Evaluation of the Wound healing Potentials of Aqueous Topical creams Containing Aqueous extract of Azadirachta indica leaves as Bioactive Ingredient
Article Information
Kenneth Chinedu Ugoeze1*, Princewill Chukwuebuka Aja1, Nkemakolam Nwachukwu1, Bruno Chukwuemeka Chinko2, Jude Nnabuife Egwurugwu3, Kennedy Emeka Oluigbo4
1Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Nigeria
2Department of Human Physiology, Faculty of Basic Medical Sciences, University of Port Harcourt, Port Harcourt, Nigeria.
3Department of Human Physiology, College of Medicine, Imo State University, Owerri, Nigeria
4Department of Clinical Pharmacy and Biopharmaceutics, Faculty of Pharmaceutical Sciences, Enugu State University of Science and Technology, Agbani City, Enugu, Nigeria
*Corresponding Author: Kenneth Chinedu Ugoeze, Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Nigeria
Received: 19 January 2021; Accepted: 02 February 2020; Published: 08 February 2021
Citation: Kenneth Chinedu Ugoeze, Princewill Chukwuebuka Aja, Nkemakolam Nwachukwu, Bruno Chukwuemeka Chinko, Jude Nnabuife Egwurugwu, Kennedy Emeka Oluigbo. Evaluation of the Wound healing Potentials of Aqueous Topical creams Containing Aqueous extract of Azadirachta indica leaves as Bioactive Ingredient. Journal of Pharmacy and Pharmacology Research 5 (2021): 176-187.
View / Download Pdf Share at FacebookAbstract
Background: Wound is one of the health indispositions with undesirable socio-economic impacts on the victim and those around them. Crude aqueous extract of Azadirachta indica leaves (AEAIL) retains verified potentials for wound healing. Developing the AEAIL into a topical aqueous cream could enhance its value in wound treatment.
Aim: The aim of this study was to formulate aqueous topical creams containing various concentrations of AEAIL as bioactive ingredients, evaluate their stability and wound healing activities in male Wistar rats using hydroxyproline (HXP) as a biochemical marker.
Materials and methods: Creams containing 1.0, 1.5, 2.0 and 3.0 % w/w of AEAIL were prepared, evaluating their stability up to 14 days and assessing their wound healing activities in male Wistar rats using DMSO, cholesterol and distilled water as controls.
Results: All the batches of creams were stable in colour, pH, viscosity, etc. and exhibited wound healing actions with the animals treated with the cream containing 1.5 % w/w of AEAIL demonstrating the highest tissue HXP level (p > 0.05). The tissue HXP levels in the animals treated with DMSO, cholesterol and distilled water were lower than those of the test creams (p < 0.05). There was significant marginal differences in percentage difference of their HXP level compared to those of the test creams (p < 0.05).
Conclusion: The aqueous extract of Azadirachta indica leaves formulated as aqueous cream was stable and retained its wound healing activities. This new formula could therefore be used in the treatment of body injuries.
Keywords
Wound healing; Aqueous cream; Azadirachta indica leaves; Bioactive ingredient; Hydroxyproline; Wistar rats
Wound healing articles; Aqueous cream articles; Azadirachta indica leaves articles; Bioactive ingredient articles; Hydroxyproline articles; Wistar rats articles
Article Details
Introduction
Wounds are damages to the outer body covering which interrupt the other soft flesh [1]. It causes social and financial infirmity to the afflicted person and those around them [2]. They may be initiated by physical, chemical, thermal, microbial or immunological abuse to the tissue [3,4] and could ordinarily be defined considering their depth, healing time, the progression of restoration, underlying pathology, the associated risk of mortality and the effect on the quality of life of the victim [5,6]. A torn, cut or pierced outer body cover is designated as an open wound. If a blunt force distress brings about a bruise, the outcome is noted as a closed wound. Those wounds described as a burn are activated by fire, heat, radiation, chemicals, electricity, or sunlight [3,4]. Restoring an injury to the body is a prolonged and complicated progression of tissue healing and transformation in reaction to an injury involving a complex series of cellular and biochemical reactions to pave way for the restoration of the injured part of a body to the re-establishment of the fundamental and serviceable constitution of the tissues as it was. It involves uninterrupted cell-cell interface and cell-matrix contacts that permit the procedure to continue in diverse corresponding segments and procedures comprising inflammation, wound contraction, re-epithelialization, tissue re-modelling and development of granulation tissue with angiogenesis. The levels of restoration of an injured body usually advance in an anticipated time frame until healing is accomplished, of which if