Gene Expression of Tyrosine Hydroxylase and Glutamate Receptors GluR1 and NR1 in Striatal Neurons of Parkinsonian Rats and Modulatory Effect of Bacoside-A, A Principle Constituent of Bacopa Monniera Linn
Article Information
Shobana Chandrasekar*
*Assistant Professor, Department of Biochemistry, School of Life Sciences, Vels Institute of Science, Technology and Advanced Studies (VISTAS), Chennai, 600 117, Tamil Nadu, India.
*Corresponding Author: Shobana Chandrasekar, Assistant Professor, Department of Biochemistry, School of Life Sciences, Vels Institute of Science, Technology and Advanced Studies (VISTAS), Chennai – 600 117, Tamil Nadu, India;
Received: 29 April 2020; Accepted: 09 May 2020; Published: 15 May 2020
Citation:
Shobana Chandrasekar. Gene expression of tyrosine hydroxylase and glutamate receptors GluR1 and NR1 in striatal neurons of Parkinsonian rats and modulatory effect of Bacoside-A, a principle constituent of Bacopa monniera Linn. International Journal of Applied Biology and Pharmaceutical Technology 11 (2020): 83-104.
View / Download Pdf Share at FacebookAbstract
Abstract
Our previous studies have provided evidences that Bacoside-A can protect the brain from 6-hydroxy dopamine (6-OHDA) induced Parkinson’s disease in rats by means of behavioral, biochemical and immunohistochemical evidences. In the present study, the modulation of the gene expression of GluR1, an α-amino-3- hydroxy-5-methyl-4-isoxazole-propionate (AMPA) glutamate receptor and NR1, N-methyl-d-aspartate (NMDA) receptor in the striatum of the 6-OHDA lesioned rat. The reverse transcriptase-polymerase chain reactions (RT-PCRs) showed significant reduction in GluR1 mRNA expression but a significant increase of NR1 mRNA expression in the striatal tissues of the lesioned side which was brought back to normalcy by Bacoside-A treatment after 2 weeks. Impaired Mitochondrial Complex I activity and MTT assay in 6-OHDA induced rats was attenuated by Bacoside – A treatment. At the neurochemical level, the decreased level of BDNF in 6-OHDA induced rats was significantly increased by Bacoside-A treatment. There were no significant changes observed in GDNF and NT-3 levels. Elevated levels of TNF – α and IL-6 were found to be reduced after Bacoside-A treatment. Bacoside-A treatment significantly caused a marked upregulation of tyrosine hydroxylase (TH) -mRNA expression confirming the anti-parkinson's effect of Bacoside-A. Collectively, our findings provide valid evidence on the neuroprotective effect of Bacoside-A in rat brain, which shows the neuro-therapeutic potential in mitigating 6 - OHDA induced neuronal dysfunctions.
Keywords
Bacoside-A; 6- hydroxy dopamine; Neuroprotection; BDNF; GluR1; NR1
Bacoside-A articles, 6- hydroxy dopamine articles, Neuroprotection articles, BDNF articles, GluR1 articles, NR1 articles
Article Details
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder having resting tremor, muscular rigidity, postural abnormalities and bradykinesia. Examination of PD brains reveals several abnormalities, mainly the degeneration of dopamine containing neurons of the nigrostriatal system (Hornykiewicz and Kish, 1986). A similar loss in nigro-striatal dopaminergic neurons is produced on administration of the neurotoxin 6-hydroxy dopamine (6-OHDA). In this animal model of PD, injection of 6-OHDA into the nigrostriatal pathway exhibits extensive loss of dopaminergic cells especially in the ipsilateral substantia nigra (Ungerstedt, 1971; Pycock, 1980; Carman et al., 1991). Recently, specific neurotrophic factors native to the striatum have been shown to increase following a neurotoxic lesion of the nigrostriatal pathway. In young rats with unilateral 6-OHDA lesions, brain derived neurotrophic factor (BDNF) and Glial cell line derived neurotrophic factor (GDNF) protein levels are found to be increased in the lesioned striatum and lesioned ventral midbrain when compared to BDNF protein levels in the same brain regions on the intact side (Yurek and Fletcher Turner, 2000; Zhou et al., 1996). The purpose of this study was to find out how protein levels of three different neurotrophic factors viz., BDNF, GDNF and Neurotrophin-3 (NT-3) are affected by the 6-OHDA lesion. Although the pathogenesis of the disease remains undetermined, aging, mitochondrial dysfunction, oxidative stress and apoptosis are major contributing factors. Recently, many factors suggest that inflammation, which has been the object of intense study, plays an vital role in the progression of Parkinson’s disease. Anti-inflammatory drugs represent a new kind of approach for this neuro disorder (Skaper, 2007; Hakkanson et al., 2007; Orr et al., 2002; Vijitruth et al., 2006). The levels of TNF-α and IL-6 indicated the role of neuroinflammation in the pathobiology of 6-OHDA induced Parkinson’s like symptoms. The inhibition of TNF-α and IL-6 has driven neuroprotection in rats (Hunter et al., 2007; Kang et al., 2007). Glutamatergic systems plays the significant roles in the pathology of Parkinson’s disease. During the course of Parkinson’s disease, hyper- activity of glutamatergic pathways to and within the basal ganglia, are noted (Calabresi et al., 1993; Greenamyre, 2000; Blandini et al., 1997). In addition, one of the possible causes of the specific cell death of the dopaminergic neurons in the SNc is suggested to be glutamate-mediated excitotoxicity (Olney et al., 1990; Rodriguez et al., 1998; Beal, 1998). Blockade of glutamate receptors by selective antagonists, in particular using the ionotropic glutamate receptor antagonists, ameliorates the Parkinsonian motor symptoms in animal models (Chase and Oh, 2000; Konitsiotis et al., 2000; Steece-Collier et al., 2000). Functions of glutamate are mediated by a family of glutamate receptors. N-methyl-d-aspartate (NMDA) receptors (NR1, NR2A, NR2B, NR2C, NR2D and NR3) and _ amino-3-hydroxy-5-methyl- 4-isoxazole-propionate (AMPA) receptors (GluR1, GluR2, GluR3 and GluR4) are two major groups of ionotropic glutamate receptors (Gasic and Hollmann, 1992; Nakanishi, 1992; Hollmann and Heinemann, 1994; Nakanishi et al., 1998). Modulations in the expression of NMDA and AMPA receptors are found in animal models of Parkinson’s disease by various techniques. However, there is still no consensus about the patterns of changes of the glutamate receptors in the neostriatum after dopamine denervation.
The Bacopa monnieiri (Scrophulariaceae) whole plant constitutes the well-known drug brahmi. It is astringent, febrifuge, emmenogogue, carminative, digestive, cardiotonic, diuretic, bronchodilatory, depurative, laxative, and tonic. It is used in the indigenous system of medicine for the treatment of insanity, epilepsy and as a potent nerve tonic (Warrier et al., 1994; The Wealth of India, 1988). The drug forms an important ingredient of a few Ayurvedic preparations such as Brahmirasayanam. The juice of leaves is given to children for relief in bronchitis. The paste of leaves is used for rheumatism (The Wealth of India, 1988).
The plant Bacopa monnieiri has been reported to contain steroidal and triterpenoidal saponins as major constituents along with alkaloids, sterols, triterpenes, fatty hydrocarbons glycosides and aminoacids as minor constituents (Rastogi and Mehrotra, 1995; Rastogi and Mehrotra, 1991; Rastogi and Mehrotra, 1993). The steroidal saponins, Bacoside A and Bacoside B are optical isomers (Rastogi and Mehrotra, 1998). The other bacosides have been identified as dammarane type triterpenoid saponins known as bacopasaponin A-G and bacopasides I-V. The bacopasaponins A-G and bacosides I-V, contain aglycone part and glycone moieties (Chakravarty et al., 2001; Mahato et al., 2000; Garai et al., 1996a; Garai et al., 1996b; Chakravarty et al., 2003). The triterpenoids reported from this plant Bacopa monnieiri are bacogenin A1, bacogenin A2 (Rastogi and Mehrotra, 1993). The standardized methanolic extract of this plant containing 38% of Bacoside A was reported to show antiulcerogenic activity when administered at the doses of 10-50 mg kg-1 b.w. daily for 5 days twice in different gastric ulcer models (Sairam et al., 2001). Our previous studies show the neuroprotective effect of Bacoside-A on 6-hydroxy dopamine induced Parkinson’s disease in rats by means of behavioral, biochemical and immunohistochemical studies (Shobana and Sumathi, 2013).
