Efficient Muscle Distribution Reflects the Positive Influence of Coenzyme Q10 Phytosome in Healthy Aging Athletes after Stressing Exercise
Article Information
Franchek Drobnic1, Joan Riera1, Rafael Artuch2,4, Cristina Jou3,4, Anna Codina3,4, Raquel Montero2,4, Abraham J Paredes-Fuentes2,4, Joan Carles Domingo5, Montse Banquells1, Antonella Riva6, Pietro Allegrini6, Giovanna Petrangolini6*, Stefano Togni6
1High Performance Centre (CAR), Av. Alcalde Barnils 3-5, Barcelona, Spain
2Inborn errors of metabolism Unit, Institut de Recerca Sant Joan de Déu, Barcelona, Spain
3Department of Pathology, Hospital Sant Joan de Deu, Barcelona, Spain
4CIBER de Enfermedades Raras (CIBERER), Instituto de Salud Carlos III Spain, Madrid, Spain
5Departament de Bioquímica i Biomedicina Molecular, Facultat de Biologia, Edifici Prevosti, Planta -1. Universitat de Barcelona, Spain
6Indena SpA., viale Ortles 12, Milano, Italy
*Corresponding Author: Giovanna Petrangolini, Indena SpA., viale Ortles 12, 20139 Milano, Italy
Received: 07 October 2020; Accepted: 23 October 2020; Published: 30 October 2020
Citation: Franchek Drobnic, Joan Riera, Rafael Artuch, Cristina Jou, Anna Codina, Raquel Montero, Abraham J Paredes-Fuentes, Joan Carles Domingo, Montse Banquells, Antonella Riva, Pietro Allegrini, Giovanna Petrangolini, Stefano Togni. Efficient Muscle Distribution Reflects the Positive Influence of Coenzyme Q10 Phytosome in Healthy Aging Athletes after Stressing Exercise. Journal of Food Science and Nutrition Research 3 (2020): 262-275.
View / Download Pdf Share at FacebookAbstract
Coenzyme Q10 (CoQ10) is an ubiquitously-distributed molecule with a key role in mitochondrial efficiency, involving protection against peroxidation induced by reactive oxygen species. In athletes during intense training and strenuous exercise, a reactive oxygen species overproduction occurs and can cause muscular stress and damage: a reduction of those undesired effects would be of benefit. CoQ10 antioxidant properties are described in several clinical studies, but efficacy of CoQ10 supplementation in pre-senescent athletes has not yet been clearly demonstrated. A randomized, intervention-controlled, single-center clinical trial was performed in healthy aging (pre-senescent) runners undergoing exercise training in conditions of high environmental stress. One group used an innovative food-grade CoQ10 phytosome formulation (Ubiqsome) daily for 30 days, while the control group did not take supplementation. Phytosome technique applied to CoQ10 successfully increased CoQ10 bioavailability, as previously demonstrated. CoQ10 levels and oxidative with inflammatory markers were detected in both plasma and muscle. Data obtained highlighted that 500 mg of CoQ10 phytosome (corresponding to 100 mg CoQ10), administered once a day for 30 days significantly improved CoQ10 bioavailability in healthy volunteer aging runners (50-65 years) by increasing both plasmatic and muscular CoQ10 levels, with a reduction of inflammatory cytokines and Malonyl Dialdehyde levels suggesting a protective effect induced by supplementation. The original CoQ10 phytosome formulation results to be of benefit in increasing CoQ10 plasmatic and muscular levels when CoQ10 decrease occurred for oxidative stress conditions, aging or high training.
Keywords
CoQ10, Inflammation, Muscle bioavailability, Oxidative stress, Ubiqsome
CoQ10 articles; Inflammation articles; Muscle bioavailability articles; Oxidative stress articles; Ubiqsome articles
CoQ10 articles CoQ10 Research articles CoQ10 review articles CoQ10 PubMed articles CoQ10 PubMed Central articles CoQ10 2023 articles CoQ10 2024 articles CoQ10 Scopus articles CoQ10 impact factor journals CoQ10 Scopus journals CoQ10 PubMed journals CoQ10 medical journals CoQ10 free journals CoQ10 best journals CoQ10 top journals CoQ10 free medical journals CoQ10 famous journals CoQ10 Google Scholar indexed journals Inflammation articles Inflammation Research articles Inflammation review articles Inflammation PubMed articles Inflammation PubMed Central articles Inflammation 2023 articles Inflammation 2024 articles Inflammation Scopus articles Inflammation impact factor journals Inflammation Scopus journals Inflammation PubMed journals Inflammation medical journals Inflammation free journals Inflammation best journals Inflammation top journals Inflammation free medical journals Inflammation famous journals Inflammation Google Scholar indexed journals Muscle bioavailability articles Muscle bioavailability Research articles Muscle bioavailability review articles Muscle bioavailability PubMed articles Muscle bioavailability PubMed Central articles Muscle bioavailability 2023 articles Muscle bioavailability 2024 articles Muscle bioavailability Scopus articles Muscle bioavailability impact factor journals Muscle bioavailability Scopus journals Muscle bioavailability PubMed journals Muscle bioavailability medical journals Muscle bioavailability free journals Muscle bioavailability best journals Muscle bioavailability top journals Muscle bioavailability free medical journals Muscle bioavailability famous journals Muscle bioavailability Google Scholar indexed journals Oxidative stress articles Oxidative stress Research articles Oxidative stress review articles Oxidative stress PubMed articles Oxidative stress PubMed Central articles Oxidative stress 2023 articles Oxidative stress 2024 articles Oxidative stress Scopus articles Oxidative stress impact factor journals Oxidative stress Scopus journals Oxidative stress PubMed journals Oxidative stress medical journals Oxidative stress free journals Oxidative stress best journals Oxidative stress top journals Oxidative stress free medical journals Oxidative stress famous journals Oxidative stress Google Scholar indexed journals Ubiqsome articles Ubiqsome Research articles Ubiqsome review articles Ubiqsome PubMed articles Ubiqsome PubMed Central articles Ubiqsome 2023 articles Ubiqsome 2024 articles Ubiqsome Scopus articles Ubiqsome impact factor journals Ubiqsome Scopus journals Ubiqsome PubMed journals Ubiqsome medical journals Ubiqsome free journals Ubiqsome best journals Ubiqsome top journals Ubiqsome free medical journals Ubiqsome famous journals Ubiqsome Google Scholar indexed journals aging-associated diseases articles aging-associated diseases Research