it fails, the expected healing may not be achieved, and may lead to whichever, a long-lasting wound like a venous sore or pathological damaging such as a keloid scar [7]. The first phase of wound healing controls bleeding [8,9]. The narrowing of the vascular system, platelet migration and formation of coagulated fibrin restores haemostasis shortly after vascular damage and creates room for an extracellular network for cell migration. With the help of this, mediators of wound healing draw inflammatory cells to the area of wound allowing the subsequent stage of inflammation [10]. This second phase intersects with the preceding phase involving haemostasis and clotting and is initiated in a few hours after the damage. This phase is principally distinct from the buildup of leukocytes and macrophages [9,11]. Macrophages move into the wounded spot, release growth factors like a platelet, facilitating the development of fresh connective tissue or granular tissue [9]. Macrophages equally help in the resolution of inflammation and kindle tissue regeneration, enabling the shift from the inflammatory to reparative stages of proliferation and remodelling, occurring within three weeks from the day of the damage to the skin. The third phase, proliferation is noted by granulation tissue formation, re-epithelialization of the wound surface and contraction of the wound margins [8,11]. Granulation tissue comprises macrophages, fibroblasts and immature collagen, all understood to motivate granulation tissue formation. Simultaneously, blood vessels will stimulate capillary growth. Fibroblasts around the surface of the wound kindle the manufacture of collagen, one of the key constituents of the extracellular matrix. The fourth stage of wound healing could continue for a very long time and consist of the reorganization of collagen fibres to generate new skin [8,11]. Fresh skin could advance less than a quarter of its ultimate strength within three weeks from the day of the injury and hardly ever attains the strength of the original skin [9].
Innumerable plant preparations have at one time or the other been utilized in the management of body injuries. Plant-based extracts exploited in restoring wounds support blood clotting, fight infection and quicken the healing of wounds. Phytoconstituents derived from plants need to be elucidated and investigated for their suitability for management of wounds [1].
Azadirachta indica or neem which has been useful in folk medicine has been exploited therapeutically for wounds, incisions and further skin disorders. Its flavonoids maintain antioxidant action, protecting against free radicals that damage cells and tissues. Its tannins support wound healing [12-14]. A. indica contains varieties of phytoconstituents [15]. Research records indicated that ethanolic or methanolic extracts of A. indica leaves possesses wound healing properties [16, 17]. A quantification of the phytoconstituents of the AEAIL has been conducted using the GC-FID techniques [18]. The phytomedicinal and the nutraceutical benefits of the AEAIL has also been documented [19]. A recent study using hydroxyproline (HXP) as a biochemical marker to evaluate the level of collagen formation in wound healing showed that the AEAIL possesses wound healing activity with the optimal effective concentration for wound healing in male Wistar rats established to be 1.5 % w/v of its crude extract [18]. The purpose of this study was to develop an aqueous cream containing the AEAIL and to evaluate its stability and retention of wound healing potentials in male Wistar rats. The degree of wound healing activity of the AEAIL incorporated in the topical creams will be assessed based on the rate of wound contraction, but, especially the level of tissue HXP detectable in the portion of healed wound in male Wistar rats. HXP is a non-essential amino acid derivative formed during post-translational protein modification through hydroxylation of the amino acid, proline by the enzyme prolyl hydroxylase which requires vitamin C as a co-factor. HXP is a major component of the protein, collagen and plays a key role in the stability of the collagen triple helix [20].
Materials and methods
Materials
The following materials were used for the studies as procured and include hydroxyproline assay kit (Elabscience, China), dimethyl sulphoxide (DMSO) (Sigma-Aldrich, USA), cholesterol (Molychem, India), emulsifying wax, liquid paraffin and soft paraffin (Kerax, UK).
Methods
Collection and extraction of the sample of Azadirachta indica leavesFresh neem leaves used had been identified by a Taxonomist and deposited in the University of Port Harcourt herbarium (voucher no. EH/P/070) as reported by Ugoeze et al [18,19]. The method of sample collection and processing as also reported by Ugoeze et al [18,19] was adopted.
