Emerging A New Strategy for the Antitumor Immunotherapy: Pharmacological Modulation of the Ca2+/Camp Signaling Interaction
Article Information
Afonso Caricati-Neto, Paolo Ruggero Errante, Francisco Sandro Menezes-Rodrigues, and Leandro Bueno Bergantin*
Department of Pharmacology, Escola Paulista de Medicina, Universidade Federal de São Paulo (UNIFESP), Brazil.
*Corresponding Author: Leandro Bueno Bergantin, Laboratory of Autonomic and Cardiovascular Pharmacology, Department of Pharmacology, Escola Paulista de Medicina, Universidade Federal de São Paulo (UNIFESP), 55 11 5576-4973, Rua Pedro de Toledo, 669, Vila Clementino, São Paulo - SP, Brazil, Postal Code: 04039-032
Received: 31 August 2017; Accepted: 13 September 2017; Published: 25 September 2017
View / Download Pdf Share at FacebookAbstract
Cancer is a major public health problem and the second leading cause of mortality around the world. Antitumor immunotherapy using monoclonal antibodies is considered selective and efficient in the treatment of different types of tumors, but its cost and toxic effects limit its application. Many tumor microenvironments, including lymphoma and carcinoma, are enriched in immune suppressive cells that contribute to immune exhaustion by means expression of inhibitory ligands, suppressive cytokines, and tumor-promoting factors. Antitumor therapies targeted to reduce the induction, recruitment, or suppressive activities of the immune cells have been investigated. New antitumor strategies using drugs targeted to intracellular signaling involved in cell proliferation and survival, angiogenesis, and metastasis have become promising in recent years. Thus, our discovery of the role of functional interaction between intracellular signaling pathways mediated by calcium ions (Ca2+) and cyclic adenosine monophosphate (cAMP) (Ca2+/cAMP signaling interaction) in these cellular responses, opened a great avenue for the development of new antitumor therapeutic strategies. Here, we discuss how the combined use of monoclonal antibodies with drugs that modulate the Ca2+/cAMP signaling interaction to reduce tumor growth could be a potential strategy in the antitumor immunotherapy due to the increment of antitumor efficacy and reduction of adverse effects.
Keywords
Ca2+/cAMP Signaling Interaction; Antitumor Immunotherapy
Article Details
1. Introduction
According to the World Health Organization (WHO) reports, cancer is the second leading global cause of death. These reports showed that the cancer was responsible for 8.8 million deaths in worldwide only in 2015. However, the number of new cancer cases can rise by about 70% over the next 2 decades. Conventional chemotherapy and radiotherapy have showed important limitations, mainly due to its high degree of undesirable side effects, low selectivity, and for affecting both tumor and healthy cells [1,2]. Thus, new antitumor strategies such as targeted therapies [3] and immunotherapy [4] have been proposed as monotherapy, or in combination with conventional therapies [5].
Antitumor immunotherapy using monoclonal antibodies, such as antibodies against vascular endothelial growth factor (VEGF), has been considered as satisfactory targeted and selective antitumor therapy [6]. Although this strategy has showed significant antitumor efficacy in the different tumor types, the toxic effects and high cost limits its current clinical use [7]. Thus, the efficacy, tolerability and cost of the antitumor immunotherapy to control the tumor growth, angiogenesis and dissemination constitute the major barriers for the development of effective antitumor therapy [8].
In the early stages, the mass tumor development is facilitated by diffusion of nutrients through from neighboring tissues. Thus, tumor growth depends on the process of angiogenesis, and the new formed blood vessels serve as routes for dissemination of the tumor cells to other places [9]. For tumor-induced angiogenesis occurring, ?v?3 integrins play an important role in the physical interaction with the extracellular matrix necessary for cell adhesion, migration and positioning, in addition to inducing signs for cell proliferation and survival [10]. The information transmitted by integrins from the extracellular medium to cytoskeleton proteins is mediated by several intracellular signaling, including the increase of GTPases activity, stimulation of mitogen-activated protein kinase (MAPK), alteration of cytosolic Ca2+ concentration ([Ca2+]c), and increase of substrates activated by phospholipase C (PLC) [11][12].