In the present study we have used the 6-OHDA model of PD to analyze whether Bacoside-A has neuroprotective effects. Injection of 6-OHDA into the substantia nigra causes dopamine neuron damage, which mimic early stages of Parkinson’s disease (Lee et al., 1996). The levels of BDNF, GDNF, NT-3, TNF – α and IL-6 were examined by ELISA and furthermore, the expression of tyrosine hydroxylase (TH), GluR1 and NR1 by RT-PCR as potential mechanisms of Bacoside-A neuroprotection.
Materials and methods
Chemicals
6-hydroxy dopamine (6-OHDA) was purchased from Sigma Aldrich. The primers for real time PCR were synthesized by Sigma Aldrich. Promega EmaxTM Immuno Assay System was used for the detection of all three neurotrophic factors. All other chemicals used were of analytical grade.
Animals and Treatments
Male Albino rats, 5 months old, weighing 250 – 300g were obtained from Central Animal House, Dr. ALMPGIBMS, University of Madras, Taramani campus, Chennai - 113, Tamil Nadu, India. Rats were housed separately in polypropylene cages and fed standard pellet diet, kept under hygienic conditions. Rats were kept on a 12hr light and dark cycles with free access to water ad libitum. All experiments and protocols described in the present study (IEAC No. 01/16/2012) were approved by the Institutional review committee for the use of human or animal subjects (Institutional Animal Ethics Committee (IEAC) of Dr. ALMPGIBMS, University of Madras, Taramani campus, Chennai - 113, Tamil Nadu, India). Rats were divided into six experimental groups of 6 animals each. Group I: Vehicle treated, sham operated control received 2µl of vehicle (0.1% ascorbic acid-saline) intracranially (S). Group II: Vehicle treated, lesioned with 6 hydroxy dopamine on 22nd day (L). Group III: Rats pretreated with Bacoside-A (10mg/kg) orally for 21 days; on 22nd day single dose of 6-hydroxy dopamine (12 µg of 6-OHDA/2µl in 0.1% ascorbic acid-saline) injected into right striatum (BAC1+L). Group IV: Rats pretreated with Bacoside-A (20mg/kg) orally for 21 days; on 22nd day single dose of 6-hydroxy dopamine (12 µg of 6-OHDA/2µl in 0.1% ascorbic acid-saline) injected into right striatum (BAC2+L). Group V: Rats administered with Bacoside-A (10mg/kg) alone orally for 21 days, sham – operated (BAC1+S). Group VI: Rats administered with Bacoside-A (20mg/kg) alone orally for 21 days, sham – operated (BAC2+S).
Isolation of Bacoside-A (Bacoside-A)
Bacoside – A was isolated as explained in our previous studies (Shobana and Sumathi, 2013). The plant material Bacopa monniera (Linn) was collected at Chennai, Tamil Nadu and was authenticated by Dr. A. Sasikala, Captain Srinivasa Murti Drug Research institute for Ayurveda, Arumbakkam, Chennai, Tamil Nadu. The triterpenoid saponin Bacoside-A was isolated from the plant by the procedure carried out by Deepak et al (2005). The isolation of Bacoside A was carried out using shrimp lethality assay to direct the fractionation of a methanolic proportion of methanol. The fractions eluted with chloroform:methanol 85:15 were combined and concentrated under vacuum (7 g). This fraction was subjected to flash chromatography on silica gel (100–200 mesh) and the fractions eluted with chloroform:methanol 85:15 were combined, concentrated under vacuum and crystallised from 70% methanol in water to obtain Bacoside A (2.5 g). Aqueous suspension of Bacoside-A was given orally to the animals at a dosage of 10 mg/kg, b.w/day (Anbarasi et al., 2006) and 20 mg/kg, b.w/day.
Lesioning
After 3 weeks of treatment with Bacoside-A, all animals in experimental and sham-operated groups were anaesthetized with ketamine and xylazine intraperitoneally (i.p.). Each animal was mounted on a stereotaxic stand (Instruments and Chemicals, Ambala, New Delhi), the skin overlying the skull was cut to expose it, and the coordinates for the striatum were measured accurately (antero-posterior 0.5mm, lateral 2.5mm, dorso-ventral 4.5mm relative to bregma and ventral from dura) with the tooth bar set at 0mm. Thereafter, all animals in experimental groups were lesioned by injecting 12μg 6-OHDA/2 μl in 0.1% ascorbic acid-saline into the right striatum, while the sham-operated group received 2.0μl of the vehicle. The injections were made manually, with the help of a Hamilton syringe, through the burr holes made on the skull surface in both groups. The injection rate was 1.0μl/min, and the needle was kept in place for an additional 1min before being slowly retracted. The experiments were performed in accordance with the guidelines of Institutional Animal Ethics Committee (IEAC) of Dr. ALMPGIBMS, University of Madras, Taramani campus, Chennai - 113, Tamil Nadu, India.