articles aging-associated diseases review articles aging-associated diseases PubMed articles aging-associated diseases PubMed Central articles aging-associated diseases 2023 articles aging-associated diseases 2024 articles aging-associated diseases Scopus articles aging-associated diseases impact factor journals aging-associated diseases Scopus journals aging-associated diseases PubMed journals aging-associated diseases medical journals aging-associated diseases free journals aging-associated diseases best journals aging-associated diseases top journals aging-associated diseases free medical journals aging-associated diseases famous journals aging-associated diseases Google Scholar indexed journals oxidative stress articles oxidative stress Research articles oxidative stress review articles oxidative stress PubMed articles oxidative stress PubMed Central articles oxidative stress 2023 articles oxidative stress 2024 articles oxidative stress Scopus articles oxidative stress impact factor journals oxidative stress Scopus journals oxidative stress PubMed journals oxidative stress medical journals oxidative stress free journals oxidative stress best journals oxidative stress top journals oxidative stress free medical journals oxidative stress famous journals oxidative stress Google Scholar indexed journals inflammatory markers articles inflammatory markers Research articles inflammatory markers review articles inflammatory markers PubMed articles inflammatory markers PubMed Central articles inflammatory markers 2023 articles inflammatory markers 2024 articles inflammatory markers Scopus articles inflammatory markers impact factor journals inflammatory markers Scopus journals inflammatory markers PubMed journals inflammatory markers medical journals inflammatory markers free journals inflammatory markers best journals inflammatory markers top journals inflammatory markers free medical journals inflammatory markers famous journals inflammatory markers Google Scholar indexed journals overproduction articles overproduction Research articles overproduction review articles overproduction PubMed articles overproduction PubMed Central articles overproduction 2023 articles overproduction 2024 articles overproduction Scopus articles overproduction impact factor journals overproduction Scopus journals overproduction PubMed journals overproduction medical journals overproduction free journals overproduction best journals overproduction top journals overproduction free medical journals overproduction famous journals overproduction Google Scholar indexed journals muscular stress articles muscular stress Research articles muscular stress review articles muscular stress PubMed articles muscular stress PubMed Central articles muscular stress 2023 articles muscular stress 2024 articles muscular stress Scopus articles muscular stress impact factor journals muscular stress Scopus journals muscular stress PubMed journals muscular stress medical journals muscular stress free journals muscular stress best journals muscular stress top journals muscular stress free medical journals muscular stress famous journals muscular stress Google Scholar indexed journals
Article Details
1. Introduction
Coenzyme Q10 (CoQ10 or ubiquinone) is a fat-soluble ubiquitous compound that functions as an electron carrier in the mitochondrial respiratory chain, as well as serving as an important intracellular antioxidant [1]. Several studies have demonstrated that CoQ10 is able to protect phospholipids and mitochondrial membrane proteins against peroxidation and DNA against the oxidative damage linked to lipid peroxidation [2, 3]. The CoQ10 content in the body changes in different organelles, tissues and among species [4]; even if the endogenous biosynthesis under physiological conditions is sufficient for basic needs, increasing evidence suggests that exogenous administration of CoQ10 can support and improve mitochondrial energy metabolism [5]. The production of CoQ10 is not constant throughout life, as is the functionality of organ and systems with aging. A significant reduction in the rate of CoQ10 biosynthesis is thought to occur during the physiological aging process and aging-associated diseases [6], and subjects of advanced age show a correlation between lower concentrations of coenzyme Q10 and a change in the oxidation-reduction state in favour of the oxidized form that conditions the senescent process. The decrease of CoQ10 content that may occur in mitochondria of some tissues is selective, rather than ubiquitous [4].
The ability to restore oxidative balance may be also compromised during physical activity of a certain intensity when accompanied by oxygen consumption that causes an important increase of reactive oxygen species (ROS) [7]. In the healthy individual, continued practice results in a better ability of increasing the internal antioxidant response and maintaining adequate homeostasis [8]. However, when that response may be compromised, due to an excess of physical work or a deficiency in the mechanisms of adaptation or even as a response to stress, an exogenous supplementation with effective antioxidants acts as good support [9]. There are several studies evaluating the impact on the performance of CoQ10 administration [10] using the oxidized [11] or reduced form [12-14]. The efficiency of absorption of orally administered CoQ10 is poor because of its insolubility in water, limited solubility in lipids, and relatively large molecular weight [15]. Several formulative efforts are made in order to enhance CoQ10 bioavailability [16, 17]; in particular the recently published phytosome delivery system was proposed as a new innovative formulation with ameliorated oral absorption in human volunteers [18]. In Single and repeated dose pharmacokinetic studies in healthy volunteers CoQ10 plasmatic levels significantly improved with phytosome delivery system.
The objective of the present study is to determine the biological activities of a new food-grade delivery form of CoQ10 (CoQ10 phytosome) in stressing exercise and its effect on the antioxidant capacity in aging athletes compared with controls. The physically stress adaptation response was evaluated before and after daily administration for a one-month period of 500 mg CoQ10 phytosome (containing not less than 100 mg of CoQ10).
