Environmental Influences on Atopic Eczema
Article Information
Wismmy Lee, Fihr Chaudhary, Devendra K Agrawal*
Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona CA 91766, USA
*Corresponding Author: Devendra K Agrawal, Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona CA 91766, USA.
Received: 16 May 2024; Accepted: 23 May 2024; Published: 13 June 2024;
Citation: Wismmy Lee, Fihr Chaudhary, Devendra K Agrawal. Environmental Influences on Atopic Eczema. Journal of Environmental Science and Public Health. 8 (2024): 101-115.
View / Download Pdf Share at FacebookAbstract
The health outcomes of an individual are shaped by a combination of genetic predisposition and environmental influences. While some diseases stem solely from environmental factors, others like atopic eczema, also known as neurodermatitis or atopic dermatitis, are multifaceted, with environmental variables playing a significant role in its initiation and severity. Atopic eczema is a prevalent chronic condition observed globally, particularly in Western industrialized nations where its prevalence is estimated to range from 2.5% to 3.5% in adults and 10% to 15% among children. The increasing incidence of atopic eczema in industrialized countries over recent decades suggests that this trend may be due to environmental changes rather than genetic predispositions. Therefore, by thoroughly examining environmental factors and their role in atopic dermatitis, one may be able to gain a better understanding of its disease pattern and develop possible preventative measures. This article provides a comprehensive analysis of how the surrounding environment contributes to the pathogenesis of atopic eczema.
Keywords
Air pollutants; Allergens, Atopy, Atopic dermatitis, Atopic eczema, Climate change, Environment, Home environment, Microbiome, Neurodermatitis, Nutritional factors, Structural racism.
Article Details
1. Introduction
Eczema is a common and chronic skin condition that is characterized by pruritus (itching), impaired epidermal function, and sensitization to dietary and environmental allergens mediated by immunoglobulin (Ig) E. It is a complex, multifaceted condition that is comprised of an interplay of genetic and environmental influences [1]. Atopic dermatitis is the most common type of eczema where dermatitis refers to the chronic inflammation of the skin, whereas eczema refers to inflamed skin with the symptoms of itching, a flacky or scaly rash, dry and irritated skin. However, the terms atopic eczema and atopic dermatitis are used inter-changeably by healthcare professionals and in this article.
1.1 Defining "environment"
Multiple definitions exist for the term "environment," ranging from a narrow focus on the physical surroundings of humans to a broader scope encompassing all environmental factors affecting living organisms. The present study adopts a comprehensive understanding of “environment,” encompassing several dimensions such as (psycho)social, anthropogenic, nutritional, and biologic factors [2].
Research indicates that individuals of higher socioeconomic status are more likely to report atopic dermatitis (AD), potentially leading to underdiagnosis in lower socioeconomic classes [3]. Despite this, there is evidence of a social gradient in symptom severity, with lower socioeconomic groups often experiencing more pronounced symptoms (Figure 1). Structural racism has also been linked to symptom intensity. Research conducted in the United States revealed that African American children who exhibited a higher prevalence of socioeconomic hardship experienced more pronounced symptoms of atopic eczema (AE) [4].
Previous studies have shown a positive association between prenatal stress and AE occurrence [5]. Additionally, individuals diagnosed with AE frequently indicate that stress acts as a catalyst for symptom exacerbation [6]. The presence of visible skin diseases can also elicit experiences of stigmatization due to societal beauty and health standards, heightening stress levels, resulting in a self-perpetuating cycle of negative effects. Furthermore, there is a growing trend in which health management is being perceived as a matter of personal responsibility which may exacerbate feelings of guilt and stress among individuals affected by AE.
Conflicts in personal, relationship or occupational settings can induce stress, worsening AE symptoms, as an association between psychosocial stress and AE symptom severity has been shown [6]. Conversely, positive social contexts may provide some benefit to individuals afflicted by AE by mitigating stress through social support. However, further investigation is needed to understand the degree to which social networks impacts AE positively [6].
AE imposes significant financial burdens on patients, including costs for emollients, medications, physician visits and more, often paid out of pocket [7]. The economic burden may increase with AE severity [7].
Figure 1: The development of Atopic Dermatitis (AD) involves multiple factors, including genetic susceptibility, epidermal barrier dysfunction, inflammation, and microbial dysbiosis. The term "exposome" refers to the biological response to external exposures that interact with the pathogenic pathways mentioned above and may modify disease progression and status. Disease-modifying factors are prevalent across groups, communities, and individuals. Adopting an exposomal approach to scientific inquiry enables the identification of timing and quantity of exposures that influence disease outcomes, combining epidemiology with cellular and molecular biology. This narrative review focuses on key external factors influencing AD development at the population, community, and individual level. Additionally, it explores how these factors impact known disruptions in biological pathways that are associated with AD.
1.2 Indoor and outdoor anthropogenic air pollutants
There is an elevated likelihood of children residing near heavily trafficked roadways to experience an augmented susceptibility of developing atopic illnesses. Furthermore, it has been observed that air pollutants have the potential to exacerbate the intensity of apparent adverse effects in children [8]. Indoor air quality, particularly the exposure to volatile organic compounds such as those emitted from building materials and furnishings can trigger symptoms even at pollutant concentrations below established standards [9]. Restoration activities like wall painting and introducing new furniture can further increase susceptibility to adverse effects, especially in infants [10].
Another study provides evidence suggesting that the combined impact of volatile organic compounds and allergens from house dust mites may exacerbate skin barrier impairment in individuals sensitized to allergens [11]. Environmental tobacco smoke stands out as a significant source of indoor particulates, and numerous studies suggest that tobacco smoke may enhance the development of atopic diseases in genetically susceptible individuals which highlights the interplay between genetic predisposition and environmental factors [11].
2. Biological factors and climate change
Anthropogenic air pollution is widely recognized as the primary driver for global warming, also known as climate change. The severity of AE symptoms has been correlated with seasonal changes. In children, symptoms often worsen in autumn and winter, while adults typically experience an exacerbation of symptoms during the summer. Heat and the lack of nocturnal air conditioning can further intensify itching sensations which can disrupt sleep; extreme weather conditions can further exacerbate clinical presentations [2].
Approximately 80% of adults with AE exhibit sensitization to inhalant or dietary allergens through the presence of IgE antibodies. Numerous scholarly publications have presented findings that establish a correlation between AE severity and the occurrence of sensitization. Seasonal fluctuation also exists, with an observed increase in symptoms throughout the summer, especially on high pollen days. In cases where eczema affects the head and neck, worsening AE symptoms during the pollen season have been observed to be attributed to aeroallergen sensitization [12].
AE is a condition that is influenced by environmental factors; thus, individuals may have the ability to exert some influence over a significant proportion of the environmental factors that can trigger or worsen AE. Individuals diagnosed with asthma and eczema constitute a susceptible demographic that is adversely affected by the effects of climate change, air pollution, and heightened levels of stress.