Formulation of aqueous cream containing aqueous extract of A. indica leaves
The aqueous topical creams containing various concentrations of the aqueous extract of Azadirachta indica leaves (AEAIL) were prepared using the formula in Table 1.
Table 1: Formula for the preparation of topical creams containing different concentrations of AEAIL
|
Ingredients |
Batches / Composition (% w/w) |
|||
|
A |
B |
C |
D |
|
|
AEAIL |
1.0 |
1.5 |
2.0 |
3.0 |
|
DMSO |
5.0 |
5.0 |
5.0 |
5.0 |
|
Cholesterol |
10.0 |
10.0 |
10.0 |
10.0 |
|
Glycerol |
5.0 |
5.0 |
5.0 |
5.0 |
|
Emulsifying ointment |
20.0 |
20.0 |
20.0 |
20.0 |
|
Water, q.s. |
100.00 |
100.00 |
100.00 |
100.00 |
Evaluation of the stability of creams
The cooled creams were evaluated visually with the naked eye for their appearance and colour. Their consistency was evaluated by rubbing a small portion of it between the fore and first fingers while their homogeneity was assessed in terms of creaming and phase separation. A known amount of each batch of cream was placed in a wide-mouthed plastic container and stored at -5°, ambient temperature and 40°C respectively. The colour, appearance, consistency and homogeneity of these samples were observed daily for 14 days for any changes.
The pH of the batches of the creams was determined using the pH meter (Ultrameter II, 6PFC E; Myron L, UK). They were stored at ambient temperature while their pH was monitored every other day for 14 days [21]. The creams were also evaluated for their spreadability, a procedure determined by placing a known quantity of each cream between two slides while a 100.0 g weight was placed on it for 10 s the distance shifted by the slides was noted.
Viscosity
The viscosity of the creams was evaluated every other day for 14 days using a Brookfield viscometer (Brookfield DV2TLVTJO, USA) fitted with spindle no. 62 at ambient temperature.
Evaluation of the wound healing effects of the creams using wound excision methods in male Wistar rats
Thirty-five adult male Wistar rats weighing 200-250g, sourced from the animal house of the Faculty of Pharmaceutical Sciences, University of Port Harcourt were kept in separate cages to acclimatize for two weeks with free access to standard feed and water all through and in standard conditions of temperature (25-29 ), relative humidity (55-66%) and natural dark/light cycle. Each rat was anaesthetized by 50 mg/kg ketamine intramuscularly. Shaving, cleaning of the dorsal area and excision of a 1.5 cm × 1.0 cm full-thickness open wound was made [22]. Animal maintenance was generally conducted in firm observance of the ethical requirements of the University of Port Harcourt. The investigation was conducted in line with the procedures for ethical conduct in the care and use of non-human animals in research [23]. All the experiments protocol for the animal studies were verified and approved by the Research Ethics Committee of the University of Port Harcourt with approval reference no.: UPH/CEREMAD/REC/MM71/043.
Evaluation of wound healing effects of the aqueous creams using animal model
A total of 35 adult male Wistar rats were divided into 7 groups (n = 5). Groups 1- 4 were treated with the creams containing 1.0, 1.5, 2.0 and 3.0% w/w AEAIL respectively while groups 5-7 were treated with DMSO, cholesterol (2% w/v) and distilled water respectively. DMSO and cholesterol were employed in the study to distinguish their effect on hydroxyproline formation from those of the AEAIL in wound healing. The treatment was carried out daily together with the measurement of wound contraction prior to cleaning and application of interventions till the closure of the wounds. The percentage of wound contraction was calculated using equation 1 below [24].

Determination of tissue hydroxyproline
At the complete closure of most of the wounds, the rats were sacrificed. Tissue bioassay was conducted using 100 mg of the tissues collected from the site of the healed wound of the respective rats, added to 1 ml of 6 M hydrochloric acid, boiled for 6 h and cooled. The pH was adjusted to 6.8 while the volume was made up to 10 ml using distilled water. Each sample was centrifuged and 1ml of its supernatant was used for the assay of HXP level using the HXP kits (Elabscience, China) and conducting the experiment based on the protocols outlined in the manufacturer’s manual which is in line with the methods described by Bergman and Loxley [25] after the principle that the oxidation product produced by HXP under the action of an oxidant reacts with dimethylaminobenzaldehyde (DMAB; Ehrlich’s reagent) showing a purplish red colour. The HXP was calculated by measuring the absorbance at 550 nm using a UV-VIS spectrophotometer (Jenway 6405, UK). The values were reported as µg/g dry weight of tissue.