Activation of PLC stimulates the hydrolysis of membrane phospholipids, generating inositol-1-4-5-triphosphate (IP3), and diacylglycerol (DAG) [12]. DAG facilitates the Ca2+ influx through plasma membrane voltage-activated Ca2+ channels (Cav), increasing the [Ca2+]c [12,13]. The activation of Ca2+ channels located in endoplasmic reticulum (ER) membrane by IP3 (via IP3 receptors) or Ca2+ (via ryanodine receptors) stimulate the Ca2+ release from ER to the cytosol, increasing the [Ca2+]c in specific intracellular sites [12]. Several mechanisms involved in intracellular Ca2+ homeostasis finely regulate the [Ca2+]c [12]. The Ca2+ ATPases located in ER membrane (SERCA) and plasma membrane (PMCA) transport the Ca2+ from cytosol to ER and extracellular medium, respectively, reducing the [Ca2+]c [12,13]. Several evidences suggest that the abnormal gene expression and activity of the different proteins involved in the intracellular Ca2+ homeostasis, such as Cav1.2, Cav3.2, SERCA2 and SERCA3, importantly contribute to tumor growth and dissemination due to cytosolic Ca2+ overload in tumor cells [14-18]. Thus, these proteins constitute potential molecular targets for antitumor therapy.
In addition to intracellular Ca2+ signaling, other signaling and messengers could contribute to tumor growth and dissemination such as intracellular signaling mediated by cyclic adenosine monophosphate (cAMP). Generated from the action of adenylyl cyclases (AC) on the adenosine triphosphate (ATP), the cAMP is an important intracellular messenger involved in the regulation of several cellular responses, including angiogenesis and tumor growth [19-21]. The increase of AC activity increments the cytoslic cAMP concentration ([cAMP]c) that active protein kinases, such as cAMP-dependent protein kinase (PKA), resulting several cellular responses [19-21]. The diminution of AC activity reduces the [cAMP]c and intracellular cAMP signaling that regulates the transcriptional factors, and gene activation, stimulating DNA synthesis and cell cycle [19-21]. Some evidences suggest that the increase of [cAMP]c inhibits the angiogenesis and tumor growth [21-24]. Thus, messenger involved in the intracellular cAMP signaling also constitute potential molecular targets for antitumor therapy.
It is well established that the Ca2+ finely regulates the AC activity, and consequently the [cAMP]c, virtually in all mammalian cells, characterizing the functional interaction between the intracellular signaling mediated by Ca2+ and cAMP (Ca2+/cAMP signaling interaction) [25,26]. By means the pharmacological modulation of the Ca2+/cAMP signaling interaction, we discovered that this interaction rules an important participation in the different cellular response, including in neurotransmitter/hormone exocytosis and cellular survival [26-30]. The Ca2+/cAMP signaling interaction finely controls the [Ca2+]c regulating the different steps of exocytosis, such as traffic and docking of secretory vesicle containing neurotransmitter and hormone [26-30]. In addition, the Ca2+/cAMP signaling interaction participate in the regulation of cellular survival mediated by cAMP/PKA/CREB [26-30].
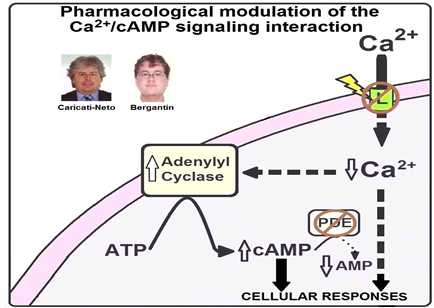
Figure 1: Pharmacological modulation of the Ca2+/cAMP signaling interaction proposed by Caricati-Neto and Bergantin [26-30]. The Ca2+/cAMP signaling interaction can be pharmacologically modulated by combined use of the the Ca2+ channel blockers (CCB) and drugs that promote the increase of [cAMP]c (cAMP-enhancer compounds). In response to the reduction of Ca2+ influx through plasma membrane voltage-activated Ca2+ channels (L) produced by CCB, the AC activity and [cAMP]c are increased. These CCB-effects can be potentiated by cAMP-enhancer compounds, such as phosphodiesterase (PDE) inhibitors.