Post-Operative Care
Recovery of anesthesia took approximately 4–5h. The rats were kept in a well-ventilated room at 25±3°C in individual cages until they gained full consciousness; they were then housed together in groups of 4 animals per cage. Food and water was kept inside the cages for the first week, allowing animals' easy access, without physical trauma due to overhead injury. Animals were then treated normally; food, water, and the bedding of the cages were changed twice per week, as usual.
Complex-I activity (NADH dehydrogenase activity)
Mitochondrial complex-I action was assessed by the spectrophotometric strategy for King and Howard (1967). The technique includes synergist oxidation of NADH to NAD with ensuing decrease of cytochrome C (cyt-C). Quickly, the response blend comprises of 350 ml of 0.2M glycylglycine support, 100ml of 1.05mM cyt-C, 100ml of 6mM NADH (in glycyl glycine cradle), 2.4ml of refined water, 10ml of test and 20ml of 0.02M sodium bicarbonate. The change in optical thickness at 550nm was recorded over 180s and action was communicated as level of hoax gathering (the mean of the trick bunch was utilized to address the information for every individual creature. At long last the mean of the amendments for the diverse treatment bunches were determined).
MTT Assay
MTT (3-(4,5-dimethyl thiazol-2-yl) - 2, 5-diphenyl tetrazolium bromide) examine is a proportion of suitable cells. In this test, yellow shading was decreased to purple shading formazan by mitochondrial reductase proteins present in living cells and a solubilizing specialist, typically dimethylsulfoxide was added to disintegrate in solvent formazan item into a hued arrangement (Mosmann, 1983). Quickly, to 100ml of test, 10ml of MTT (10mg/ml in 0.1M phosphate cradle) was included and brooded for 3h at 37C. After hatching, 200ml of dimethyl sulphoxide (DMSO) was added to stop the response. The recognition of this hued arrangement was measured at 580nm and the outcomes as far as 'cell feasibility' were communicated as level of the hoax gathering (the mean of the trick bunch was utilized to address the information for every individual creature. At long last, the mean of the remedies for the distinctive treatment bunches were determined).
Estimation of tumor necrosis factor - alpha (TNF- α) and interleukin-6 (IL-6) in striatum
The measurements of TNF-α and IL-6 were finished by rodent TNF-α and IL-6 immunoassay pack (R&D Systems, Minneapolis, MN, USA). The Quantikine rodent TNF-α and IL-6 immunoassay pack is a 4.5 h strong stage ELISA intended to gauge rodent TNF-α and IL-6 levels. It is a strong stage sandwich protein connected immuno-sorbent examine (ELISA) utilizing a plate peruser. Focuses were determined from the standard bends and action was communicated as level of hoax gathering.
Quantification of neurotrophic factors by ELISA
Animals were euthanized, cerebrums were evacuated, the striatal mind districts were dismembered on ice, and the examples were then put away at – 80oC. Consequently, each tissue test was homogenized in 400µl volumes of homogenate support (400mM NaCl, 0.1% Triton-X, 2mM EDTA, 0.1mM benzothonium chloride, 2mM benzamidine, 0.1M Phosphate cushion pH 7.4). The homogenates were centrifuged for 10min at 10,000xg at 4oC. The homogenate was isolated into 100µl copy tests and neurotrophic factor content was resolved utilizing a counter acting agent sandwich position: removed neurotrophic factors from each example were caught with a monoclonal immune response against BDNF, GDNF, or NT-3; the caught BDNF was then bound to a second, explicit, polyclonal neutralizer (pAb) against BDNF, GDNF, or NT-3. Subsequent to washing, the measure of explicitly bound pAb was identified utilizing an animal varieties explicit enemy of IgY immunizer conjugated to horse radish peroxidase (HRP) as a tertiary reactant. Unbound conjugate was expelled by washing and, following a hatching period with a chromogenic substrate, the shading change was estimated in a microplate peruser (450nm). The measure of neurotrophic factor was relative to the shading change produced in an oxidation-decrease response; the promega E-max Immuno Assay System was utilized for the recognition of all the three neurotrophic factors. The unwavering quality of the neurotrophic factor measures went from 97 to 99% dependent on relapse investigation.