Urbanization has led to biodiversity decline and lifestyle changes, and the human immune system has not yet undergone sufficient adaptation to effectively respond to recent circumstances. While it may not be possible to fully undo the effects of urbanization, integrating natural elements into urban environments, such as design strategies incorporating abundant green spaces, is recommended. Additionally, the presence of a varied microbiome and adherence to a varied diet has been shown to contribute to overall well-being. Monitoring airborne pollen and pollutant levels and correlating them with symptom severity is crucial, along with avoiding offending agents when possible. There currently exists a need for the promotion of research on the protective effects of natural environments and social contexts in mitigating AE manifestations.
3. The impact of the home environment on atopic eczema
A growing body of academic literature points to environmental factors as possible causes of the increasing incidence of atopic eczema in industrialized nations. In a Nottingham-based study, researchers employed a descriptive case-control methodology to investigate this phenomenon. By employing odds ratios and population attributable risk percentages, researchers analyzed the risk associated with reported cases of eczema in children. The study revealed that children were more likely to develop atopic eczema if exposed to high levels of indoor humidity, had their bedrooms heated with electric space heaters and slept on synthetic pillows [13]. Households where regular vacuuming was practiced demonstrated a lower incidence of AE compared to those where it was not [13].
4. The role of air pollutants/atmospheric pollution
AD can be triggered by various factors, one of which is atmospheric or air pollution, a relatively recently recognized environmental contributor likely stemming from increased industrialization in urban areas resulting in the release of pollutants into the environment. Currently, the precise mechanism of how these contaminants contribute to AD development is unclear. An investigation into the connection between environmental toxins and AD has been presented in this article.
Given that approximately 33% of individuals who have been diagnosed with AD occur have encountered disease onset within their first year of life, it is crucial to consider the potential consequences of prenatal exposure to air pollution on AD. A study involving 469 pregnant women investigated the effects of fine air pollutant exposure and their children’s occurrence of AD symptoms within their first year of life [14]. The children were also assessed for any long-term consequences of their prenatal exposure to air contaminants and tobacco smoking [14]. The children were examined at three-month intervals throughout the course of the child’s first year of life [14]. The findings indicated that increased prenatal exposure to fine particulate matter doubled the risk of AD symptoms during the child’s first year of life [14].
A study conducted in Sweden found an association between AD and reduced ventilation within residential residences, namely within the beds that were designated for use by children [15]. In addition, a German study discovered a correlation between indoor renovation activities, such as painting, furniture installation, and floor covering, and the development of AD throughout an individual’s lifetime, including the antenatal period, infancy, and early childhood. This may possible be due to higher concentrations of volatile organic compounds in the environment [16]. Additionally, a study in Taiwan examined 317,926 young people and found a significant and positive link between eczema and traffic-related air pollutants, such as carbon monoxide and nitrogen oxides (NOx), in both males and females [17]. Similarly, a study conducted on a sample of 4907 French children revealed correlations between the lifelong presence of atopic eczema and urban air pollutants. Particulate matter having a diameter of 10 micrometers or less (PM10), benzene, and NOx were all included in this group of contaminants [18].
A study based on United States population data found a substantial statistical link between the average annual levels of nitrogen dioxide (NO2), sulfur dioxide (SO2), and sulfur trioxide (SO3) and the incidence of childhood AD [19]. A German study demonstrated a correlation between the prevalence of AD in children aged one to six and the proximity of their residences to major roads [20].
Atopic dermatitis is an allergic condition that can be influenced by pollutants in both indoor and outdoor environments. These substances can communicate with the epidermis by binding to the stratum corneum, the outermost layer of the epidermis. In some cases, they can even penetrate the bloodstream via dermal capillaries [21]. Skin biopsy analysis in a study involving 75 individuals with AD also showed a connection between the severity of eczema and the occurrence of damage caused by reactive oxygen species [22].
5. What epidemiological evidence is there linking the environment you live in with eczema?
The observed increase in the frequency of eczema has prompted investigation into the hygiene hypothesis as a possible explanation. However, even though this concept was first investigated sixty years ago, the physical environment regarding AD is still considered to be relatively understudied. Thus, it is necessary to do a thorough investigation of the many physical characteristics such as temperature, ultraviolet (UV) radiation, humidity, and the amount of time spent indoors and their effects on AD.
The limited availability of conclusive epidemiological evidence linking physical environmental factors to eczema can largely be attributed to the insufficient number of adequately large study populations available to investigate these factors as well as the difficulty in distinguishing the individual contributions of possible risk factors. One study emphasizes that the epidermis is a crucial protective layer that acts as a physical barrier against potential damage that may be caused by the surrounding environment [23]. The ability of epidermis to maintain its structural integrity is largely dependent on the presence of an intact stratum corneum (SC) and the strong connections that can be found within the stratum granulosum. When mutations in filaggrin (FLG) were discovered to cause loss of function in the context of atopic eczema in 2006, it rekindled academic interest in the epidermal barrier's role in the development of eczema and has led to the formulation of new hypothesis about how the epidermal barrier contributes to eczema [23]. There exists an approximately 3.3 times increased risk of eczema occurrence in individuals with a single mutation in the FLG gene compared to control, and there has been a significant association between fluctuations in mutation numbers and an increased propensity to develop eczema. Environmental variables that interact with the primary barrier protein may also increase the likelihood of developing eczema and make the condition more severe. The ability of the SC to acclimatize to major changes in its physical environment is essential to the organism's ability to survive, particularly regarding significant shifts in temperature, humidity, and exposure to UV radiation. The protein filaggrin has been hypothesized to play a pivotal part in the mechanism by which dry climate conditions impair the skin's barrier function.
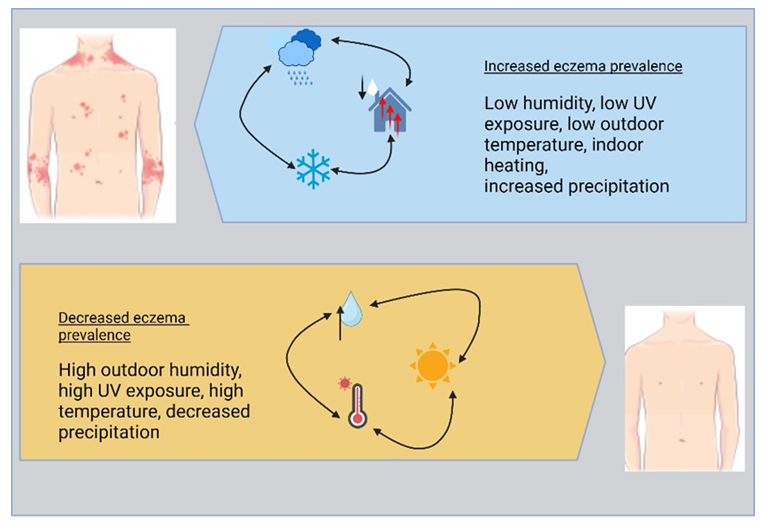
UV light has a deep and complex interaction with the epidermis, and these photon-epidermal interactions may play a significant impact in eczema pathogenesis [24]. Further research is needed to elucidate the fundamental mechanisms that relate eczema with climatic and environmental conditions, with such research having the potential to pave the way for the creation of preventative and early intervention techniques and treatment regimens that may be curated to one’s climatic circumstances. Studies in the United States have shown a connection between outside weather conditions and eczema incidence, with higher relative humidity, UV index, mean temperature, less rainfall, and fewer days of central heating being associated with lower eczema rates [23].