Several evidences support that the abnormal intracellular signaling mediated by Ca2+ and cAMP could be involved in tumor growth and dissemination [31-37]. As previously mentioned, the abnormal gene expression and activity of the different proteins involved intracellular Ca2+ homeostasis contribute to tumor growth [14-18]. In addition, the increase of [cAMP]c inhibits the angiogenesis and tumor growth [21-24]. Thus, we have proposed that the combined use of monoclonal antibodies with drugs that modulate the Ca2+/cAMP signaling interaction to reduce tumor growth could be potential strategy in the antitumor immunotherapy due to increment of antitumor efficacy and reduction of adverse effects [31-37]. Figure 1 shows how the Ca2+/cAMP signaling interaction could be pharmacologically modulated by the combined use of the Ca2+ channel blockers (CCB) and drugs that promote the increase of [cAMP]c (cAMP-enhancer compounds).
2. Pharmacological modulation of the Ca2+/cAMP signaling interaction as a new therapeutic strategy in tumor immunotherapy
In tumor cells, the intracellular Ca2+ signaling pathways are remodeled, or deregulated, changing their physiology, and distinguish them from non-malignant cells [38,39]. This remodeling, or deregulation, provides means by which cancer cells can overcome systemic anticancer defense mechanisms [38,39]. In addition, this remodeling or deregulation can lead to genetic diversity found in cancer tissues thereby providing effective cellular strategies to the selection pressure to acquire specific traits [38,39].
Several evidences suggest that the cytosolic Ca2+ overload due to abnormal gene expression and activity of the different types of Ca2+ channels importantly contribute to tumor growth and dissemination due to cytosolic Ca2+ overload in tumor cells [14-18]. Evidences suggest that Ca2+ channels TRP and Orai participate in the intracellular Ca2+ signaling involved the physiological angiogenesis processes [17]. Thus, the Ca2+ channels have become important molecular targets in tumor cells and the drugs that interfere with the Ca2+ channels could be useful in the treatment of different types of tumor [18,31-37,40,41].
L-type Ca2+ channels has been implicated in the development and progression of several tumors, and a recent meta-analysis of microarray datasets showed that mRNA gene profile of the L-type Ca2+ channels in different types of cancer [42-47]. For example, it was showed that the L-type Ca2+ channels are significantly up-regulated in colon and esophageal cancer [43-47]. Thus, pharmacological blockade of these channels could be used as a therapeutic strategy for antitumor therapy. In fact, some studies showed that the L-type CCB, such as amlodipine, mibefradil and NNC-55-0396, inhibit the proliferative response in different tumor cells [18,40,41]. It was suggested that these L-type CCB can directly modify the transcription of genes and their products, e.g., the proteolytically cleaved 75 kDa C-terminal fragment of Cav1.2, a Ca2+ channel associated transcriptional regulator (CCAT), which translocates to the nucleus altering the transcription of several genes, including Myc, Bcl-associated death promoter (Bad) and artemin [42]. Nuclear CCAT levels increase or decrease in response to low and high intracellular Ca2+, respectively [42]. It was suggested that L-type CCB NNC 55-0396 inhibits tumor angiogenesis by suppression of hypoxia-inducible factor-1alpha signal transduction via both proteasome degradation, and protein synthesis pathways [18]. The L-type CCB Amlodipine inhibited both in vitro and in vivo the growth of human epidermoid carcinoma A431 cells, via arresting cell cycle at G1 phase, and reducing phosphorylation of retinoblastoma protein, expression levels of cyclin D1 and cyclin dependent kinase [40].
Novel splice variants of T-type Ca2+ channels are commonly detected in human glioma, breast, ovarian, prostate colon and esophageal cancer cells [43-47]. Cav3.1 transcripts predominate in the normal adult brain, but human glioma and glioma cell lines contain Cav3.1 as predominant splice and Cav3.1 as a novel splice variant, which is absent in normal brain Ca2+ channels [43-47]. Mibefradil, a T- and L-type CCB, reduced tumor size, to improve the survival rate in glioma animal model as well as in a patient derived pancreas xenograft animal model [43,48]. A novel mibefradil-derived compound NNC-55-0396 inhibited angiogenesis in tumor cells, becoming a promising chemotherapy drug [18,43].