2.2.6 Statistical analysis
The figures were presented as a mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed followed by Fisher’s Least Significant Difference (LSD) post hoc test to determine the level of significance.
Results and discussion
Evaluation of the aqueous creams
A homogeneous greenish smooth and consistently stable creams were obtained. It is essential to consider a suitable pharmaceutical formulation that enhances the optimal delivery of the active constituent leading to the efficacy of the preparation. Such formulations should be considered as suitable for the management of open wounds, which among other features, should be easily spread with emollient characteristics. The AEAIL was formulated as a cream employing the principles of oil-in-water emulsions [26]. The formula and the constituents employed in the preparation of the batches of creams were shown in Table 1. The incorporation of the emulsifying ointment enhances the stability of the oil-in-water cream formed, establishing the hydrophilic component. Its presence with water and glycerol provide effective emollient and moisturizing effect which enables the reduction of dryness and irritation of the damaged skin as an occlusive barrier is formed on the skin to inhibit the escape of moisture from the skin. Hydration of the stratum corneum permits the opening up of intra and intercellular channels for ease of penetration of active ingredients into the cells and injured tissues. An effective topical dermatologic formulation is also certain since water, glycerol and the emulsifying ointment forms the hydrophilic component of the formulation which serves as a vehicle to solubilize and disperse the extract in the non-aqueous phase of the cream and also supports the mixability and penetration of the extract in the hydrophobic component of the skin [27]. The DMSO and cholesterol contained in the formulation act as amphipathic surfactants to provide further stability to the formulation especially imparting hydrophobic and hydrophilic properties to the cream and enhance the stability of the plant extract acting as the active constituent to retain its activity in the environment of the various adjuvants. In addition to its action as an amphipathic surfactant, DMSO also acts as a penetration enhancer [28, 29] which is expected to improve the penetration of the plant extract into the tissue to boost activity. Additional aspects of the oil-in-water based cream take account of easy washability and high skin pore occlusion efficiency. Mostly, occlusion of wounds has been recognized to expressively decrease inflammation which amounts to decline in pains and scaring [30]. Decrease of pain and inflammation as well as speeding up of wound healing has been improved with moist healing environment and such conditions have been attained using oil-in-water creams which tallies with an occlusive formulation [31,32].
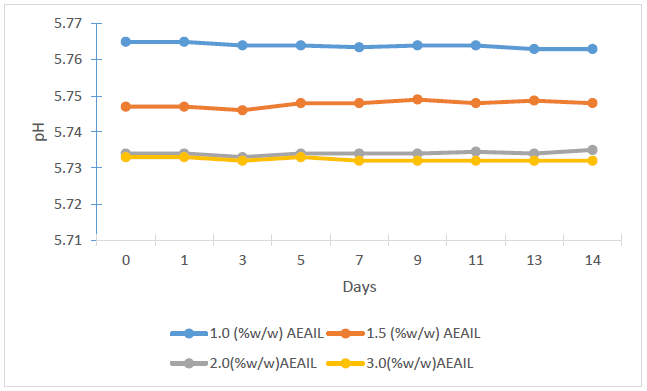
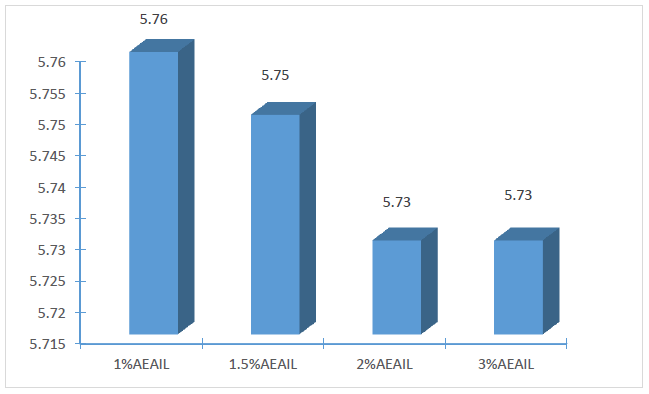
The stability of the cream is critical to its effectiveness and safety. The appearance and consistency of the formulations were used to assess the stability of the creams as apparent instability may appear as a change in colour and/or consistency. Considering these features, there were no indications of coalescence, change in colour or inconsistency in the creams in the various stress situations they were exposed to. Spoilage or instability in some creams could occur as alterations in pH which gives rise to unwelcome experiences like skin irritation [33]. The influence of storage time on the pH of the creams is shown in Figure 1. A statistically significant variation in the pH of the creams were recorded (p < 0.05), with pH decreasing as the concentration of the AEAIL increased from 1.0 – 2.0% w/w (p < 0.05) with no statistical difference in the pH of the creams containing 2.0 and 3.0 % w/w of the AEAIL (p > 0.05) (Figure 2). The mean pH of 5.760±0.001, 5.750±0.005, 5.740±0.001 and 5.730±0.001 was recorded for the batches of creams containing 1.0, 1.5, 2.0 and 3.0% w/w of AEAIL respectively as at the 14th day of storage. These values, however, are very close to the pH of the skin of 5.7 for an average adult [34]. A stable pH of the respective batches of creams was recorded (Figure 1) as the storage progressed to the 14th day signifying the stability of the creams containing different concentration of extract.