Figure 2: The Role of Allergens in Atopic Eczema. Allergens in acute atopic dermatitis (AD) trigger the development of Th2 cells and the release of pro-inflammatory cytokines, including interleukin 1 (IL-1), IL-6, and tumor necrosis factor (TNF)-α, from dendritic cells (DCs). Thymic stromal lymphopoietin (TSLP), which is generated from damaged keratinocytes, also produces DCs that are specialized for Th2 cell development [31]. IL-4 and IL-13 inhibit the activation of genes involved in the innate immune response and enhance vulnerability to skin infections. The secretion of the Th1-like cytokines IL-12 and IL-18 triggers the transition from Th2 cells to Th1 cells, resulting in the development of the chronic phase of AD [32]. The release of remodeling-related cytokines, such as transforming growth factor (TGF)-β1 and IL-11 [33], the attraction of eosinophils, and the generation of IL-5 all contribute to the persistence of chronic AD.
6. Role of Allergens in Atopic Eczema
Over the past three decades, there has been an uptick in the prevalence of allergic diseases, particularly among the youth with the United States experiencing a considerable surge. While the rate of increase of asthma prevalence has stabilized in developed countries, the increased rate of atopic dermatitis has not slowed down. This trend suggests environmental factors may exert varying degrees of influence on the clinical manifestations of atopic disorders (Figure 2).
In a 2020 study, the role that immune cells play in both the development and progression of eczema was investigated [25]. Researchers concluded that immune cells were found to be involved in both the pathogenesis and progression of AD and its involvement is complex and impacted by a variety of factors such as genetic modifications, epidermal dysfunction, inflammation, and infections [25]. Studies have uncovered the significant role that gene mutations in the epidermal protein filaggrin play in the development of AD and their contribution to the disease's pathophysiology [25]. However, there is still a lack of clarity regarding the connection between FLG mutations and the risk of developing AD.
The research also found that the inflammatory response in people with AD is influenced by the localized utilization of proteins within the body, which may lead to inflammation at both systemic and localized levels. Moreover, the immune system significantly influences AD etiology, with IgE receptors in the skin potentially triggering inflammation in the gastrointestinal tract. This process, which may be aided by the absorption of proteins into the circulatory system, may be triggered by an allergic reaction. Researchers concluded that immune cell influence is modified by both the environment and immune system and plays a role in the development of AD early in the disease process [25]. Overall, the study underscored the complexity of immune cell involvement in AD development and progression, and the role that IgE specifically plays in disease progression [25].
7. Outdoor air pollutants on atopic eczema
Outdoor air pollutants have a considerable short-term and long-term impact on the prevalence of AD in adults and contribute to the onset, severity, and progression of the symptoms associated with AD. A 2022 study highlights the importance of considering air pollution within the treatment regimens of adults with AD [26]. Traffic-related air pollution, particularly particulate matter with a diameter of 2.5 micrometers or less (PM2.5) and nitrogen dioxide (NO2), has been linked to increased frequency and incidence of AD in adults [27]. PM2.5 was significantly associated with a twofold increase in incident AD risk [27]. Time-series research has shown that some air pollutants are linked to an increase frequency in medical visits, either in outpatient or emergency room settings. Airborne environmental irritants may aggravate AD symptoms, and this link is most clear when considering only a single lag in the model, with the most severe impacts being seen on day 0 of the lag [26]. In addition, the influence of particulate air pollutants remained statistically significant even after controlling for exposure to other air pollutants [27, 28, 29]. These findings suggest that the influence of outdoor air pollutants on the worsening of AD symptoms may be both immediate and constant across a wide range of difference types of air pollutants. The behavior that was found might be explained by a common and direct pathogenic mechanism, such as a high degree of oxidative stress [30]. In conclusion, outdoor air pollutants may have a collective role in aggravating symptoms of AD within a relatively short timescale.
8. Indoor Environmental Factors influence atopic eczema
8.1 The Relationship Between Eczema and Home and Lifestyle Factors
Several research studies [16, 31, 32, 33] have suggested a potential link between the prevalence of eczema in children and the residential and home environments in which they live. Additionally, it has been discovered that some dietary habits, such as the consumption of vegetables and fruits [34], as well as lifestyle factors, such as parental smoking [35], can potentially increase the probability of acquiring eczema. However, previous research has yielded inconclusive findings, likely due to the existence of numerous confounding variables related to diet and lifestyle.
There is a significant association between the presence of confirmed eczema in children and both the residential and domestic environments in which they are raised, as well as the lifestyles of their caregivers [36]. Factors influencing the prevalence of childhood eczema include repainting the child's room, house size, building age, dwelling style, bedclothes airing practices, TV/computer use/duration, and the presence of pets in the household [36]. Childhood eczema has significant relationships with characteristics of the home, such as its dimensions and architectural style [31, 36]. Living in multi-story residences (seven stories of higher) or separate properties like villas or townhouses was associated with increased infantile eczema vulnerability, and was consistent with prior studies [37, 38]. Proximity to highways may also contribute to childhood eczema onset, maintaining statistical significance even after controlling for possible confounders, except for nitrogen dioxide (NO2) [36]; this discovery is consistent with the findings of earlier research [39].
Composite wood and cement flooring was found to be significantly linked to increased probabilities of infantile eczema, compared to stone or tile flooring. The process of painting a child's bedroom and rich wood was linked to an increase in the risk of eczema [34, 36]. In addition, there was a statistically significant correlation between damp and moldy conditions in the nursery with children with mold or damp spots in their bedrooms having double the eczema incidence, observed across all models [36]. This observation also agrees with prior research results [40].
Lifestyle factors such as the frequency with which a child’s room was cleaned, the drying of bedclothes, the presence of pets in the household, and the amount of time watching television/using a computer and their effect on AD was examined. Lifestyle aspects such as inconsistent bedroom cleanliness were significantly associated with increased juvenile eczema risk [36]. Frequency of bedsheet airing, dog ownership, and daily screen time were linked to infantile eczema risk [36]. These findings align with a previous 2013 study which showed dog ownership as a risk factor for infantile eczema [44]. The findings revealed in this study are also consistent with those found in earlier studies from Shanghai and Japan [41]. Insufficient room cleaning and bedclothes airing were associated with higher childhood eczema likelihood [42].
Studies in Baotou City revealed a statistically significant increased relationship between humidifier use and childhood eczema prevalence [43]. Another independent study found similar results, concluding that using a humidifier was linked to an increase in eczema [34]. However, this finding could be related to the fact that cities in the South tend to have a more humid environment overall.