Proangiogenic events mediated by Ca2+ have been investigated in tumor-derived endothelial cells [17]. Abnormal expression and function of Transient Receptor Potential (TRP) channels is involved in the alterations of intracellular Ca2+ signaling in endothelial cells from human breast and kidney tumors and [17]. TRP channels subfamily includes a number of putative proangiogenic channels [17]. It was suggested that pharmacological blockade of these Ca2+ channels could significantly reduce the vascularization in these tumors [17]. Thus, antitumor immunotherapy using monoclonal antibodies against VEGF, has been considered as satisfactory targeted and selective antitumor therapy [6]. These findings support that the pharmacological modulation of the different types of Ca2+ channels in the tumor cells combined with monoclonal antibodies against VEGF could be a novel alternative for antitumor immunotherapy.
Several evidences suggest that the drugs that modulate the intracellular cAMP signaling could be used to inhibit the angiogenesis and tumor growth. It also was showed that the increase of [cAMP]c produced by phosphodiesterase (PDE) inhibitors suppress the endothelial extracellular matrix remodeling [22]. In addition, it was showed that PDE inhibitors reduce the intracellular signaling mediated by PI3K/AKT to down-modulate VEGF secretion and vessel formation in vitro, and stimuling the lower synthesis of VEGF and diminishing the microvessel density in animal model of diffuse large B-cell lymphoma [23]. Some studies showed that the association of PDE2 and PDE4 inhibitors with curcumin significantly reduced the VEGF production, angiogenesis and tumor growth [24].
It was showed that the increase of [cAMP]c induced by AC activator forskolin produced significant antitumor effects [49]. The 8-Cl-cAMP, and the PKA I-selective cAMP analogs (8-piperidinoadenosine - 3',5'-cyclic monophosphate (8-PIP-cAMP) and 8-hexylaminoadenosine - 3',5'-cyclic monophosphate (8-HA-cAMP) produced a significant antiproliferative effects in human cancer cell lines [50]. The anti-proliferative effect of the PKA I-selective cAMP analogs was attributed to growth arrest, while the 8-Cl-cAMP appears be due to pro-apoptotic effect [50]. It also observed that the PKA I-selective cAMP analogs, but not 8-Cl-cAMP, inhibited ERK phosphorylation, whereas 8-Cl-cAMP alone induced a progressive phosphorylation of the p38 MAPK via activation of AMPK by its metabolite 8-Cl-adenosine [51]. Pharmacological inhibition of the p38 MAPK prevented the pro-apoptotic effect of 8-Cl-cAMP [51]. These findings suggest that 8-Cl-cAMP and the PKA I-selective cAMP analogs could be used in human tumor therapy.
Interestingly, the 8-Cl-cAMP and PKA type I-selective cAMP analogs (8-PIP-cAMP and 8-HA-cAMP) also showed a potent antiproliferative effect in medullary thyroid cancer cell lines [52]. It was showed that the 8-Cl-cAMP significantly inhibited the transition of cell population from G2/M to G0/G1 phase and from G0/G1 to S phase [51]. In addition, the 8-Cl-cAMP induced apoptosis in medullary thyroid cancer cell lines [52]. This finding demonstrated that cAMP analogs, particularly 8-Cl-cAMP, significantly suppress cell proliferation in medullary thyroid cancer cell lines and provide rationale for a potential clinical use of drugs that interfere with intracellular signalling mediated by cAMP/PKA in the antitumor therapy. Although the role of intracellular cAMP signaling in tumor cells has been poorly investigated, the drugs that interfere in the intracellular cAMP concentration have been proposed as potential adjuvant, chemotherapeutic or chemopreventive agents in different types of cancer, including hepatocellular carcinoma [53].
