean pupal duration reached a maximum of 14 days in 1.3% treatment for 72 hrs group.
3.3 Treatment of the fifth larval instar of S. littoralis
Newly molted fifth instar larvae of S. littoralis had been divided into 12 groups each group containing 60 larvae fed on an artificial diet containing 0.7, 1, 1.3% of methanolic chinaberry leaves extracts for 24, 48, 72 hrs., group for each concentration. The control groups were fed on an artificial diet only. It is shown from Table 2 and Figures 11-19 that the metamorphosis in all larval groups that exposed to Melia azedarach leaves extracts of concentrations 0.7, 1, 1.3% was affected. The mean normal duration of the fifth larval instar reached 3.2days in the control groups compared with 4.1, 3.9, 4.4 days in 0.7, 1, 1.3% groups; respectively. The mean duration of the treated fifth larval instar was increased with the increase of M. azedarach concentration and with the increase of the period of the treatment. The mortality percentage in the fifth larval instar were 6.7, 48.3, 43.3% for 0.7, 1, 1.3% groups; respectively. The mean duration of the sixth larval instar varied from the control in increasingly manner reaching a maximum of 7.9 days in 1.3% treatment for 72hrs. group. The mean duration of the sixth larval instar resulting from the treated fifth larval instar were 7.1, 7.1, 7.9 days for 0.7, 1.0, 1.3 groups; respectively compared to 6.2 days for the control group. The mean duration of the sixth larval instar varied from the control in increasingly manner reaching a maximum of 7.9 days in 1.3% treatment for 72hrs group.
The sixth larval instar mortality were 31.7, 38.3, 41.7 in 0.7, 1, 1.3 treated groups; respectively. In Table 2, the higher sixth larval instar percentage mortality is 41.7% in 1.3% treatment for 72 hrs group. In Table 2 the pre-pupal mortality percentage reached up to 6.7, 8.3, 12 for 0.7, 1.0, 1.3% group; respectively. The percentages of larval-pupal intermediates mortality were reached in 0.7, 1, 1.3% group, 6.7, 5, 6.7; respectively. The maximum larval-pupal intermediates mortality percentage was in 1.3% treated group, 6.7. The mean duration of the pupal period was increased with the increase in concentration from 9.2 days in the control group to 11.3 in concentration from 9.2days in the control group to 11.3, 17.3, 15 days for 0.7, 1, 1.3% group; respectively. The pupal duration reached a maximum of 15 days in 1.3% treatment for 72hrs group. Utilization of the leaves methanolic chinaberry extract to different formative phases of cotton leaf worm revealed that the fourth and fifth instar of Spodoptera littoralis were the most defenseless stages for the chinaberry extract treatment. The effect of the methanolic chinaberry extract on the larval instars was not absolute since it relied on the time of larval instar and the grouping of the extraction and the duration of the treatment period on the instar. The abnormality forms acquired were ordered into three main groups; larval irregularities; pupal anomalies and anomalous moths. In the present examination, no additional larval instar had been seen in the present treatment. Individuals of the first larval instar were divided into groups, each consisted of 60 larvae. The first group was benefited from typical counterfeit eating routine until the finish of the analysis. This group represents the control group. The other groups were fed on normal diet mixed with 0.7%, 1%, 1.3% of the leaves extract for 24, 48, 72 hours, then the larvae were transferred to the normal artificial eating regimen to complete their life span, these groups represent the treated groups. The experiment was repeated multiple times and the mean was recorded. The treatment of the fourth larval instars by Zanzalacht leaves extract caused larval mortality, pupal and adult malformations.