Eczema in children is known to have complex causes, although such causes have not been fully explored. The pathogenic processes underlying this disease may involve the dysregulation of cytokines and inflammatory mediators [45]. In addition, there is convincing evidence showing that indoor exposure to exogenous microbiota can cause changes in the intestinal microbiota of children, hence modifying their immunological activities [46]. This was shown by demonstrating that indoor exposure to exogenous microbiota can generate changes in the intestinal microbiota of children [46]. In addition, it is important to note that interior wood products and wall coatings can emit organic pollutants like organophosphates or brominated flame retardants. This is something that should be taken into consideration. These drugs have been proven to have negative effects on the gut microbiota and have the potential to affect the functionality of the immune system [47]. However, to prevent the development and progression of eczema, it is critical to ensure that the skin's natural barriers remain intact [48]. There is a possibility that the structural integrity of the stratum corneum could be compromised using humidifiers and the presence of dogs in home contexts. In addition, the results of our research indicated that there may be a connection between the consumption of fast food and its effect on one's health. This association may be due to several different mechanisms including changes in intestinal microbiota composition, the consumption of fatty acids, and the subsequent incidence of inflammation [49].
8.2 Role of the skin's microbiome in the manifestation of atopic eczema
Although dysbiosis is a hallmark of AD, the precise makeup of skin microbiome communities and the causal link between dysbiosis and eczema remain poorly understood. This section aims to explore both the skin microbiome profile in AD patients and the potential link between dysbiosis and AD. The amount of research into the skin's microbiome has increased noticeably. This is likely because different types of bacteria can flourish in the skin's many ecological niches. Age, gender, and environmental exposure can all have an impact on the skin microbiome's different niches and communities. The significance of the skin microbiome on essential host functions, such as immunity and colonization by pathogenic microbes, is gradually coming into focus as more research is conducted in this area. An exhaustive study of the skin microbiome profile in AD has not yet been carried out, despite the potential significance of skin dysbiosis and the microbiome in the development of new treatment strategies (Figure 3). This section gives a comprehensive investigation of the skin microbiome profile in individuals who have AD, and it also raises questions regarding the possible existence of causal linkages between skin microbiota and disease development. Through an in-depth analysis of relevant scientific literature, this research aims to delineate the composition of the skin microbiome in both animal models and eczema patients, thereby offering deeper insights into the condition.
There is a significant link between the presence of Staphylococcus aureus and the severity of atopic dermatitis. The prevalence of S. aureus is notably higher on skin affected by AD in comparison to unaffected skin. When compared to unaffected regions, the prevalence of S. aureus is higher in portions of the skin that are affected by the condition, particularly in places where inflammation is present. During flareups, there was a discernible increase in prevalence of S. aureus that was noticed among those who had not been given any medication. Two additional species belonging to the genus Staphylococcus, specifically S. epidermidis and S. hemolyticus, demonstrated a significantly increased abundance in affected areas [50]. Research indicates that the bacterial diversity on AD-afflicted skin was substantially reduced when compared to control with further decrease in diversity observed during AD flare-ups [50]. This decrease in diversity involves several species that belong to the genus Streptococcus, Propionibacterium, Acinetobacter, Corynebacterium, and Prevotella. This drop cannot be solely attributed to an increase in the number of S. aureus bacteria. On facial skin affected by AD, the presence of the acne-causing bacteria Propionibacterium acnes was observed to be less prevalent than on control skin. In addition to this, there was a negative correlation found between the amount of Propionibacterium acnes present in the skin and the severity of the disease. Surprisingly, although there was a decrease in the levels of Corynebacterium during bouts of Eczema aggravation, there was an increase in the antecubital fossa of those who had primary immunodeficiency. After a flare-up event, there was an observed increase in the relative abundance of species that had a decrease in the size of their population. An examination of the fungal microbiome found that patients with AD had a lower abundance of Malassezia spp. compared to healthy individuals, while at the same time there was an increase in M. dermatitis and a wider diversity of non-Malassezia spp [50]. Human AD skin is characterized by a wider variety of non-Malassezia fungal species as well as a narrower range of bacterial species [50].
Like humans, these findings were observed in dogs. Dogs diagnosed with AD [51] and mice lacking Adam17 [52] exhibited reduced bacterial diversity, elevated levels of Staphylococcus and S. aureus during the initiation of eczematous inflammation, and heightened presence of Corynebacterium Spp.
There is a substantial correlation between the relative presence of Acinetobacter spp. on the skin and the expression of anti-inflammatory molecules in individuals without pre-existing health conditions [35]. Following an outbreak of eczema, there is a significant increase in the incidence of Acinetobacter which points to the possibility of an anti-inflammatory role of Acinetobacter in the setting of eczema [35].
Individuals diagnosed with AD had a greater risk of becoming colonized with Staphylococcus compared to people who did not have the condition [53]. Lesional skin was found to have a higher odds ratio than non-lesional skin, indicating susceptibility of affected skin to microbial colonization and further exacerbation. Staphylococcal species play a key part in the worsening of AD, and it is possible that they may play a function in the disease's onset [50].
The microbiome is becoming increasingly recognized as a potential target for both the prevention and treatment of AD (Figure 3). However, despite the development of innovative approaches to research methodology, our comprehension of the impact of dysbiosis in AD remains incomplete, directing areas for further research.
Figure 3: Dysbiosis of the skin microbiome in atopic dermatitis (AD). Genetic abnormalities in both structural (e.g., FLG and SPINK5) and immunological (e.g., IL-4 and IL-13) genes related to the skin barrier result in heightened disruption of the skin barrier. A weakened skin barrier response increases the vulnerability of the skin to allergens. Furthermore, the skin experiences dehydration and pruritus, exacerbating the strain effects on the already compromised skin barrier. In summary, the weakened skin barrier results in elevated skin pH, modified keratinocyte adhesion qualities, heightened serine protease activity, and inflammation. An acute Th2 response is believed to attenuate certain antimicrobial peptide (AMP) responses as well. These events contribute to the imbalance of the skin microbiome in individuals with AD, resulting in increased colonization by S. aureus and reduced diversity of the overall microbial population. The presence of S. aureus on the skin is believed to worsen the severity of AD by releasing several virulence factors that harm the skin barrier and promote inflammation.
9. The Impact of Climate on Eczema
9.1 Climatic factors are associated with childhood eczema prevalence
Eczema can be triggered and exacerbated by numerous factors, one of which being climate change (Figure 4). The prevalence of eczema and elements of climate were examined in a 2013 study that looked at data from the United States National Survey of Children’s Health [24]. The population-based sample involved 79,667 participants with 10,072 experienced eczema and found reduced eczema incidence in areas with high UV index, humidity, temperature, reduced precipitation, and fewer days of central heating use [24]. The relative humidity of the outside air may act as a barrier against the development of eczema or its progression [23]. Another study conducted in 12 countries from Western Europe found that the prevalence of asthma symptoms increased be 2.7% with an increase of annual meal indoor humidity of 10% according to written survey and that the prevalence of eczema symptoms correlated negatively with mean annual outdoor temperature [55]. However, interestingly, one preliminary experiment studying a small number of children diagnosed with AD found a correlation between increased instances of disease exacerbations and higher temperatures, humid conditions, excessive amount of perspiration, and psychological stress [56]. It is important to note that these connections were found within a time window ranging from 0 to 4 days after exposure [56].