Due to involvement of Ca2+ and cAMP in the regulation of the several cellular responses, including angiogenesis and tumor growth, the proteins involved in the Ca2+/cAMP signaling interaction have been considered potential pharmacological targets in the antitumor therapy, in combination with conventional chemotherapy, radiotherapy and immunotherapy. It is important to mention that the cancer therapy with drugs that modulate the Ca2+/cAMP signaling interaction could be useful to control growth of cancer with high rates of resistance to conventional immunotherapy, to decrease dose of monoclonal antibodies intravenously infused, and their adverse effects. Thus, the pharmacological modulation of the Ca2+/cAMP signaling interaction in tumor cells could open a great avenue for the development of new antitumor therapeutic strategies to reduce tumor growth by combined use of the CCB, drugs that interfere with cAMP signaling and anti-VEGF monoclonal antibodies. This new strategy could promote important advances in the antitumor immunotherapy, increasing its efficacy and reducing side effects.
References
- Bray F, Ferlay J, Laversanne M, Brewster DH, Gombe Mbalawa C, et al. Cancer incidence in five continents: Inclusion criteria, highlights from volume X and the global status of cancer registration. Int J Cancer 137 (2015): 2060-2071.
- Azar FE, Azami-Aghdash S, Pournaghi-Azar F, Mazdaki A, Rezapour A, et al. Cost-effectiveness of lung cancer screening and treatment methods: a systematic review of systematic reviews. BMC Healt Serv Res. 17 (2017): 413.
- Larkin J, O´Reilly A. The safety of nivolumab for the treatment of metastatic melanoma. Expert Opin Drug Saf 16 (2017): 955-961.
- Hahan AW, Gill DM, Pal SK, Agarwal N. The future of imune checkpoint cancer therapy after PD-1 and CTLA-4. Immunotherapy 9 (2017): 681-692.
- Visconti R, Morra F, Guggino G, Celetti A. The between now and then of lung concer chemotherapy and immunotherapy. Int J Mol Sci 18 (2017): E1374.
- Ronca R, Benkheil M, Mitola S, Struyf S, Liekens S. Tumor angiogenesis revisited: Regulators and clinical implications. Med Res Rev (2017).
- Diaz RJ, Ali S, Qadir MG, De La Fuente MI, et al. The role of bevacizumab in the treatment of glioblastoma. J Neurooncol (2017).
- de Miguel-Luken MJ, Mansinho A, Boni V, Calvo E. Immunotherapy-based combinations: current status and perspectives. Curr Opin Oncol (2017).
- De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumor angiogenesis. Nat Rev Cancer (2017).
- Demircioglu F, Hodivala-Dilke K. ?v?3 integrin and tumor blood vessels-learning from the past to shape the future. Curr Opin Cell Biol 42 (2016): 121-127.
- Atkinson SJ, Ellison TS, Steri V, Gould E, Robinsosn SD. Redefining the role(s) of endotheial ?v?3-integrin in angiogenesis. Biochem Soc Trans 42 (2014): 1590-1595.
- Pechkovsky DV, Scaffidi AK, Hackett TL, Ballard J, Shaheen F, et al. Transforming growth factor beta 1 induces alphavbeta3 integrin expression. In human lung fibroblast via a beta3 integrin-, c-Src-, and p38 MAPK-dependent pathway. J Biol Chem 283 (2008): 12898-12908.
- Nakamura Y, Fukami K. Regulation and physiological functions of mammalian phospholipase C. J Biochem 161 (2017): 315-321.
- Busselberg D, Florea AM. Targeting intracellular calcium signaling ([Ca2+]i) to overcome acquired multidrug resistance of cancer cells: A mini-review. Cancer (2017): E48.
- Parkash J, Asotra K. Calcium wave signaling in cancer cells. Life Sci 87 (2010): 587-595.
- Monteith GR, Davis FM, Roberts-Thomson SJ. Calcium channels and pumps in cancer: changes and consequences. J Biol Chem 287 (2012): 31666-31673.
- Munaron L, Genova T, Avanzato D, Antoniotti S, Fiorio Pla A. Targeting calcium channels to block tumor vascularization. Recent Pat Anticancer Drug Discov 8 (2013): 27-37.