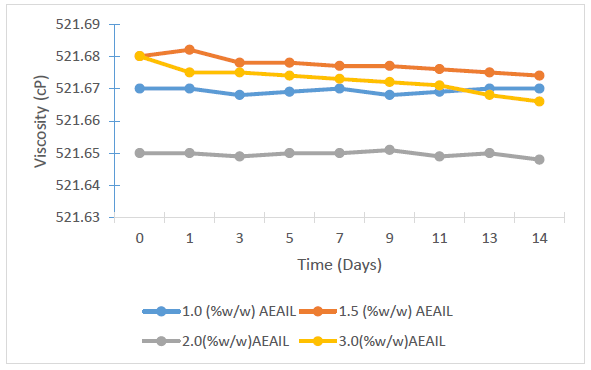
The viscosities of the respective batches of creams are presented in Figure 3, showing variations in the viscosities of the batches of creams containing different concentrations of the AEAIL (p < 0.05), though there was no consistent pattern of variation of their viscosities. The viscosities of the batches of creams were stable.
Evaluation of the wound healing effects of the creams
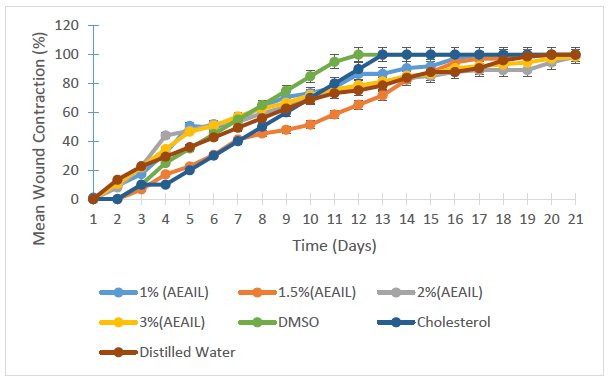
Figure 4 shows the pattern of contraction of wounds following the treatment with the creams containing 1.0, 1.5, 2.0 and 3.0% w/w of AEAIL and DMSO, cholesterol and distilled water serving as controls. There was a continuous contraction of the various wounds with complete wound closure achieved in the 20, 19, 12, 13 and 20th day of treatment for the creams containing 1.0 and 1.5% w/w AEAIL, DMSO, cholesterol and distilled water respectively. As at the 21st day of treatment, those treated with the creams containing 2.0 and 3.0% w/w of AEAIL showed 98.67 and 98.66% closure respectively.