A correlation was found between humidity level and UV index, two measures of environmental exposure, and the incidence of childhood eczema in the United States [23]. Data from the Weather Service and the National Climate Data Centre collected in 2006-2007 and the 2007 National Survey of Children’s Health were utilized to compile data that was used in the following study. The results of the study revealed there was a discernible reduction in the number of cases of eczema that occurred whenever there was an increase in the average yearly relative humidity and UV index within the higher quartile range [23]. In areas with greater air temperatures in the highest quartile, the incidence of eczema was found to be decreased compared to areas with a mean annual heating degree day count that fell within the third quartile that showed increased incidence. This suggests that the environment may play a role in triggering disease in children who are predisposed to developing AD.
10. Nutritional Factors and Atopic Eczema
This section analyzes the available evidence regarding dietary and nutritional factors that are hypothesized to play a role in the development of eczema.
In a study conducted in the United Kingdom in 2007, researchers explored the relationship between pregnant mothers' consumption of oily fish and the onset of eczema in their children by the age of five [57]. According to their findings, maternal consumption of oily fish may be correlated with a reduced incidence of eczema in offspring [57]. No significant link was found between the occurrence of eczema and the other allergic food groups investigated in the study [57]. Similar results were found in another study that looked at the effects of prenatal fish oil supplementation in a cohort of 83 women which showed a statistically significant reduced IL-10 neonatal cytokine response to allergens [58]. Interestingly, although no difference was found in the frequency of atopic dermatitis of the children by 1 year of age, the fish oil group experienced less severe symptoms of disease vs control [58].
There is a positive correlation between an increased maternal consumption of meat and an increased susceptibility of children to allergic rhinitis, wheezing, and AD [59]. This is consistent with another study that gives findings suggesting the impact of prenatal maternal consumption of foods on the likelihood of allergy-related consequences in their children [60].
Recently, a meta-analysis found that lower maternal vitamin D blood levels during pregnancy were associated with an increased risk of eczema in their children [61]. However, another meta-analysis of observational studies found no statistically significant association between prenatal vitamin D status, as measured by levels of circulating 25-hydroxyvitamin D in maternal blood during pregnancy or cord blood at birth, and the risk of Eczema in children between the ages of 1 and 9 years [62]. Thus, conflicting evidence for the association of prenatal vitamin D levels and eczema risk exist. Prenatal vitamin D levels were also found to be correlated with an increased risk of AD in high latitude regions [62]. This finding indicates the significance of regional and geographic variations regarding AD. Recently, a significant amount of research has been put into determining whether there is a link between the prevalence of eczema and the serum levels of vitamin D. Researchers discovered an inverse correlation between eczema severity and serum 25-hydroxyvitamin D levels, the form of vitamin D found in the blood. However, this association has not been consistently replicated in other studies [67, 68].
There is conflicting evidence regarding the possible link between breastfeeding and an increased risk of allergic reactions. While other studies have suggested that breastfeeding may offer some protection against the development of eczema, while other studies have shown that nursing has no discernible effect and may even increase the risk of developing eczema [63]. One 2005 study examined a birth cohort of 4,089 infants and found that exclusive breastfeeding for a minimum duration of four months was correlated with a decreased risk of developing eczema in children by the age of four [64]. This decrease in risk was observed independently of whether the children in the study had a history of atopy, allergic sensitization, or asthma in their families [64]. A meta-analysis of 18 prospective studies agrees and adds to this evidence showing that exclusive breastfeeding for the first three months after delivery has been linked to a reduced risk of eczema in infants, even with a family history of atopy present [65]. In contrast however, another study states that the practice of exclusively breast-feeding an infant for at least three months before the onset of eczema did not reveal any substantial preventive effects against the condition [66]. Thus, more research may be warranted to elucidate the impact of breastfeeding on atopic dermatitis in children.
11. Structural racism and its impact on atopic dermatitis
In the United States, individuals who identify as Black, Latinx, and Indigenous are subject to an inequitable distribution of the disease burden associated with atopic dermatitis. The term "structural racism" refers to the wide variety of societal processes that keep discriminatory practices alive by constructing and reinforcing unequal systems through conscious policies and practices that are sanctioned by governmental organizations and institutions. This helps to ensure that discriminatory practices continue unabated. The phenomenon under examination is firmly rooted at multiple strata, including the areas of economics, education, healthcare, and the judicial system. Consequently, it causes discrepancies within both the tangible and intangible parts of society.
In this section, a conceptual framework is presented, and current research is drawn upon to highlight the role that structural racism plays in causing disparities regarding atopic disorders. Specifically, this section focuses on the role that structural racism plays in the United States. It investigates the ways in which residential segregation, financial position, and mass incarceration are all contributing factors in creating these inequities. These factors may affect both the innate and adaptive immune responses and worsen the physiological stress responses, which may ultimately result in a disproportionate burden of disease for racial and ethnic populations.
Utilizing racial taxonomies in research and clinical applications has been a common practice within the field of medicine throughout the course of history [69]. This has resulted in medicine playing an important part in the propagation of the concept of race and the distinctions that relate to it.
Figure 5 depicts how the concept of race, and the subsequent development of a perceived hierarchy are founded in the maintenance of structural racism across multiple domains, including legal, political, cultural, and economic institutions. This is illustrated by the fact that structural racism still exists today. The continuation of this practice has led to the racial segregation of residential areas, the unequal distribution of socioeconomic opportunities, and the pervasive use of the penal system. The existence of structural racism and the intermediary mechanisms that are linked with it are what give rise to racial injustices. These inequities, in turn, lead to the continuation of interpersonal racism. Interpersonal racism and discrimination have a substantial impact on the unequal availability of resources that promote health, such as disease prevention measures and access to healthcare. These experiences also add to the development of chronic psychosocial stress and make the effects of structural racism even worse, as explained in Figure 5.
It is postulated that structural racism acts as a catalyst for the creation of proximal routes of inequity within the boundaries of our theoretical framework. These proximal routes of inequity then exert their impact on both the physical and social settings of their respective environments (Figure 5). Blacks, Latinxs, and Indigenous peoples are disproportionately affected by environmental injustices such as pollution, unsafe working conditions, and substandard living quarters [70, 71]; these risk factors have been shown to have a significant correlation with the development of atopic dermatitis.
It has been shown that the prevalence of atopic dermatitis in Black children is roughly 1.7 times higher compared to White children throughout the early childhood phase [72]. Empirical data exists indicating a heightened likelihood of sustaining prolonged and severe illness among black children [8, 9, 63]. While the incidence of atopic dermatitis in Latinx newborns is comparable to that of White children during the initial stages of infancy, Latinx children are more prone to enduring persistent and severe manifestations of the condition [23].
There are still persistent racial and ethnic inequalities in the prevalence of asthma and atopic dermatitis, even though standard socioeconomic factors were controlled in the study [73]. This persistent gap has spurred debate regarding the fundamental origin of this residual difference, and it has been highlighted as evidence in favor of the biological determinism hypothesis [73]. Despite this, little evidence exists to support the hypothesis that biological differences across racial groups are to blame for the observed disparities in the incidence and severity of diseases. Variations in the prevalence and morbidity of atopic dermatitis between Black and White individuals could be linked to other factors not related to genetic heritage, polygenic risk scores, or genetic skin pigment scores [74]. There are persistent inequalities in asthma and atopic dermatitis among various racial and ethnic groups [75]. These differences are presumably the result of social and structural variables that have not been considered, including the implications of structural racism.