- Kim KH, Kim D, Park JY, Jung HJ, Cho YH, et al. NNC 55-0396, a T-type Ca2+ channel inhibitor, inhibits angiogenesis via supression of hypoxia-inducible factor-1alpha signal transduction. J Mol Med 93 (2015): 499-509.
- Krasteva PV, Sondermann H. Versatile modes of cellular regulation via cyclic dinucleotides. Nat Chem Biol 13 (2017): 350-359.
- Xiao LY, Kan WM. Cyclic AMP (cAMP) confers drug resistance against DNA damaging agents via PKAIA in CML cells. Eur J Pharmacol 794 (2017): 201-208.
- Neto A, Ceol CJ. Melanoma-associated GRM3 variantes dysregulate melanosome trafficking and cAMP Signaling. Pigment Cell Melanoma Res (2017): 24.
- Yun S, Budatha M, Dahlman JE, Coon BG, Cameron RT, et al. Interaction between integrin ?5 and PDE4D regulates endotelial inflammatory signalling. Nat Cell Biol 18 (2016):1043-1053.
- Suhasini NA, Wang L, Holder KN, Lin AP, Bhatnagar H, et al. A phosphodiesterase 4B-dependent interplay between tumor cells and the microenvironment regulates angiogenesis in B-cell lymphoma. Leukemia 30 (2016): 617-626.
- Abusnina A, Keravis T, Zhou Q, Justiniano H, Lobstein A, et al. Tumor groth inhibition and anti-angiogenic effects using curcumin correspond to combined PDE2 and PDE4 inhibition. Thromb Haemost 113 (2015): 319-328.
- Bergantin LB, Souza CF, Ferreira RM, Smaili SS, Jurkiewicz NH, Caricati-Neto A, Jurkiewicz A. Novel model for "calcium paradox" in sympathetic transmission of smooth muscles: role of cyclic AMP pathway. Cell Calcium 54(2013): 202-212.
- Caricati-Neto A, García AG, Bergantin LB. Pharmacological implications of the Ca2+/cAMP signalling interaction: from risk for antihypertensive therapy to potential beneficial for neurological and psychiatric disorders. Pharmacol Res Perspect 3 (2015): 1-10.
- Caricati-Neto A, Bergantin LB. New therapeutic strategy of Alzheimer´s and Parkinson´s diseases: Pharmacological modulation of neural Ca2+/cAMP intracellular signaling interaction. Asian J Pharm Pharmacol 2 (2016)): 136-143.
- Bergantin LB, Caricati-Neto A. Challenges for the pharmacological treatment of neurological and psychiatric disorders: Implications of the Ca2+/cAMP intracellular signalling interaction. Eur J Pharmacol 788 (2016): 255-260.
- Bergantin LB, Caricati-Neto A. Recent advances in pharmacotherapy of neurological and psychiatric disorders promoted by discovery of the role of Ca2+/cAMP signaling interaction in the neurotransmission and neuroprotection. Adv Pharmac J 1 (2016): 66-69.
- Bergantin LB, Caricati-Neto A. From discovering “calcium paradox” to Ca2+/cAMP interaction: Impact in human health and disease. Scholars Press (2016).
- Caricati-Neto A, Errante PR, Bergantin LB. A novel alternative for cancer therapy: Pharmacological modulation of Ca2+/cAMP intracellular signaling interaction. Am J Pharmacol Pharmacother 4 (2017): 20-34.
- Errante PR, Menezes-Rodrigues FS, Leite AA, Caricati-Neto A, Bergantin LB. New antitumoral pharmacological strategies involving Ca2+/cAMP signaling pathways. J Cancer Epidem Prev 2 (2017): 1-6.
- Errante PR, Caricati-Neto A, Bergantin LB. Insights for the inhibition of cancer progression: Revisiting Ca2+ and cAMP signalling pathways. Adv Cancer Prev 2 (2017): 1-2.
- Errante PR, Menezes-Rodrigues FS, Caricati-Neto A, Bergantin LB. The pharmacological modulation of Ca2+/cAMP intracellular signaling pathways and traditional antitumoral pharmaceuticals: a plausible multitarget combined therapy? J Clin Exper Oncol 6 (2017): 1-3.