Results of tissue assay of hydroxyproline
The results of the tissue HXP assay is presented in Table 2 showing the mean tissue HXP levels obtained due to the treatment of various wounds using the creams containing 1.0, 1.5, 2.0 and 3.0% w/w AEAIL and DMSO, cholesterol and distilled water as controls. The results showed that the highest tissue HXP level was obtained from the group of animals treated with the cream containing 1.5% w/w of the AEAIL, though there was no statistical difference in the mean tissue HXP levels of this and those obtained from the groups treated with the creams containing 1.0, 2.0 and 3.0% w/w of AEAIL (p > 0.05), but, there was a significant difference in the mean tissue HXP levels for the test creams and the entire control groups (p < 0.05) with marked percentage differences in their HXP levels (Table 2). Detection of statistically significant elevated mean tissue HXP in the male Wistar rats treated with the various creams compared to the control groups was an indication of the retention of the wound healing activity of the AEAIL in the presence of the adjuvants employed in the formulation of the creams. This shows that the mean tissue HXP levels detected in the groups treated with the test creams were due to their contents of the AEAIL. In an earlier study, our research team confirmed the wound healing potential of crude AEAIL in male Wistar rats using HXP as a biochemical marker and established its optimal wound healing concentration as 1.5% w/v [18]. The results of the present study have further confirmed that a minimal concentration of 1.5% w/w of AEAIL among its other concentrations used as bioactive ingredients in the formulation of topical aqueous creams retained its wound healing activity having caused the formation of new collagen as the wound healing progressed as indicated by the highest level of tissue HXP. The mechanism of wound healing of the AEAIL may be attributed to its various phytoconstituents [18] based on their anti-oxidant, anti-inflammatory properties, etc.
Conclusion
Aqueous extract of Azadirachta indica leaves could therefore be useful as a bioactive constituent in the development of an aqueous topical cream useful in the treatment of body injuries.
Table 2: Difference in the mean tissue hydroxyproline levels of treated groups compared to the control groups (DMSO, cholesterol and distilled water)
|
Sample |
Tissue HXP (µg/g) |
Variation of HXP of test samples from controls |
Remark |
||
|
DMSO |
Cholesterol |
Dist. water |
|||
|
1.0% AEAIL |
1.5767±0.03 |
20.82% |
24.98% |
49.99% |
< 0.05 |
|
1.5% AEAIL |
1.6300±0.09 |
24.90% |
29.20 |
55.06 |
< 0.05 |
|
2.0% AEAIL |
1.4753±0.27 |
13.05% |
16.94 |
40.34 |
< 0.05 |
|
3.0% AEAIL |
1.5594±0.04 |
19.49% |
23.61 |
48.34 |
< 0.05 |
|
DMSO |
1.3050±0.02 |
- |
- |
- |
- |
|
Cholesterol |
1.2616±0.03 |
- |
- |
- |
- |
|
Dist. Water |
1.0512±0.02 |
- |
- |
- |
- |
References
- Thakur R, Jain N, Pathak R, et al. Practices in wound healing studies of plants. Evidence-based Complementary and Alternative Medicine 2011 (2011).
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004: A joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. Journal of the American Academy of Dermatology 55 (2006): 490-500.
- Shuid AN, Yusof AA, Anwar MS. The effects of Carica papaya Linn. latex on the healing of burn wounds in rats. Jurnal Sains Kesihatan Malaysia (Malaysian Journal of Health Sciences) 3 (2005).
- Jalalpure S, Agrawal N, Patil M, et al. Antimicrobial and wound healing activities of leaves of Alternanthera sessilis Linn. International Journal of Green Pharmacy 2 (2008): 141.
- Fletcher J. Differences between acute and chronic wounds and the role of wound bed preparation. Nursing Standard (through 2013) 22 (2008): 62.
- Reddy M. Skin and wound care: important considerations in the older adult. Advances in Skin & Wound Care 21 (2008): 424-436.
- Martin P. Wound healing--aiming for perfect skin regeneration. Science 276 (1997): 75-81.
- Guo SA, DiPietro LA. Factors affecting wound healing. Journal of Dental Research 89 (2010): 219-229.
- Singer AJ, Clark RA. Cutaneous wound healing. New England Journal of Medicine 341 (1999): 738-746.
- Boateng JS, Matthews KH, Stevens HN, et al. Wound healing dressings and drug delivery systems: a review. Journal of Pharmaceutical Sciences 97 (2008): 2892-2923.
- Hanson D, Langemo D, Thompson P, et al. Understanding wound fluid and the phases of healing. Advances in Skin & Wound Care 18 (2005): 360-362.
- Joshi AR, Joshi K. Ethnomedicinal plants used against skin diseases in some villages of Kali Gandaki, Bagmati and Tadi Likhu watersheds of Nepal. Ethnobotanical Leaflets 2007 (2007): 27.
- Arora N, Bansal MP, Koul A. Azadirachta indica Exerts Chemopreventive Action Against Murine Skin Cancer: Studies on Histopathological, Ultrastructural Changes and Modulation of NF-κB, AP-1, and STAT1. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics 19 (2011): 179-191.