Limited research has been conducted to investigate the frequency and intensity of atopic dermatitis within the Indigenous population residing in the United States. The investigation of this research domain holds significant importance, particularly in relation to the recognition and alleviation of risk factors that may possess distinct characteristics peculiar to this demographic.
12 Conclusions and Future Directions
In conclusion, atopic eczema (AE), also known as neurodermatitis or atopic dermatitis, is a prevalent chronic skin condition worldwide which affects an estimated 2.5% to 3.5% of adults and 10% to 15% of children in Western industrialized countries. Academic research suggests that environmental factors may be to blame for the upward trend in the prevalence of atopic eczema in developed countries. Environmental factors include physical surroundings, anthropogenic, nutritional, and biogenic factors. Research underscores the disproportionate burden borne by individuals from lower socioeconomic backgrounds and marginalized communities, who often experience more pronounced AE symptoms. Psychosocial stress has been found to have a positive association with the likelihood of experiencing AE, especially regarding to societal beauty standard. Anthropogenic air pollutants, both emitted outside and indoors, have been found to increase the susceptibility of children in developing atopic disease. Moreover, environmental elements such as temperature, UV radiation, and air quality directly impact the skin's barrier function, thereby influencing AE development and severity.
Atopic dermatitis (AD) is an allergic and immunologic disorder that can be influenced by various environmental factors, both within and outside the home. Studies have found a correlation between reduced ventilation in residential homes, indoor renovation activities, and the occurrence of AD during various stages of life. The presence of volatile organic compounds in the indoor environment is a likely culprit for this link. A study in Taiwan found a significant positive correlation between AD and air pollutants from traffic, such as carbon monoxide and nitrogen oxides, in both males and females. A study conducted in France revealed correlations between the lifelong presence of eczema and urban air pollutants. A correlation was observed between the prevalence of childhood AD and the average yearly levels of nitrogen dioxide (NO2), sulfur dioxide (SO2), and sulfur trioxide (SO3).
The hygiene hypothesis has been investigated as a potential explanation for the observed rise in the frequency of these ailments. However, the study of the physical environment is still relatively understudied, with limited research on physical characteristics such as temperature, UV radiation, humidity, and time spent indoors. The epidermis, a crucial protective layer, plays a key role in the development of eczema. The involvement of immune cells in the development and progression of eczema is complex and influenced by various circumstances. Outdoor air pollutants significantly impact the prevalence of AD in adults, contributing to the onset, severity, and progression of symptoms associated with AD.
Atopic eczema is influenced by indoor environmental factors, including dietary habits, lifestyle factors, and the environment in which children live. Factors such as repainting a child's room, house size, age, and lifestyle can increase the likelihood of acquiring eczema. Living in a residential structure with multiple levels or near highways can also increase vulnerability to infantile eczema. The use of composite wood and cement as flooring materials has been linked to increased infantile eczema. A lack of consistency in cleanliness, frequent bedsheet air outs, presence of dogs, and prolonged exposure to television or computer use are also linked to eczema. Humidifier use has been found to have a positive association with childhood eczema, but no significant association was found in Shanghai and Japan.
Fast food consumption may also contribute to eczema, possibly due to changes in intestinal microbiota, fatty acid consumption, and inflammation. The skin microbiome plays a crucial role in the development of AD, a condition characterized by dysbiosis. However, there is limited understanding of the specific composition of skin microbiome communities and the causal relationship between dysbiosis and eczema. This research aims to provide a description of the skin microbiome profile in individuals with AD and investigate the possibility of a causal relationship between dysbiosis and eczema.
Staphylococcus aureus is significantly linked to the severity of AD, with higher prevalence observed in those with AD compared to control skin. The presence of Streptococcus, Propionibacterium, Acinetobacter, Corynebacterium, and Prevotella species decreases when the skin is afflicted by AD. Eczema can be influenced by various factors, including climate change. Studies have shown an inverse relationship between the incidence of eczema and temperature, time spent in the sun, precipitation, and outside humidity.
Relative humidity and UV index are associated with the prevalence of childhood eczema in the United States, with a significant reduction in cases when there was an increase in the average yearly relative humidity and UV index within the higher quartile range. Environmental variables can make the symptoms of eczema worse, potentially leading to chronic illnesses and episodes of greater severity. The skin microbiome is becoming more recognized as a potential target for both the prevention and treatment of AD. A study conducted in the United States from 2006 to 2007 found a correlation between prevalent climatic conditions and an increased risk of developing eczema. Environmental factors, such as temperature, humidity, ultraviolet index, and heating degree days, were also influenced by eczema. Nutritional factors have been hypothesized to play a role in the development of eczema, with some studies finding no significant link between the occurrence of eczema and any other allergic food groups investigated.
A positive link was found between increased maternal consumption of meat and an increased susceptibility of children to allergic rhinitis, wheezing, and AD. However, inconsistent findings on the impact of prenatal maternal consumption on allergy-related consequences in children were found. Lower maternal vitamin D blood levels during pregnancy were also linked to an increased risk of eczema in children. There is conflicting data about the possible link between breastfeeding and an increased risk of allergic reactions. A 2005 birth cohort study found that exclusive breastfeeding for a minimum duration of four months was related to a lower risk of developing eczema by the time children reached the age of four. Structural racism is a significant issue in the United States, particularly in relation to atopic dermatitis, affecting individuals who identify as Black, Latino, and Indigenous. This racism is perpetuated through various societal processes, leading to racial segregation, unequal distribution of socioeconomic opportunities, and the pervasive use of the penal system.
This chronic skin condition is not solely determined by genetic factors, and it is evident that environmental shifts play a significant role in its prevalence. From socioeconomic disparities to air pollution, prenatal stress, and even climate change, these environmental elements have a profound impact on AE.
The data suggests that individuals from lower socioeconomic backgrounds and marginalized communities are more likely to experience pronounced AE symptoms.
Prenatal stress, exposure to indoor pollutants, and stressful social environments contribute to the severity of the condition. Additionally, the skin's barrier function, impacted by factors like temperature and UV radiation, plays a key role in AE development.
While genetics and biology undoubtedly play a role, the evidence overwhelmingly supports the notion that we have the power to influence and mitigate the prevalence and severity of AE through environmental factors. Thus, strategies to address these issues must encompass not only medical interventions but also efforts to address social and environmental determinants. As we gain a deeper understanding of how the environment affects AE, we can develop more targeted and effective preventive measures and treatments, ultimately improving the quality of life for those affected by this condition.
Author Contribution:
Concept and design: WL, FC, DKA; Review of literature: WL, FC; Drafting the article and figure preparation: WL, FC; Revising and editing the manuscript: DKA; Final approval of the article: WL, FC, DKA.
Funding:
The research work of DKA is supported by the R01 HL144125 and R01 HL147662 grants from the National Institutes of Health, USA. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Competing interests:
All the authors have read the manuscript and declare no conflict of interest. No writing assistance was utilized in the production of this manuscript.
Consent for publication:
All the authors have read the manuscript and consented for publication.