2. Materials and Methods
2.1 Clinical study
This is a randomized, interventional, controlled, single-center trial registered at ClinicalTrials.gov with the identifier NCT03893864. Twenty healthy male runners (performing regular exercise for more 4 hours per week), between 50 and 65 years old, non-smoking volunteers with no known musculoskeletal or inflammatory pathology were recruited for the study. Subjects had to have maximal oxygen consumption (VO2max) of at least 45 ml/kg, as assessed by a maximal treadmill exercise test. Exclusion criteria were: treatment with anti-inflammatory/analgesic/antioxidant drugs or vitamins or antioxidant supplementation in the previous month, abnormal liver or renal function tests, laboratory findings suggestive of an active inflammatory or infectious process and presence of any known disease. All subjects underwent a comprehensive medical history and physical examination by a sports medicine physician to ensure proper trial eligibility. Twelve subjects participated as an intervention group and eight as a control group. The participants were not acclimatized to heat and the study was conducted in the months of March-April with an average temperature in the area of 15ºC and 18ºC, respectively. Subjects agreed to avoid the use of vitamin/mineral supplements during the entire 30-day study and completed a 7-day food record before the first exercise test session. They were also instructed to maintain the same diet and training load during the study and to have a relaxed training day before each test. All the subjects were informed of the purpose, requirements and possible risks of the study before giving their written consent to participate. The study was carried out in accordance with the Declaration of Helsinki and was approved by the local Ethics Committee of the Direcció General de l’Esport de la Generalitat de Catalunya with the number 07/2018/CEICGC.
2.2 Experimental design
Each subject performed two experimental sessions separated by a 5-week period, during which only the subjects in the intervention group were administered with the nutritional supplement. Two weeks before the first test, each subject performed an incremental maximal test until exhaustion on motorized treadmill (EG2, Vitoria, Spain) to determine maximal VO2max, using a computerized metabolic cart (Master Screen CPX, Erich Jaeger, Wuerzburg, Germany). The velocity corresponding to 60% (V60), 70% (V70), and 80 % (V80) of their VO2max was calculated by linear interpolation of data from the maximal exercise test. On day 1 of the study the subjects came to the laboratory at 9:00 AM after an overnight fast and having consumed at least 500 ml of water since waking up. Dry nude body weight was measured before and after the session. The subjects entered into the climatic chamber programmed at 35°C temperature and 50% humidity, and the heart rate (HR) transmitter was placed. Subjects ran continuously on the treadmill at speed V60 for 5 minutes, V70 for 5 minutes and V80 for 5 minutes for three consecutive bouts, separated by two minutes of recovery in between the slopes. During the stress test, water was provided “at libitum” in 50 ml bottles at room temperature and the amount of water consumed was measured and repeated in the second test session of study. To measure basal HR, a Polar®heart watch system (Polar Electro Inc, Kempele, Finland) was used, and results were recorded every 5 minutes during the test and after 5 minutes of recovery. A micro-sample of blood (20 µl) was obtained from the ear lobe to measure plasma lactate concentration ([La]) by Miniphotometer (Dr. Lange® Berlin, Germany), at rest (L0) and at 15 (L15), 32 (L32) and 49 min (L49). The Borg scale was used to assess subjective perception of effort at 15, 32, and 49 minutes.
2.3 Supplement
Subjects were randomized into two groups, one of 12 subjects who were instructed to take Coenzyme Q10 phytosome, 500 mg film-coated tablet (Ubiqsome®, Indena SpA) [18] containing not less than 100 mg of CoQ10 Ubiquinone (in the morning, just before breakfast) for 30 days. The control group, 8 subjects, did not take the supplementation.
2.4 Muscle biopsies
Muscle biopsies were performed 5 days before the first and second session tests at the climate chamber, in order to obtain samples for CoQ10 concentration analysis in muscle and muscular characteristics, as previously described [19]. For histological examinations muscle biopsy was orientated and snap-frozen in liquid nitrogen-chilled isopentane and stored at −80ºC until its use. The frozen muscle tissue was evaluated using a standard panel of muscle stains using standard guideless for haematoxylin and eosin, modified Gomori trichrome, nicotinamide adenine dinucleotide dehydrogenase-tetrazolium reductase (NADH-TR), succinic dehydrogenase (SDH), cytochrome oxidase (COX). Additional muscle samples for immunohistochemistry were available for fast and slow myosin heavy chain (NCL-MHCn, Novocastra Laboratories, Newcastle Upon Tyne, UK) antibodies were applied to 10-μm-thick cryosections, and revealed by immunoperoxidase techniques. Light microscopy images were acquired with a DP72 digital camera on a BX41 bright-field light microscope using cellSens Entry 1.4 software (all by Olympus Corp.).
2.5 Analysis
Plasma and muscle CoQ10 values were quantified by high-performance liquid chromatography (HPLC) with electrochemical detection, as previously reported [19-21]. One week prior to the study day, routine laboratory analyses (complete blood count, erythrocyte sedimentation rate [ESR], C-reactive protein [CRP], aspartate aminotransferase [AST], alanine aminotransferase [ALT], gamma glutamyl transferase [GGT], alkaline phosphatase, urea, creatinine, uric acid, total cholesterol, HDL-C, LDL-C, triglycerides, sodium, calcium, magnesium, vitamin D, serum iron, transferrin, ferritin) were performed to assess eligibility. Blood samples were collected from antecubital vein, immediately before the experimental exercise and exposure in the climate chamber and at 30 minutes after the end of the running test. Biochemical measurements included levels of CoQ10, the Total Capacity Assay (TAC) and Malonyl Dialdehyde (MDA). TAC in plasma samples was measured using the OxiSelect™ Total Capacity Assay kit (STA-360, Cell Biolabs Inc., San Diego, CA, USA). Uric acid equivalent was used to calculate copper-reducing equivalent values (µM copper-reducing equivalent). MDA levels, expressed as µmol/L (µM) concentration, were measured colorimetrically using the OxiSelect™ TBARS Assay Kit (MDA Quantitation, STA-330, Cell Biolabs Inc., San Diego, CA, USA).Cytokines IL-6, IL-8, IL-10, TNF-α and MCP-1 in plasma were simultaneously analysed with Bead-Based Multiplex Assays using the Luminex technology (Millipore, US). Results obtained are expressed as pg/ml.