The mean percentage of larval mortality as well as the pupal and adult malformations (Table 1) concerning variations from the norm. The malformed pupae stayed accordingly as such without further advancement until they died. It is noticed that the malformed adults and pupae had been developed and resulted from the normally appeared pupae and larvae. The results given in (Table 1 and Figure 1) demonstrated a delay in the rate of the development of the larvae treated with the three concentrations of Melia azedarach leaves extracts. Newly molted fifth instar larvae of S. littoralis had been divided into groups each group comprising sixty larvae fed on artificial diet containing 0.7%, 1%, 1.3% of methanolic chinaberry leaves for 24hrs., 48hrs., 72hrs./each concentration. The control groups were fed on artificial diet only. The mean duration of treated fifth larval instar was increased with the increase of Melia azedarach concentrations. Shalaby et al. [29] revealed that larvae of the tobacco horn worm, Manduca sexta attempted to shed into a supernumerary larval instar after treatment with low concentrations of neem seed extract. Sieber and Rembold [30] worked on the effect of azadirachtin on the shedding of Locusta migratoria. He observed that treated larvae remained in the pharate condition unable to shed their old cuticle. Deformed pupae were obtained by Bidmon et al. [31], after injection the blowfly larvae Calliphora vicina by azadirachtin. Abnormalities in the larvae, pupae and adults of the house fly Musca vicina were observed by Radwan [2] after treatment with different concentrations of chinaberry leaf and fruit extracts. Jotwani and Srivastava [32] dealt with hatchlings larvae of Chilo partellus utilizing neem kernel suspension and by Hyde et al. [33] utilizing neem extract for controlling some hemipterous rice pests. Schmutterer and Rembold [34] detailed that azadirachtin is said to act like a shedding hormone mimic.
Rembold et al. [35] treated Locusta migratoria nymph with 4 µg azadirachtin/g fresh weight, within the first two days subsequent to shedding. The nymphs were incapable of shedding their old cuticle. Prabhaker et al. [36] dealt with the larvae of cabbage Looper trichoplusiani by neem seed extract, joined into an artificial diet, the pupae were deformed. Herbicides and insecticides are broadly utilized in current agribusiness. It has been accounted for in different investigations that use of insecticides can increase tolerance of herbivorous insects to insecticides. To enhance food production, pesticides are broadly utilized by farmers for crop protection. Structurally diverse chemicals have been designed as insecticides and herbicides to kill or suppress pests such as weeds and insects. Insecticides and herbicides may contaminate different environments through spray drift, volatilization, leaching and drainage [6, 7]. GSTs in insects are associated with inactivation of endogenous metabolites and detoxification of xenobiotics [8, 11]. S. littoralis is a serious polyphagous insect pest that considerably reduces yields of numerous economically important crops such as tobacco and peanut [13]. Pest management against S. littoralis infestation has turned out to be progressively troublesome everywhere throughout the world in light of the fact that most regularly utilized this pest. Different examinations announced that S. littoralis developed a high resistance to insecticides such as organophosphates and pyrethroids [12, 13, 37].
The expansion in prohemocyte cells specifically will affect the expansion in the quantity of plasmatocytes and both of these cells will be engaged with the mechanism of the insect's immune system. The comparison of the average hemocytic type of S. litura larvae when given the extract of M. jalapa happens differently. The outcomes demonstrated a distinction in the normal kind of hemocytes with various convergences of M. jalapa. The extensive number of lysosomes with high compound substance on plasmatocytes fills in as an impetus for remote substances [38]. The humoral insusceptible reaction in insects plays a major role in the immune system by activating various enzymatic and non-enzymatic reactions used by the body for the recognition of foreign agents and developing resistance to them. The mechanism relies upon the capacity of the cells to recognize foreign agents through receptors on their membranes. There are eight receptors associated with the humoral immune mechanism: thioester-containing proteins (TEPs), LPS-binding protein, peptidoglycan recognitions proteins (PGRPs), gram-negative bacteria binding proteins (βGRPs), hemolin (immunoglobulin superfamily), and Bombyx mori multibinding protein. The introduction of the foreign agents perceived by these receptors impact the cell’s response by stimulating the induction and secretion of antimicrobial peptides and starting the melanization procedure [39].
Receptors are an essential piece of the safe immune defense mechanisms in organisms. Lectin receptors, which are proteins that bind carbohydrates [40] are the principle factors activating phenol oxidation in the hemolymph plasma [39]. Immulectin in granular cells and eonocytoids increment exemplification movement [40]. A receptor’s capacity to perceive a foreign agent induces the primary receptor to initiate an immune response. Lectins are equipped for inciting cell and humoral successions in the immune system, can be utilized as a marker for the acknowledgment of foreign agents/pathogens and subsequent signal transductions. A concentration of 0.2% is subsequently considered as the ideal fixation to animate the resistant reaction, as shown by the immune response, as indicated by the rapid increase in the number of hemocytes produced (P<0.05) at this concentration toxicity at high concentrations causes a change in an insect’s enzymatic and coordination systems. The activation of PO is the main enzymatic reaction important to the humoral response sequence. This enzyme plays an important role in melanogenesis in invertebrates [41, 42]. In conclusion; the present results indicated that Zanzalacht leaves extract has a great potential for becoming an important bio-insecticide against S. littoralis.