GluR1 and NR1 mRNA expression by Reverse transcriptase-polymerase chain reaction
Fourteen days after medical procedure, 6-OHDA-lesioned and age coordinated ordinary rodents were executed, and striatal tissues were analyzed. All labware was treated with diethyl pyrocarbonate and autoclaved before use. Striatal tissues were homogenized in TriZOL reagent. After hatching for 5 min at room temperature, chloroform was included for stage detachment. The upper watery stage was gathered and all out RNA was encouraged by blending in with isopropyl liquor. The RNA pellet was washed once with 75% ethanol and was air-dried. It was at last redissolved in sans rnase water. The A260/A280 proportions were resolved with readings somewhere in the range of 1.6 and 1.8 utilizing a spectrophotometer (UV-1601, Shimadzu). Semi-quantitative RT-PCR was directed utilizing the housekeeping quality glyceraldehyde-3-phosphate dehydrogenase (G3PDH) as an inside standard for PCR preliminaries, for example a "preliminary dropping" strategy (Wong et al., 1994). RT-PCR was performed utilizing G3PDH (200 bp: 5'- TTCCTACCCCCAATGTATCC-3', 5'- CCCCAGCATCAAAGGTG-3'; 437 bp: 5' ATGGTGAAGGTCGGTGTGAAC-3', 5'- GCTGACAATCTTGAGGGAGT-3'), NR1 (5'- AACCTGCAGAACCGCAAG-3', 5'- GCTTGATGAGCAGGTCTATGC-3'), GluR1 (5'- AGGTTTGCTTTGTCACAA-3', 5'- CTTCTCCAGGTCCTGAAA-3'), preliminary combines as portrayed in past investigations (Yoshioka et al., 1996; Lai et al., 2000; Sze et al., 2001). The quantity of cycles was differed to decide the ideal number that would permit identification of the enhanced items, while saving intensification for these qualities in the log stage. Diverse enhancement cycles were utilized (NMDA receptor subunits with G3PDH: NR1: 30 cycles; AMPA receptor subunits with G3PDH: G3PDH: 33 cycles; GluR1: 35 cycles). All out RNA was weakened to 1µg/µl in sans rnase water, blended in with 0.5µg of pd(N)6 and 47µl of without rnase water to a last volume of 49µl in a response tube containing RT-PCR dots. One of the above preliminary sets was added to give a last volume of 50µl. The response was hatched at 42oC for 30 min, trailed by 95oC for 5 min to inactivate the RT and to totally denature the format. Responses were run for the ideal cycles with 55oC tempering cycle (1 min), 72oC expansion cycle (1 min), and a 95oC denaturing cycle (50s). The G3PDH preliminary set was added into the response as indicated by its relating pre-aligned cycle number. Control enhancements were done either without RT or without RNA. The PCR items were stacked on agarose gels (1%) containing ethidiume bromide and electrophoresis was performed at 100V for 30 min. Picture of the DNA gels after RT-PCR examinations were carefully caught by a gel documentation framework (Ultra Violet Products C-80). The optical thickness of each band was estimated by a picture examining programming (Metamorph). The powers of GluR1 and NR1 groups of the striatal tissues were standardized utilizing the forces acquired in the G3PDH groups and afterward communicated as a proportion.
Result
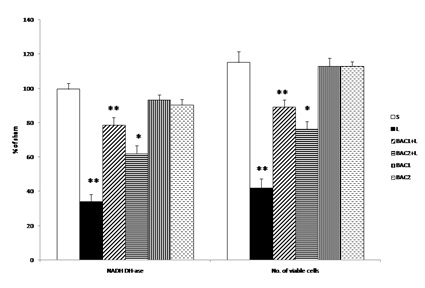
Data represents mean ± S.D (n = 6 in each group). NADH-DHase and MTT assay expressed as percentage of sham. S: Vehicle treated, sham operated control received 2µl of vehicle (0.1% ascorbic acid-saline) intracranially. L: Vehicle treated, lesioned with 6 hydroxy dopamine on 22nd day. BAC1+L: Rats pretreated with Bacoside-A (10mg/kg) orally for 21 days; on 22nd day single dose of 6-hydroxy dopamine (12 µg of 6-OHDA/2µl in 0.1% ascorbic acid-saline) injected into right striatum. BAC2+L: Rats pretreated with Bacoside-A (20mg/kg) orally for 21 days; on 22nd day single dose of 6-hydroxy dopamine (12 µg of 6-OHDA/2µl in 0.1% ascorbic acid-saline) injected into right striatum. BAC1: Rats administered with Bacoside-A (10mg/kg) alone orally for 21 days, sham – operated. BAC2: Rats administered with Bacoside-A (20mg/kg) alone orally for 21 days, sham – operated. ** p<0.01; *p<0.05; L group compared with S; BAC1+L, BAC2+L compared with L by one way ANOVA with Tukey’s post hoc test.