2.6 Statistical evaluation
Data are expressed as means ± standard deviation (S.D.). Between- and within-group changes of all variables of CoQ10 on different tissues, inflammatory cytokines, MDA and total antioxidant capacity levels were analysed with a three-way mixed-design analysis of variance (ANOVA) followed by Tukey-Kramer test for pairwise comparisons. Significance was set at p<0.05. Analysis of CoQ10 plasma and muscle levels was made by t test for paired data.
3. Results
There were no statistically significant baseline differences between subjects in the CoQ10 group (n=11) and control group (n=8), their mean baseline characteristics are presented in Table 1. The CoQ10 plasmatic levels (detected by HPLC) before allocation showed similar values in both groups at baseline in agreement with those reported in other studies [22-24]. All the subjects in the intervention group, except two, completed the study. One withdrew for personal reasons after his enrolment and the second subject withdrew before the second session at the chamber due to a muscular injury related to his professional work. There were 11 evaluable healthy volunteers in the supplemented group. A statistically significant increase in CoQ10 levels was detected in the supplementation group after one month of CoQ10 phytosome supplementation, both before and after exercise, while in the control group CoQ10 levels are not modified, as shown in Figure 1. Muscle CoQ10 values, quantified by HPLC, showed a statistically significant increase (36%) in CoQ10 phytosome group after 30-day of supplementation in respect to day 1 (Figure 2).
Morphological observation (Figure 3) of the muscular pre- and post-treatment biopsies showed a conserved pattern without variability in fiber size or presence of internal nuclei, a characteristic of unhealthy processes. Accumulation of subsarcolemmal mitochondria, without ragged red fibers (sign of mitochondrial dysfunction) was observed and the intermiofibrillar pattern showed disruption with core structures in oxidative stains (NADH, SDH and COX). No COX negative fibers were observed. A checkerboard mosaic pattern of type 1 and 2 fibers, with tendency to small groups and isolated co-expression, was displayed, but without significant differences between pre- and post-treatment biopsies. Exercise variables through the stress protocol at the climate chamber pre- and post-intervention are reported in Table 2.
In both groups, the heart rate reached in the last block of exercise was close to 100% of the maximum heart rate of each subject. The stress test in the climatic chamber significantly increased the values of HR, lactate concentration and Borg Scale during the three exercise blocks in both groups. The lactate concentration dropped significantly after one month’s supplementation of CoQ10 phytosome, but not in the control group. Perceived stress decreased significantly but no differences by group were detected. A significant difference in each increase in speed with respect to the previous measurement (both in the control and in CoQ10 supplemented groups) was observed, but no significant differences between groups were detected, also due to the high variability. In the second and third series a statistically significant reduction in lactate concentration was observed in post-intervention values in respect to pre-intervention values only in phytosome-treated group. The perception of fatigue (evaluated by Borg scale) increased as the working time increased in exposure to heat and humidity. The second test was best perceived with values of statistical significance in the second and third series for both groups. In all the series no significant differences between the groups were observed.
The effect of supplementation with CoQ10 phytosome on the oxidative damage marker Malonyl Dialdehyde (MDA) and the Total Capacity Assay (TAC) is presented in Table 3, together with values of inflammatory cytokines. High values in the TAC assay reflect high antioxidant capacity (i.e. greater protection). Exercise under stress conditions increased MDA levels after the stress exposition in both groups at Day 1 of the study, as expected. After one month, in the supplemented group the MDA basal and after exercise plasma levels are significantly decreased, while the control group show the similar basal levels and the increase of the oxidative marker. The total oxidative pattern always decreased after exercise in both groups due to the consumption of the stock that it was available to combat stress. This stock significantly improved (p<0.001) above the values prior to the first test after exercise in CoQ10 phytosome group, while values of the control group were not significantly modified. In terms of inflammatory pattern, reported in Table 3, it is possible to observe that at Day 1 (Pre-intervention) the levels of cytokines IL-6, IL-8, IL-10 and TNFα are increased after exercise in both groups. It is noteworthy that after supplementation the control group behaved similarly to the pre-intervention status, while the phytosome group showed a general reduction of cytokines values after exercise. In supplementation group a statistically significant difference post- vs pre-intervention was observed for IL-6 and IL-10.
|
Group |
Age years |
Height cm |
Weight kg |
BMI kg/m2 |
max HR b/min |
VO2 Max ml/kg/min |
V60 km/h |
V70 km/h |
V80 km/h |
|
CoQ10 |
54.6 ± 4.3 |
175.3 ± 3.7 |
76.8 ± 8.3 |
25.0 ± 2.7 |
168.3 ± 10.0 |
46.1 ± 6.1 |
10.6 ± 1.3 |
11.3 ± 1.4 |
12.1 ± 1.5 |
|
Control |
50.1 ± 7.8 |
175.1 ± 8.3 |
72.9 ± 11.5 |
23.6 ± 2.4 |
175.8 ± 11.9 |
47.8 ± 6.4 |
10.1 ± 1.5 |
10.8 ± 1.6 |
11.5 ± 1.8 |
The values are expressed as mean ± standard deviation, n=11 (CoQ10-supplemented group) and n=8 (control)
Table 1: Subject characteristics.
The values are presented as mean ± standard deviation. Statistical analysis: t test for paired data; *p<0.001 compared to Day 0 and to control
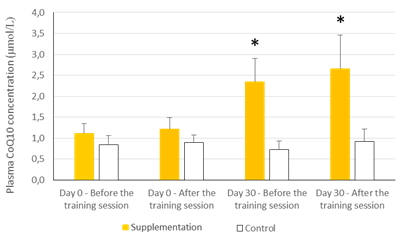
Figure 1: Effects of exercise and supplementation on CoQ10 plasma levels.
The values are presented as mean ± standard deviation. Statistical analysis: t test for paired data; *p<0.05 compared to basal levels
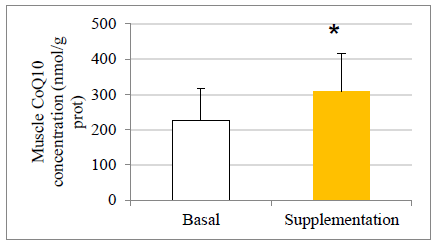
Figure 2: Effect of exercise and CoQ10 phytosome supplementation on CoQ10 concentration in muscle.