References
- Armes NJ, Wightman JA, Jadhav DR, et al. Status of insecticide resistance in Spodoptera litura in Andhra Pradesh, Indian. Pestic Sci 50 (1997): 240-248.
- Radwan EH. Effect of crude methanolic extracts of leaves and fruits of chinaberry tree Melia azedarach L. on the adult house fly Musca domestica vicina Macq. MSc Thesis Faculty of Sci. Univ. of Alex (1999).
- Armenta R, Martínez A, Chapman T, et al. Impact of a nucleopolyhedrovirus bio-insecticide and selected synthetic insecticides on the abundance of insect natural enemies on maize in southern Mexico. J Econ Entomol 96 (2003): 649-661.
- Gupta RK, Raina JC, Arora RK, et al. Selection and field effectiveness of nucleopolyhedrovirus isolates against Helicoverpa armigera (Hubner). Int J Virol 3 (2007): 45-59.
- Elvira S, Williams T, Caballero P. Juvenile hormone analog technology: Entomol 108 (2010): 1345-1357.
- Radwan EH, Youssef NS, Hashem HO, et al. The Effects of Zanzalacht on the Gonotrophic cycle of the Adult House fly Musca Domestica. Journal of plants and animal ecology 1 (2019): 23-39.
- Le TH, Lim ES, Lee SK, et al. Effects of glyphosate and methidathion on the expression of the Dhb, Vtg, Arnt, CYP4 and CYP314 in Daphnia magna. Chemosphere 79 (2010): 67-71.
- Li XC, Schuler MA and Berenbaum MR. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annual Review of Entomology 52 (2007): 231-253.
- Deng HM, Huang YF, Feng QL, et al. Two epsilon glutathione S-transferase cDNAs from the common cutworm, Spodoptera litura: characterization and developmental and induced expression by insecticides. Journal of Insect Physiology 55 (2009): 1174-1183.
- Zhang JQ, Ge PT, Li DQ, et al. Two homologous carboxylesterase genes from Locusta migratoria with different tissue expression patterns and roles in insecticide detoxification. Journal of Insect Physiology 77 (2015): 1-8.
- Salim AMA, Shakeel M, Ji JY, et al. Cloning, expression, and functional analysis of two acetylcholinesterase genes in Spodoptera litura (Lepidoptera: Noctuidae). Comparative Biochemistry and Physiology, Part B 206 (2017): 16-25.
- Tong H, Su Q, Zhou XM, et al. Field resistance of Spodoptera litura (lepidoptera: noctuidae) to organophosphates, pyrethroids, carbamates and four newer chemistry insecticides in Hunan, China. Journal of Pest Science 86 (2013): 599-609.
- Shad SA, Sayyed AH, Fazal S, et al. Field evolved resistance to carbamates, organophosphates, pyrethroids, and new chemistry insecticides in Spodoptera litura Fab. (Lepidoptera: Noctuidae). Journal of Pest Science 85 (2012): 153-162.
- Schoonhoven LM, van Loon JJA, and Dicke M. Insect-Plant Biology, 2nd Edn. Oxford: Oxford University Press (2005).
- Chapman RF. Contact chemoreception in feeding by phytophagous insect. Annu Rev Entomol 48 (2003): 455-484.
- Shapiro M. Enhancement in activity of homologous and heterologous baculoviruses infectious to beet armiworm (Lepidotera: Noctuidae) by an optical brightener. Journal of Economic Enthomology 93 (2000): 572-574.
- Leng P, Zhang Z, Pan G, et al. Applications and development trends in biopesticide. African J of Biotechn 10 (2011): 19864-19873.
- Kandagal AS and Khetagoudar MC. Study on larvicidal activity on weed extract against Spodoptera litura. 476 Journal of Environmental Biology 34 (2011): 253-257.
- Horne PA and Page J. Integrated pest management for crops and pastures. Victoria: Landlink Press. 481 (2008): 136.
- Schreiner I. Cluster caterpillar (Spodoptera litura [Fabricius]). Agricultural Pest of the Pacific ADAP (2000).