- Viji CS, Trikkurmadom SA, Rajalekshmi et al. Collagen—Azadirachta indica (neem) leaves extract hybrid film as a novel wound dressing: in vitro studies. Int J Pharm Sci Rev Res 32 (2015): 193-199.
- Govindachari TR, Suresh G, Gopalakrishnan G, et al. Identification of antifungal compounds from the seed oil ofAzadirachta Indica. Phytoparasitica 26 (1998): 109-116.
- Barua CC, Talukdar A, Barua AG, et al. Evaluation of the wound healing activity of methanolic extract of Azadirachta Indica (Neem) and Tinospora cordifolia (Guduchi) in rats. Pharmacologyonline 1 (2010): 70-77.
- Osunwoke Emeka A, Olotu Emamoke J, et al. The wound healing effects of aqueous leave extracts of Azadirachta indica on wistar rats. J Nat Sci Res 3 (2013): 181.
- Ugoeze KC, Aja PC, Nwachukwu N, et al. Assessment of the phytoconstituents and optimal applicable concentration of aqueous extract of Azadirachta indica leaves for wound healing in male Wistar rats. Thai Journal of Pharmaceutical Sciences (TJPS) 45 (2021).
- Ugoeze KC, Oluigbo KE, Chinko BC. Phytomedicinal and Nutraceutical Benefits of the GC-FID Quantified Phytocomponents of the Aqueous Extract of Azadirachta indica leaves. Journal of Pharmacy and Pharmacology Research 4 (2020): 149-163.
- National Center for Biotechnology information (2021). Pubchem Compound Summary for CID 5810, Hydroxyproline. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/hydroxyproline [accessed 17.01.21].
- Akhtar N, Khan BA, Haji M, et al. Evaluation of various functional skin parameters using a topical cream of Calendula officinalis extract. African Journal of Pharmacy and Pharmacology 5 (2011): 199-206.
- Suguna L, Singh S, Sivakumar P, et al. Influence of Terminalia chebula on dermal wound healing in rats. Phytotherapy Research 16 (2002): 227-231.
- Guidelines for Ethical Conduct in the Care and Use of Nonhuman Animals in Research. Washington DC, USA: American Psychological Association, 2012. Available from https://www.apa.org/science/leadership/care/guidelines. Retrieved June 13, 2019.
- Kirker KR, Luo Y, Nielson JH, et al. Glycosaminoglycan hydrogel films as bio-interactive dressings for wound healing. Biomaterials 23 (2002): 3661-3671.
- Bergman I, Loxley R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Analytical Chemistry 35 (1963): 1961-1965.
- De Vringer T, De Ronde HA. Preparation and structure of a water-in-oil cream containing lipid nanoparticles. Journal of Pharmaceutical Sciences 84 (1995): 466-472.
- Roberts MS, Pugh WJ, Hadgraft J. Epidermal permeability: penetrant structure relationships. 2. The effect of H-bonding groups in penetrants on their diffusion through the stratum corneum. International Journal of Pharmaceutics 132 (1996): 23-32.
- Junyaprasert VB, Singhsa P, Jintapattanakit A. Influence of chemical penetration enhancers on skin permeability of ellagic acid-loaded niosomes. Asian Journal of Pharmaceutical Sciences 8 (2013): 110-117.
- Moghbel A, Faghiri A. Influence of dimethyl sulfoxide as a penetration enhancer of piroxicam gel through biological skin. Iranian Journal of Pharmaceutical Sciences 2 (2006): 177-184.
- Hultén L. Dressings for surgical wounds. The American Journal of Surgery 167 (1994): S42-S45.
- Metzger S. Clinical and financial advantages of moist wound management. Home Healthcare Now 22 (2004): 586-590.
- Atiyeh BS, Amm CA, El Musa KA. Improved scar quality following primary and secondary healing of cutaneous wounds. Aesthetic Plastic Surgery 27 (2003): 411-417.
- Kikwai L, Babu RJ, Prado R, et al. In vitro and in vivo evaluation of topical formulations of spantide II. Aaps Pharmscitech 6 (2005): E565-E572.
- Kristeen C. About Skin pH and Why It Matters. Healthline, March 19, 2019. Available from: https://www.healthline.com/health/whats-so-important-about-skin-ph [accessed 15.01.21].


 Impact Factor: * 2.5
Impact Factor: * 2.5 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 