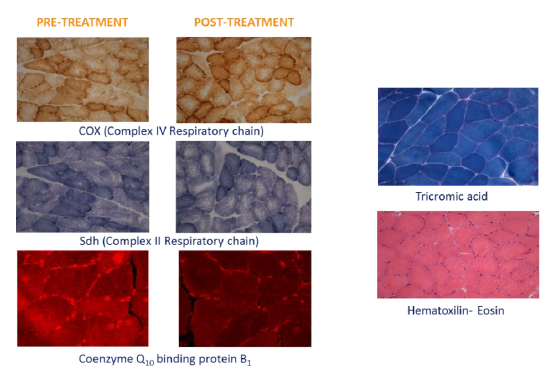
Figure 3: Representative histological sections of muscle pre and post supplementation.
|
HR (b.min-1) |
Treatment Group |
Stage |
Pre intervention |
Post intervention |
|
Control |
V60 |
154 ± 15 |
148 ± 16≠ |
|
|
V70 |
165 ± 14* |
163 ± 12* |
||
|
V80 |
169 ± 12 |
166 ± 10* |
||
|
CoQ10 |
V60 |
151 ± 12 |
147 ± 11≠ |
|
|
V70 |
163 ± 12* |
159 ± 11* |
||
|
V80 |
169 ± 14* |
167 ± 13* |
||
|
[La] mmol.l-1 |
Control |
V60 |
3.96 ± 1.17 |
3.33 ± 1.18 |
|
V70 |
5.57 ± 3.05 |
4.43 ± 2.13* |
||
|
V80 |
5.85 ± 3.3 |
4.71 ± 1.84 |
||
|
CoQ10 |
V60 |
3.54 ± 1.57 |
3.21 ± 1.47 |
|
|
V70 |
4.30 ± 2.17* |
3.74 ± 2.30≠ |
||
|
V80 |
5.75 ± 2.09* |
4.50 ± 2.23*≠ |
||
|
Borg Scale |
Control |
V60 |
12.6 ± 0.5 |
12.0 ± 0.81 |
|
V70 |
15.7 ± 1.1* |
14.4 ± 1.1*≠ |
||
|
V80 |
17.3 ± 1.0* |
15.7 ± 1.6*≠ |
||
|
CoQ10 |
V60 |
12.0 ± 1.3 |
11.0 ± 1.2 |
|
|
V70 |
14.3 ± 1.6* |
13.1 ± 0.6*≠ |
||
|
V80 |
16.9 ± 0.7* |
14.9 ± 2.1*≠ |
The values are presented as mean ± standard deviation. HR: Heart Rate; [La] Lactate Concentration; Statistical analysis: Three-way ANOVA of repeated measures. I: Treatment effect (control/CoQ10); S: Session effect (Before exercise/After exercise); T: Time effect (Start/End treatment); * p<0.05: Session Exercise effect; ≠ p<0.05: Time effect (Start/End treatment). IxT; IxS; SxT are no significant
Table 2: Effect of exercise and supplementation on physiological variables.
|
Markers |
Group |
Pre Intervention |
Post intervention |
ANOVA |
||
|
Before Ex. |
After Ex. |
Before Ex. |
After Ex. |
|||
|
MDA |
Control |
2.22 ± 0.80 |
3.49 ± 0.98≠ |
2.16 ± 0.87∞ |
3.81 ± 1.30≠∞ |
S, TxI S,T, SxT |
|
CoQ10 |
2.43 ± 0.45 |
2.94 ± 0.47≠ |
1.81 ± 0.58∞ |
2.15 ± 0.63≠∞ |
||
|
TAC |
Control |
154.7 ± 49.6 |
134.8 ± 49.4 |
154.7 ± 32.4 |
141.8 ± 28.8 |
S,T, I SxI, TxI |
|
CoQ10 |
127.7 ± 25.5 |
81.3 ± 19.1*≠ |
208.8 ± 60.2*∞ |
133.7 ± 33.2*≠∞ |
||
|
IL6 |
Control |
0.01 ± 0.01 |
0.29 ± 0.44 |
0.01 ± 0.02 |
0.36 ± 0.45 |
S, I, SxI, TxI |
|
CoQ10 |
0.04 ± 0.11 |
1.48 ± 1.26*≠ |
0.24 ± 0.73 |
0.55 ± 0.98∞ |
||
|
IL8 |
Control |
0.87 ± 1.00 |
1.81 ± 1.66 |
0.69 ± 0.53 |
1.85 ± 0.42≠ |
S, I, SxI, TxI |
|
CoQ10 |
2.49 ± 1.6 |
4.63 ± 1.9*≠ |
3.08 ± 1.34* |
3.86 ± 1.20 |
||
|
IL10 |
Control |
1.51 ± 3.20 |
4.41 ± 5.42≠ |
2.95 ± 2.87 |
10.21 ± 9.11≠ |
S, I SxI, TxI |
|
CoQ10 |
2.63 ± 2.4 |
24.5 ± 18.9*≠ |
3.09 ± 2.35 |
12.78 ± 8.61≠∞ |
||
|
TNFa |
Control |
8.60 ± 2.83 |
10.06 ± 3.10≠ |
9.79 ± 3.97 |
11.56 ± 4.87 |
S, I SxI, TxI |
|
CoQ10 |
10.87 ± 4.18 |
14.70 ± 4.90*≠ |
11.02 ± 2.95 |
13.44 ± 5.32 |
||
|
MCP-1 |
Control |
265 ± 88 |
345 ± 175≠ |
230 ± 113 |
314 ± 151≠∞ |
S, I, T |
|
CoQ10 |
273 ± 67 |
372 ± 93≠ |
278 ± 58* |
349 ± 114≠ |
||
The values are presented as mean ± standard deviation. MDA and TAC values are expressed as µmol/L, while cytokines as pg/mL. Statistical analysis: Three-way ANOVA for repeated measures. p<0.05. I: Treatment effect (control/CoQ10); S: Session effect (Before exercise/After exercise); T: Time effect (Start/End treatment); SxT: Interaction between session effect and Time effect; SxI: Interaction effect between session effect and Treatment; TxI: Interaction effect between Time effect and Treatment. *indicates significant differences between control and CoQ10. ≠ indicates significant differences between before and after exercise. ∞ indicates significant differences between pre and post intervention
Table 3: Effect of exercise and supplementation on oxidative markers and inflammatory cytokines release.