- Lacey LA, Grzywacz D, Shapieoilan DI, et al. Insect pathogens as biological control agent: back to the future. Journal of Invertebrate Pathology 132 (2015): 1-41.
- Sigh B and Kaur A. Control of insect pest in crop plants and stored food grains using plant saponins: a review. 511 LTW-Food Science Technology 87 (2017): 93-101.
- Elbarky NM, Dahi HF, and El-Sayed YA. Toxicicological evaluation and biochemical impacts for radient as a new generation of spinosyn on Spodoptera littoralis (Boisd.) larvae. Egypt. Acad J Biolog Sci 1 (2008): 85-97.
- Steel RGD and Torrie JH. Principles and procedures of statistics. McGraw-Hill stimulus. J Insect Physiol 44 (1960): 471-481.
- Hegazy EM. Further studies on certain natural enemies attacking the cotton leafworm in Alexandria. PhD. Thesis, Fac. Agri. Alex. Univ (1976).
- Ascher KRS, and Gsell R. The effect of neem seed kernel extract on Epilachna varivestis Muls. larvae. Z. Pflkrankh. Pflschutz 88 (1981): 764-767.
- Schneider BH and Erml K. Quantitative determination of azadirachtin from neem seeds using high performance liquid chromatography. Pro.3rd Int. Neem Conf. Nairobi (1986): 161-170.
- Snedecor GW and Cochram WE. Statistical methods. Iowa State University Arnes (1967).
- Shalaby AM, Elzayat MA, Hashem HO, et al. Effect of certain juvenile hormone on the development of the cotton leafworm Spodoptera littoralis (Biosd.). Bull.Fac. Sci. Alex. Univ 27 (1987): 141-156.
- Sieber KP and Rembold H. The effects of azadirachtin on the rndocrine control of moulting in Locusta migratoria. J Insect Physiol 29 (1983): 523-527.
- Bidmon HJ, Kauser G, Mobus P, et al. Effect of azadirachtin on blowfly larvae and pupae. Proc. 3rd Int. Neem Conf., Nairobi (1986): 253-271.
- Jotwani MG, and Srivastava KP. A review of neem reseach in India in relation to insects.Proc 2nd Int Neem Conf Rauischholzhausen (1983): 43-56.
- Heyde JVD, Saxena RC, and Schmutterer H. Neem oil and neem extracts as potential insecticides for control of hemipherous rice pests. Proc. 2nd Int. Neem Conf (Rauischholzhausen, 1983): 377-390.
- Schmutterer H and Rembold H. Zur wirkung einiger Reinfraktionen aus samen von Azadirachta indica auf Frassaktivitat und Metamorphose von Epilachna varivestis (Col.: Coccinellidae)Z.Angew. Entomol 89 (1980): 179-188.
- Rembold H, Forster H, Czoppelt CH, et al. The azadirachtins, A group of insect growth regulators from the neem tree. Proc. 2nd Int. Neem Conf., Rauischholzhausen (1983): 153-162.
- Prabhaker N, Coudriet DL, Kishaba AN, et al. Laboratory evaluation of neem-seed extract against larvae of the cabbage looper and beet armyworm (Lepidoptera: Noctuidae). J Econ Entomol 79 (1986): 39-41.
- Gillot C. Entomology. (3rd Edn.) University of Saskatchewan-Springer Netherlands (2005): XVIII, 500 832.
- Qamar A and Jamal K. Differential hemocyte counts of 5th instar nymps and adults of Dysdercus cingulatus Fabr (Hemiptera: Pyrrhocoridae) treated with acephate an organophosphorus insecticide, Biology and Medicine 1 (2009): 116-121.
- Marmas VJ and Lampropoulou M. Regulators and signalling in insect hemocyte immunity. Cellular Signalling 21 (2009): 186-195.
- Yu QX. A novel C-type immulectin-3 from Manduca sexta is translocated from hemolimph into the cytoplasma hemocyte. Journal of Insect Biochemistry and Molecular Biology 35 (2005): 285-295.
- Nappi AJ and Christensen BM. Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochemistry and Molecular Biology 35 (2005): 443-459.
- Suryani I and Anggraeni T. The effect of leaf bio-pesticide Mirabilis jalapa and fungi Metarhizium anisopliae to immune response and mortality of Spodoptera exigua Instar IV. AIP Conference Proceeding: Bandung (2014).


 Impact Factor: * 4.2
Impact Factor: * 4.2 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 