- Errante PR, Menezes-Rodrigues FS, Leite AA, Caricati-Neto A, Bergantin LB. The second messengers Ca2+ and cAMP as potential therapeutic targets for the control of cancer progression. Adv Cancer Prev 2 (2017): 1-2.
- Errante PR, Leite AA, Menezes-Rodrigues FS, Caricati-Neto A, Bergantin LB. A novel potential therapeutic target as adjuvant treatment for cancer: the pharmacological interference on the Ca2+/cAMP cellular signaling pathways. Enliven: Chall Cancer Detec Ther 1 (2017): 1-2.
- Menezes-Rodrigues FS, Errante PR, Caricati-Neto A, Bergantin LB. Extracellular cyclicAMP/Adenosine signaling pathway: a potential pharmacological target for therapeutic intervention in chronic lymphocytic leukemia. Adv Cancer Prev 2 (2017): 1-2.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 100 (2007): 57-70.
- Wang XT, Nagaba Y, Cross HS. The mRNA of L-type calcium channel elevated in colon cancer: protein distribution in normal and cancerous colon. Am J Pathol 57 (2007): 1549-62.
- Yoshida J, Ishibashi T, Nishio M. G1 cell cycle arrest by amlodipine, a dihydropyridine Ca2+ channel blocker, in human epidermoid carcinoma A431 cells. Biochem Pharmacol 73 (2007): 943-53.
- Krouse AJ, Gray L, Macdonald T, et al. Repurposing and rescuing of mibefradil, an antihypertensive, for cancer: a case study. Assay Drug Dev Technol 13 (2015): 650-653.
- Gomez-Ospina N, Tsuruta F, Barreto-Chang O, et al. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell 127 (2006): 591-606.
- Kale VP, Amin SG, Pandey MK. Targeting ion channels for cancer therapy by repurposing the approved drugs. Biochim Biophys Acta (BBA) ?Biomembr 1848 (2015): 2747-2755.
- Dziegielewska B, Gray LS, Dziegielewski J. T-type calcium channels blockers as new tools in cancer therapies. Pflugers Arch 466 (2014): 801-810.
- Ohkubo T, Yamazaki J. T-type voltage-activated calcium channel Cav3.1, but not Cav3.2, is involved in the inhibition of proliferation and apoptosis in MCF-7 human breast cancer cells. Int J Oncol 41 (2012): 267-275.
- Gackičre F, Bidaux G, Delcourt P, et al. CaV3.2 T-type calcium channels are involved in calcium-dependent secretion of neuroendocrine prostate cancer cells. J Biol Chem 283 (2008): 10162-10173.
- Latour I, Louw DF, Beedle AM, et al. Expression of T-type calcium channel splice variants in human glioma. Glia 48 (2004): 112?119.
- Garrido-Laguna I, Tan AC, Villarroel MC, et al. Activity of the T-type calcium channel antagonist Mibefradil in pancreatic cancer xenografts. Clin Cancer Res 14 (2008): 49-49.
- Murray F, Insel PA. Targeting cAMP in chronic lymphocytic leukemia: A pathway-dependent approach for the treatment of leukemia and lymphoma. Expert Opin Ther Targets 17 (2013): 937-949.
- Sapio L, Gallo M, Illiano M. The natural cAMP elevating compound forskolin in cancer therapy: is it time? J Cell Physiol 232 (2017): 922-927.
- Lucchi S, Calebiro D,Filippis T, et al. 8-chloro-cyclic AMP and protein kinase A I-selective cyclic AMP analogs inhibit cancer cell growth through different mechanisms. PLoS One 6 (2017): e20785.
- Dicitore A, Grassi ES, Caraglia M, et al. The cAMPanalogs have potent anti-proliferative effects on medullary thyroid cancer cell lines. Endocrine. 51 (2016): 101-112.
- Massimi M, Cardarelli S, Galli F, et al. Increase of intracellular cyclic AMP by PDE4 inhibitors affects HepG2 cell cycle progression and survival. J Cell Biochem 118 (2017): 401-411.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