4. Discussion
The potential benefits of CoQ10 supplementation have been recognized in health and disease with particular reference to cardiovascular benefits and neurodegenerative diseases [25, 26], where CoQ10 has become an increasingly popular ingredient for dietary supplements in recent years. That was basically related to the beneficial effects attributed to its fundamental role in mitochondrial energy production [15, 24]. CoQ10 in its pure form is a crystalline powder insoluble in water and with limited solubility in lipids, and therefore it is poorly absorbed. Therefore the choice of an appropriate formulation for oral ingestion would impact CoQ10 bioavailability [17], since CoQ10 is slowly absorbed in the gastrointestinal tract, due to its high hydrophobicity and relatively large molecular weight. The formulation exploited in the present study is a food grade delivery system CoQ10 phytosome (Ubiqsome) with already described improved oral absorption in human volunteers [18]. A randomized, interventional, controlled, single-center trial was performed with CoQ10 phytosome (500 mg/day) supplementation for one month using healthy aging athletes, in comparison with non-supplemented control healthy aging athletes, and the results obtained support the increased CoQ10 plasma and muscle levels related to an antioxidant response and reduction of inflammatory markers after supplementation.
The observed plasma CoQ10 values at basal in both groups are in agreement with those reported in literature (0.38-1.34 mmol/L or 101-283 mmol/mol cholesterol [22]) for healthy adult subjects and perfectly matched with the values displayed in Bhagawan and Chopra [24]. After one month of phytosome supplementation, CoQ10 plasma concentrations were significantly increased, 2.1 times compared to that observed for similar doses administered as oil suspension [27] with a fatty diet, or for a longer time period, i.e. 2 months [28]. Regarding the quality of the study sample, it is important to underline that in other literature studies [28, 29] factors that can modify CoQ10 absorption (like sex, age, lipid and alcohol consumption) were not controlled, while in our study the subjects were homogeneous, i.e. all males, of a similar age range, active athletes, without metabolic or inflammatory alterations, following an adequate diet without any other kind of supplementation or unhealthy habits, so data obtained from the present study are cleaner from other interference factors.
It can be noted that after exercise there was an increase in CoQ10 plasmatic concentrations both in the control and in the supplemented groups. Interestingly, that increase, which could be understood as an effect associated with the antioxidant response, was significantly higher in the supplemented subjects (P<0.05). During aging, a decrease in CoQ10 content may occur in mitochondria of some tissues in certain species. It has also been experimentally shown that physiological concentrations of CoQ10 in mitochondria do not exceed those required for kinetic saturation of NADH-Q-oxidoreductase, suggesting that CoQ10 is rate limiting in the electron transport chain [30]. Therefore, a decrease of CoQ10 below the physiological levels can potentially affect mitochondrial activity. The gold standard procedure for biochemical diagnosis of CoQ10 deficiency is the analysis of CoQ10 concentration in urine [20] . In the present study, for the first time, CoQ10 levels were measured in muscle by using two different techniques, HPLC and immunofluorescence. Basal CoQ10 values in the muscle detected by HPLC analysis resulted in the range of 121-451 nmol/g protein, in agreement with those described elsewhere [31, 32]. These data give value to the study, by providing reference muscle concentrations in a controlled study with healthy individuals, aging (an age not yet studied), practitioners of a continuous activity of a certain intensity that requires respiratory chain enzymes activities. These values at the muscular level were all within the reference range, showing an increase after supplementation. Indeed, after one month CoQ10 supplementation, a significant increase in CoQ10 concentrations in muscle of about 1.4 times in respect to day 0 was observed. A significant increase in CoQ10 muscle concentrations was also observed when CoQ10 levels were detected by immunofluorescence technique (data not shown), strongly supporting evidence seen by HPLC detection.
It is likely that muscle tissue cells demand of CoQ10 is more efficient than other living cells that have fewer metabolic needs, and in fact supplementation did not significantly modify CoQ10 levels in urinary epithelial cells, mononuclear or platelets [19], in agreement with the idea that in muscle tissue the CoQ10 mitochondrial concentration seems not to possess saturation kinetics [33]. An increase in CoQ10 muscle concentration after exercise in CoQ10 phytosome-supplemented subjects [11] was previously observed, but no further studies had confirmed the data, up to the present study, that support and extend evidence for the first time in aging athletes.
As we know, athletes may have CoQ10 levels below those desired, due to increased metabolic stress and a higher formation of free radicals and aging process that can induce a significant and general reduction in the CoQ10 biosynthesis. Karlsson [34] observed that muscle ubiquinone was higher in individuals with high muscle tissue respiration and/or signs of an elevated peripheral (local) oxygen availability, and those with better aerobic physical condition. Interestingly in our study, the subjects with higher aerobic physical condition incorporated higher amounts of CoQ10, implying that the respiratory chain might use an extra CoQ10 amount, when available, to increase antioxidant supplies and so to improve that functional response.
Although this opinion is not consolidated [33] there seems to be a positive relationship between the CoQ10 levels in muscle and the muscle percentage of type I fibers, as an image of the quality of endurance of the individual [34], even Karlsson and colleagues evaluated fast twitch fibers altogether. Conversely, when aging individuals are supplemented, it seems that CoQ10 improves the presence of type IIb (fast) fibers instead of type I (slow) fibers, suggesting that CoQ10 influences older subjects (in aging an atrophy occurred mainly involving reduction in type II fibers). An antiaging effect may be thus justified by previously published data supporting that CoQ10 treatment can influence the fiber type composition by acting as gene regulator [35].