References
- Sohn A, Frankel A, Patel RV, et al. Eczema. Mt Sinai J Med 78 (2011): 730-739.
- Luschkova D, Zeiser K, Ludwig A, et al. Atopic eczema is an environmental disease. Allergol Select 5 (2021): 244-250.
- Ofenloch R, Schuttelaar M, Bruze M, et al. Socioeconomic Status and the Prevalence of Skin and Atopic Diseases in Five European Countries. Acta Derm Venereol 99 (2019): 309-314.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatric Dermatol 37 (2018): 142-146.
- Braig S, Weiss JM, Stalder T, et al. Maternal prenatal stress and child atopic dermatitis up to age 2 years: The Ulm SPATZ health study. Pediatric Allergy Immunol 28 (2016): 144-151.
- Zeiser K, Hammel G, Kirchberger I, et al. Social and psychosocial effects on atopic eczema symptom severity - a scoping review of observational studies published from 1989 to 2019. J European Acad Dermatol Venereol 35 (2020): 835-843.
- Zink A, Arents B, Fink-Wagner A, et al. Out-of-pocket Costs for Individuals with Atopic Eczema: A Cross-sectional Study in Nine European Countries. Acta Dermato Venereol 99 (2019): 263-267.
- Kim J, Kim EH, Oh I, et al. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol 132 (2013): 495-498.
- Kim EH, Kim S, Lee JH, et al. Indoor Air Pollution Aggravates Symptoms of Atopic Dermatitis in Children. Plos One 10 (2015): e0119501.
- Herbarth O, Fritz GJ, Rehwagen M, et al. Association between indoor renovation activities and eczema in early childhood. Int J Hygiene Environ Health 209 (2006): 241-247.
- Peden DB. The epidemiology and genetics of asthma risk associated with air pollution. J Allergy Clin Immunol 115 (2005): 213-219.
- Werfel T, Heratizadeh A, Niebuhr M, et al. Exacerbation of atopic dermatitis on grass pollen exposure in an environmental challenge chamber. J Allergy Clin Immunol 136 (2015): 96-103.
- Mcnally N, Williams H, Phillips D. Atopic eczema and the home environment. British J Dermatol 145 (2001): 730-736.
- Jedrychowski W, Perera F, Maugeri U, et al. Effects of Prenatal and Perinatal Exposure to Fine Air Pollutants and Maternal Fish Consumption on the Occurrence of Infantile Eczema. Int Arch Allergy Immunol 155 (2011): 275-281.
- Bornehag CG, Sundell J, Weschler CJ, et al. The Association between Asthma and Allergic Symptoms in Children and Phthalates in House Dust: A Nested Case-Control Study. Environ Health Perspectives 112 (2004): 1393-1397.
- Herbarth O, Fritz GJ, Rehwagen M, et al. Association between indoor renovation activities and eczema in early childhood. Int J Hygiene Environ Health 209 (2006): 241-247.
- Lee YL, Su HJ, Sheu HM, et al. Traffic-Related Air Pollution, Climate, and Prevalence of Eczema in Taiwanese School Children. J Investigative Dermatol 128 (2008): 2412-2420.
- Schultz ES, Litonjua AA, Melén E. Effects of Long-Term Exposure to Traffic-Related Air Pollution on Lung Function in Children. Curr Allergy Asthma Rep 17 (2017).
- Kathuria P, Silverberg JI. Association of pollution and climate with atopic eczema in US children. Pediatric Allergy Immunol 27 (2016): 478-485.
- Morgenstern V, Zutavern A, Cyrys J, et al. Atopic Diseases, Allergic Sensitization, and Exposure to Traffic-related Air Pollution in Children. Am J Respiratory Critical Care Med 177 (2018): 1331-1337.
- Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol 134 (2015): 993-999.
- Niwa Y, Sumi H, Kawahira K, et al. Protein oxidative damage in the stratum corneum: evidence for a link between environmental oxidants and the changing prevalence and nature of atopic dermatitis in Japan. British J Dermatol 149 (2003): 248-254.
- Silverberg JI, Simpson EL. Associations of Childhood Eczema Severity. Dermatitis 25 (2014): 107-114.
- Langan SM, Irvine AD. Childhood Eczema and the Importance of the Physical Environment. J Investigative Dermatol 133 (2013): 1706-1709.
- Asilsoy S, Al S. The Role of Allergens in Atopic Dermatitis. J Behcet Uz Child Hosp 10 (2020): 197-202.
- Chen H, Hung WT, Tang KT. The relationship between outdoor air pollutants and atopic dermatitis of adults: A systematic review and meta-analysis. Asian Pacific J Allergy Immunol 40 (2021): 295-307.
- Zhang L, Jing D, Lu Q, et al. NO2 exposure increases eczema outpatient visits in Guangzhou, China: an indication for hospital management. BMC Public Health 21 (2021).
- Nakhjirgan P, Mahmoodi M, Kashani H, et al. Air pollution and exacerbation of skin itching and sleep disturbance in Iranian atopic dermatitis patients. J Environ Health Sci Engineering 17 (2019): 811-816.
- Guo Q, Xiong X, Liang F, et al. The interactive effects between air pollution and meteorological factors on the hospital outpatient visits for atopic dermatitis in Beijing, China: a time-series analysis. J Eur Acad Dermatol Venereol 33 (2012): 2362-2370.
- Krutmann J, Liu W, Li L, et al. Pollution and skin: From epidemiological and mechanistic studies to clinical implications. J Dermatol Sci 76 (2014): 163-168.
- Liu W, Huang C, Hu Y, et al. Associations of building characteristics and lifestyle behaviors with home dampness-related exposures in Shanghai dwellings. Building Environ 88 (2015): 106-115.
- Zhang M, Wu Y, Yuan Y, et al. Effects of home environment and lifestyles on prevalence of atopic eczema among children in Wuhan area of China. Chinese Science Bulletin 58 (2013): 4217-4222.
- Norbäck D, Lu C, Zhang Y, et al. Sources of indoor particulate matter (PM) and outdoor air pollution in China in relation to asthma, wheeze, rhinitis and eczema among pre-school children: Synergistic effects between antibiotics use and PM10 and second-hand smoke. Environ Internat 125 (2019): 252-260.
- Cai J, Liu W, Hu Y, et al. Household environment, lifestyle behaviors, and dietary habits in relation to childhood atopic eczema in Shanghai, China. Int Arch Occup Environ Health 90 (2016): 141-159.
- Ehlayel MS, Bener A. Duration of breast-feeding and the risk of childhood allergic diseases in a developing country. Allergy Asthma Proc 29 (2018): 386-391.
- Liu Y, Sun S, Zhang D, et al. Effects of Residential Environment and Lifestyle on Atopic Eczema Among Preschool Children in Shenzhen, China. Front Public Health 10 (2022).
- Cai J, Huang C, Liu W, et al. Associations Between Home Dampness-related Indicators and Eczema among Preschool Children in Shanghai, China. Procedia Engineering 121 (2015): 1948-1953.
- Pan D, Liu S, Huang D, et al. Effects of household environmental exposure and ventilation in association with adverse birth outcomes: A prospective cohort study in rural China. Sci Total Environ 822 (2018): 153519.