Evidence of a direct nature supporting an antioxidant role for coenzyme Q10 has been derived from a number of studies using subcellular systems. The measures of TAC, and the production of MDA, are efficient tools to estimate oxidative stress, defined as a disturbance in the pro-oxidant versus anti-oxidant balance in favour of the former. This is an interesting process, in which the oxidative capacity of the exercise is not always easy to demonstrate [13] and the use of antioxidants not always proven.
In our study, the CoQ10 supplementation group significantly and positively changed their baseline TAC and MAD values prior to the test (MAD was decreased and TAC was increased). This fact encourages us to argue that in humans, correct supplementation may possibly increase CoQ10 levels to optimal values for each subject, better regulating oxidative stress and autophagy in some unhealthy processes. In the same way, administration of CoQ10 partially reverses the severe decline of respiratory metabolism of cardiac tissue that occurs in aging animals [36]. Therefore Kroger and Klingenberg [37] suggested that CoQ10 acts as a homogeneous pool during electron transport. It is possible that some part of that pool, in slow equilibrium with the electron, may be involved in an antioxidant role, without affecting the respiratory function of the mitochondria. The increase in this CoQ10 concentration storage would support a better disposition to act in the antioxidant process.
This oxidative and antioxidant response to a stress has been previously demonstrated in similar studies [10, 38], even if not shown by others [11, 13]. We previously discussed the difficulty of comparing all studies due to the number of variables affecting the final results. In the present study, the response of both groups was similar in the first phase, showing a decrease in post-exposure antioxidant capacity. We observed that basal TAC levels increased in the supplemented group, but not in controls.
This study exposed a stressing exercise in trained subjects that caused a strong request for the protection of antioxidant and anti-inflammatory systems. This is perhaps the reason for a somewhat different response between the two groups in our study.
It is difficult to separate the oxidative process of aging from that associated with exercise. The complex interplay between age-associated reduction in habitual physical activity and the intrinsic aging processes complicates the interpretation of the aetiology of physical function decline. Assuming aging, and that the different structures of the respiratory chain system started to age from 40-50 years, [39] our study involved pre-senescence subjects; however, from results obtained by tests performed on those subjects, it appeared that those athletes had adequate redox capacity, but also responded to the CoQ10 supplementation.
On the other hand, the release of cytokines resulted highly modified in subjects supplemented with CoQ10; in particular Interleukins (IL-6, IL-8 and IL-10) reduction was observed after exercise after 30 days of CoQ10 phytosome supplementation, indicating a protective effect of the supplement. Those data are in agreement with the decrease of IL-6, IL-8, IL-10 and TNF-α production after CoQ10 administration (for 14 days) in Elite swimmers [40].
5. Conclusion
The new CoQ10 formulation demonstrated efficient improvement of CoQ10 bioavailability, not only in plasma but above all in the muscle. This increase correlated with an improvement in the plasma antioxidant activity capacity and functionality under normal and stress conditions; CoQ10 muscle concentration correlated to the baseline concentration in muscle and on aerobic capacity, as well as the bioavailability of CoQ10 at cellular sites. Thus, the new food-grade lecithin-based formulation of CoQ10, Ubiqsome, administered once a day for 30 days significantly improved CoQ10 bioavailability in healthy volunteers aging runners (50-65 years) by increasing both plasmatic and muscular CoQ10 levels, confirming that phytosome technology applied to a chemical product and not only to natural substances is a powerful delivery system suitable for athletes After one month of CoQ10 phytosome supplementation the inflammatory response and anti-oxidant pattern under stress conditions was improved, as suggested by a reduction of inflammatory cytokine and MDA levels. CoQ10 phytosome supplementation results in the benefit of increasing CoQ10 plasmatic and muscular levels, in situations where CoQ10 reduction occurred due to oxidative stress conditions, aging or high training.
Acknowledgements
We would like to express our gratitude to Dr. Paola Misiano for her valuable editorial support. This research did not receive any specific grant from funding agencies in the public, or not-for-profit sectors.
Conflict of Interest
AR, PA, GP and ST are employees of Indena.
References
- Crane FL, Sun IL, Sun EE. The essential functions of coenzyme Q. Clin Investig 71 (1993): 55-59.
- Forsmark-Andrée P and Ernster L. Evidence for a protective effect of endogenous ubiquinol against oxidative damage to mitochondrial protein and DNA during lipid peroxidation. Mol Aspects Med 15 (1994): 73-81.
- Guillermo López-Lluch, Juan Carlos Rodríguez-Aguilera, Carlos Santos-Ocaña, et al. Is coenzyme Q a key factor in aging? Mech Ageing Dev 131 (2010): 225-235.
- Rajindar S. Sohal, Sergey Kamzalov, Nathalie Sumien, et al. Effect of coenzyme Q10 intake on endogenous coenzyme Q content, mitochondrial electron transport chain, antioxidative defenses, and life span of mice. Free Radic Biol Med 40 (2006): 480-487.
- Carlos Santos-Ocaña, Thai Q Do, Sergio Padilla, et al. Uptake of exogenous coenzyme Q and transport to mitochondria is required for bc1 complex stability in yeast coq mutants. J Biol Chem 277 (2002): 10973-10981.
- Battino M, Ferri E, Gorini A, et al. Natural distribution and occurrence of coenzyme Q homologues. Membr Biochem 9 (1990): 179-190.
- Jackson MJ and McArdle A. Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J Physiol 589 (2011): 2139-2145.
- Gomez-Cabrera MC, Domenech E, Viña J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44 (2008): 126-131.
- Long He, Ting He, Shabnam Farrar, et al. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol Biochem 44 (2017): 532-553.
- Alvaro Sarmiento, Javier Diaz-Castro, Mario Pulido-Moran, et al. Short-term ubiquinol supplementation reduces oxidative stress associated with strenuous exercise in healthy adults: A randomized trial. Biofactors 42 (2016): 612-622.
- Matthew Cooke, Mike Iosia, Thomas Buford, et al. Effects of acute and 14-day coenzyme Q10 supplementation on exercise performance in both trained and untrained individuals. J Int Soc Sports Nutr 5 (2008): 8.
- Patrick Orlando, Sonia Silvestri, Roberta Galeazzi, et al. Effect of ubiquinol supplementation on biochemical and oxidative stress indexes after intense exercise in young athletes. Redox Rep 23 (2018): 136-145.
- Richard J Bloomer, Robert E Canale, Cameron G McCarthy, et al. Impact of oral ubiquinol on blood oxidative stress and exercise performance. Oxid Med Cell Longev 2012 (2012): 465020.
- Alvaro Sarmiento, Javier Diaz-Castro, Mario Pulido-Moran, et al. Coenzyme Q10 Supplementation and Exercise in Healthy Humans: A Systematic Review. Curr Drug Metab 17 (2016): 345-358.
- Bhagavan HN and Chopra RK. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res 40 (2006): 445-453.
- Abdel-azim Zaghloul, Bill Gurley, Mansoor Khan et al. Bioavailability assessment of oral coenzyme Q10 formulations in dogs. Drug Dev Ind Pharm 28 (2002): 1195-1200.
- Chopra RK, Goldman R, Sinatra ST, et al. Relative bioavailability of coenzyme Q10 formulations in human subjects. Int J Vitam Nutr Res 68 (1998): 109-113.
- Giovanna Petrangolini, Massimo Ronchi, Elisabetta Frattini, et al. A New Food-grade Coenzyme Q10 Formulation Improves Bioavailability: Single and Repeated Pharmacokinetic Studies in Healthy Volunteers. Curr Drug Deliv 16 (2019): 759-767.
- Abraham J Paredes-Fuentes, Raquel Montero, Anna Codina, et al. Coenzyme Q10 treatment monitoring in different human biological samples. Antioxidants 9 (2020): 979.
- Dèlia Yubero, Raquel Montero, Maria Ramos, et al. Determination of urinary coenzyme Q10 by HPLC with electrochemical detection: Reference values for a paediatric population. Biofactors 41 (2015): 424-430.
- Delia Yubero, Raquel Montero, Judith Armstrong, et al. Molecular diagnosis of coenzyme Q10 deficiency. Expert Rev Mol Diagn 15 (2015): 1049-1059.
- Montero R, Yubero D, Salgado MC, et al. Plasma coenzyme Q10 status is impaired in selected genetic conditions. Sci Rep 9 (2019): 793.
- Yubero D, Allen G, Artuch R, et al. The Value of Coenzyme Q10 Determination in Mitochondrial Patients. J Clin Med 6 (2017): 37.
- Bhagavan HN and Chopra RK. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 7 (2007): 78-88.
- Langsjoen PH and Langsjoen AM. Overview of the use of CoQ10 in cardiovascular disease. Biofactors 2-4 (1999): 273-284.
- Beal MF. Coenzyme Q10 as a possible treatment for neurodegenerative diseases. Free Radic Res 36 (2002): 455-460.
- Cestmír Zita, Kim Overvad, Svend Aage Mortensen, et al. Serum coenzyme Q10 concentrations in healthy men supplemented with 30 mg or 100 mg coenzyme Q10 for two months in a randomised controlled study. Biofactors 18 (2003): 185-193.
- Kaikkonen J, Nyyssönen K, Porkkala-Sarataho E, et al. Effect of oral coenzyme Q10 supplementation on the oxidation resistance of human VLDL+LDL fraction: absorption and antioxidative properties of oil and granule-based preparations. Free Radic Biol Med 22 (1997): 1195-1202.
- Jari Kaikkonen, Tomi-Pekka Tuomainen, Kristiina Nyyssonen, et al. Coenzyme Q10: absorption, antioxidative properties, determinants, and plasma levels. Free Radic Res 36 (2002): 389-397.
- Estornell E, Fato R, Castelluccio C, et al. Saturation kinetics of coenzyme Q in NADH and succinate oxidation in beef heart mitochondria. FEBS Lett 311 (1992): 107-109.
- Yubero D, Montero R, Artuch R, et al. Biochemical diagnosis of coenzyme q10 deficiency. Mol Syndromol 5 (2014): 147-155.
- Andrew J Duncan, Simon J R Heales, Kevin Mills, et al. Determination of coenzyme Q10 status in blood mononuclear cells, skeletal muscle, and plasma by HPLC with di-propoxy-coenzyme Q10 as an internal standard. Clin Chem 51 (2005): 2380-2382.
- Lenaz G, Fato R, Castelluccio C, et al. An updating of the biochemical function of coenzyme Q in mitochondria. Mol Aspects Med 15 (1994): 29-36.
- Karlsson J, Lin L, Sylvén C, et al. Muscle ubiquinone in healthy physically active males. Mol Cell Biochem 156 (1996): 169-172.
- Linnane AW, Kopsidas G, Zhang C, et al. Cellular redox activity of coenzyme Q10: effect of CoQ10 supplementation on human skeletal muscle. Free Radic Res 36 (2002): 445-453.
- Beyer RE. The participation of coenzyme Q in free radical production and antioxidation. Free Radic Biol Med 8 (1990): 545-565.
- Kröger A and Klingenberg M. Further evidence for the pool function of ubiquinone as derived from the inhibition of the electron transport by antimycin. Eur J Biochem 39 (1973): 313-323.
- Díaz-Castro J, Guisado R, Kajarabille N, et al. Coenzyme Q(10) supplementation ameliorates inflammatory signaling and oxidative stress associated with strenuous exercise. Eur J Nutr 51 (2012): 791-799.
- Jerome L. Fleg, Christopher H. Morrell, Angelo G. Bos, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 112 (2005): 674-682.
- Emami A. The Impact of Pre-Cooling and CoQ10 Supplementation on Mediators of Inflammatory Cytokines in Elite Swimmers. Nutr Cancer 72 (2020): 41-51.


 Impact Factor: * 3.8
Impact Factor: * 3.8 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 