- Robinson CL, Baumann LM, Romero K, et al. Effect of urbanisation on asthma, allergy and airways inflammation in a developing country setting. Thorax 66 (2011): 1051-1057.
- Taylor-Robinson D, Williams H, Pearce A, et al. Do early-life exposures explain why more advantaged children get eczema? Findings from the U.K. Millennium Cohort Study. British J Dermatol 174 (2016): 569-578.
- Nakhjirgan P, Mahmoodi M, Kashani H, et al. Air pollution and exacerbation of skin itching and sleep disturbance in Iranian atopic dermatitis patients. J Environ Health Sci Engineering 17 (2019): 811-816.
- Miyake Y, Ohya Y, Tanaka K, et al. Home environment and suspected atopic eczema in Japanese infants: The Osaka Maternal and Child Health Study. Pediatric Allergy Immunol 18 (2007): 425-432.
- Wang L, Huang X, Sundell J, et al. Impact of home humidifier on children’s eczema. Chinese Sci Bulletin 61 (2016): 1721-1727.
- Clayton T, Asher IM, Crane J, et al. Time trends, ethnicity and risk factors for eczema in New Zealand children: ISAAC Phase Three. Asia Pacific Allergy 3 (2013): 161-178.
- Rojahn TB, Vorstandlechner V, Krausgruber T, et al. Single-cell transcriptomics combined with interstitial fluid proteomics defines cell type-specific immune regulation in atopic dermatitis. J Allergy Clin Immunol 146 (2020): 1056-1069.
- Ta LDH, Tay CJX, Lay C, et al. Household environmental microbiota influences early-life eczema development. Environ Microbiol 23 (2021): 7710-7722.
- Roman P, Cardona D, Sempere L, et al. Microbiota and organophosphates. NeuroToxicol 75 (2019): 200-208.
- Kelleher MM, Cro S, Cornelius V, et al. Skin care interventions in infants for preventing eczema and food allergy. Cochrane Database of Systematic Rev 2021 (2021).
- Rutter CE, Silverwood RJ, Williams HC, et al. Are Environmental Factors for Atopic Eczema in ISAAC Phase Three due to Reverse Causation? J Investigative Dermatol 139 (2015): 1023-1036.
- Bjerre R, Bandier J, Skov L, et al. The role of the skin microbiome in atopic dermatitis: a systematic review. British J Dermatol 177 (2017): 1272-1278.
- Meason-Smith C, Diesel A, Patterson AP, et al. What is living on your dog’s skin? Characterization of the canine cutaneous mycobiota and fungal dysbiosis in canine allergic dermatitis. FEMS Microbiol Ecol 91 (2015): fiv139.
- Kobayashi T, Glatz M, Horiuchi K, et al. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity 42 (2015): 756-766.
- Totté J, van der Feltz W, Hennekam M, et al. Prevalence and odds of Staphylococcus aureuscarriage in atopic dermatitis: a systematic review and meta-analysis. British J Dermatol 175 (2016): 687-695.
- Suárez-Varela MM, García-Marcos Alvarez L, Kogan MD, et al. Climate and prevalence of atopic eczema in 6- to 7-year-old school children in Spain. ISAAC PhASE III. Int J Biometeorol 52 (2008): 833-840.
- Weiland SK. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med 61 (2004): 609-615.
- Langan S, Bourke J, Silcocks P, et al. An exploratory prospective observational study of environmental factors exacerbating atopic eczema in children. British J Dermatol 154 (2006): 979-980.
- Willers SM, Devereux G, Craig LCA, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax 62 (2007): 773-779.
- Dunstan JA, Mori TA, Barden A, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy. J Allergy Clin Immunol 112 (2003): 1178-1184.
- Baïz N, Just J, Chastang J, et al. Maternal diet before and during pregnancy and risk of asthma and allergic rhinitis in children. Allergy, Asthma Clin Immunol 15 (2019).
- Netting MJ, Middleton PF, Makrides M. Does maternal diet during pregnancy and lactation affect outcomes in offspring? A systematic review of food-based approaches. Nutrition 30 (2014): 1225-1241.
- Wei Z, Zhang J, Yu X. Maternal vitamin D status and childhood asthma, wheeze, and eczema: A systematic review and meta-analysis. Pediatric Allergy Immunol 27 (2016): 612-619.
- Pacheco-González RM, García-Marcos L, Morales E. Prenatal vitamin D status and respiratory and allergic outcomes in childhood: A meta-analysis of observational studies. Pediatric Allergy Immunol 29 (2018): 243-253.
- Kim JH. Role of Breast-feeding in the Development of Atopic Dermatitis in Early Childhood. Allergy, Asthma Immunol Res 9 (2017): 285.
- Kull I, Böhme M, Wahlgren CF, et al. Breast-feeding reduces the risk for childhood eczema. J Allergy Clin Immunol 16 (2005): 657-661.
- Gdalevich M, Mimouni D, David M, et al. Breast-feeding and the onset of atopic dermatitis in childhood: A systematic review and meta-analysis of prospective studies. J Am Acad Dermatol 45 (2001): 520-527.
- Yang Y, Tsai C, Lu. Exclusive breastfeeding and incident atopic dermatitis in childhood: a systematic review and meta-analysis of prospective cohort studies. British J Dermatol 161 (2009): 373-383.
- Chiu YE, Havens PL, Siegel DH, et al. Serum 25-hydroxyvitamin D concentration does not correlate with atopic dermatitis severity. J Am Acad Dermatol 69 (2013): 40-46.
- D’Auria E, Barberi S, Cerri A, et al. Vitamin D status and body mass index in children with atopic dermatitis: A pilot study in Italian children. Immunol Lett 181 (2017): 31-35.
- Witzig R. The Medicalization of Race: Scientific Legitimization of a Flawed Social Construct. Ann Internal Med 125 (1996): 675.
- Bailey ZD, Feldman JM, Bassett MT. How Structural Racism Works — Racist Policies as a Root Cause of U.S. Racial Health Inequities. New England J Med 384 (2021): 768-773.
- Rosenbaum E. Racial/Ethnic Differences in Asthma Prevalence: The Role of Housing and Neighborhood Environments. J Health Social Behavior 49 (2018): 131-145.
- Kim Y, Blomberg M, Rifas-Shiman SL, et al. Racial/Ethnic Differences in Incidence and Persistence of Childhood Atopic Dermatitis. J Investigative Dermatol 139 (2019): 827-834.
- Daya M, Barnes KC. African American ancestry contribution to asthma and atopic dermatitis. Ann Allergy, Asthma Immunol 122 (2019): 456-462.
- Abuabara K, You Y, Margolis DJ, et al. Genetic ancestry does not explain increased atopic dermatitis susceptibility or worse disease control among African American subjects in 2 large US cohorts. J Allergy Clin Immunol 145 (2020): 192-198.
- Martinez A, de la Rosa R, Mujahid M, et al. Structural racism and its pathways to asthma and atopic dermatitis. J Allergy Clin Immunol 148 (2021): 1112-1120.






 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 