Fig. 1 shows the effect of Bacoside - A on alteration in the mitochondrial enzyme complex activity and MTT assay in 6-OHDA induced rats. Challenge with 6-OHDA significantly impaired mitochondrial enzyme complex activities (decreased NADH- dehydrogenase activity and number of viable cells) as compared to sham group. Bacoside - A treatment at a dose of 10mg/kg b.w. significantly (p<0.01) restored mitochondrial enzyme complex activity and viable cells than 20mg/kg b.w. dosage as compared to 6-OHDA induced group. However, Bacoside - A alone treated groups did not show any significant changes.
Data represents mean ± S.D (n = 6 in each group). S: Vehicle treated, sham operated control received 2µl of vehicle (0.1% ascorbic acid-saline) intracranially. L: Vehicle treated, lesioned with 6 hydroxy dopamine on 22nd day. BAC1+L: Rats pretreated with Bacoside-A (10mg/kg) orally for 21 days; on 22nd day single dose of 6-hydroxy dopamine (12 µg of 6-OHDA/2µl in 0.1% ascorbic acid-saline) injected into right striatum. BAC2+L: Rats pretreated with Bacoside-A (20mg/kg) orally for 21 days; on 22nd day single dose of 6-hydroxy dopamine (12 µg of 6-OHDA/2µl in 0.1% ascorbic acid-saline) injected into right striatum. BAC1: Rats administered with Bacoside-A (10mg/kg) alone orally for 21 days, sham – operated. BAC2: Rats administered with Bacoside-A (20mg/kg) alone orally for 21 days, sham – operated. ** p<0.01; *p<0.05; L group compared with S; BAC1+L, BAC2+L compared with L by one way ANOVA with Tukey’s post hoc test.
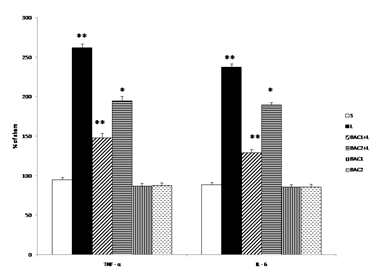
Effect of Bacoside-A on the levels of TNF - α and IL –6 in 6-OHDA induced rats is given in Fig. 2. Intrastriatal 6-OHDA injection resulted in significant increase (p<0.01) in TNF- α and IL - 6 levels as compared to sham group. Bacoside -A (10mg/kg b.w) treatment significantly (p<0.01) attenuated TNF- α and IL - 6 levels than Bacoside –A dosage of 20mg/kg b.w (p<0.05) as compared to 6-OHDA induced group. However, Bacoside - A treatment in healthy animals did not influence these parameters significantly as compared to sham group.
Data represents mean ± S.D (n = 6 in each group). S: Vehicle treated, sham operated control received 2µl of vehicle (0.1% ascorbic acid-saline) intracranially. L: Vehicle treated, lesioned with 6 hydroxy dopamine on 22nd day. BAC1+L: Rats pretreated with Bacoside-A (10mg/kg) orally for 21 days; on 22nd day single dose of 6-hydroxy dopamine (12 µg of 6-OHDA/2µl in 0.1% ascorbic acid-saline) injected into right striatum. BAC2+L: Rats pretreated with Bacoside-A (20mg/kg) orally for 21 days; on 22nd day single dose of 6-hydroxy dopamine (12 µg of 6-OHDA/2µl in 0.1% ascorbic acid-saline) injected into right striatum. BAC1: Rats administered with Bacoside-A (10mg/kg) alone orally for 21 days, sham – operated. BAC2: Rats administered with Bacoside-A (20mg/kg) alone orally for 21 days, sham – operated. ** p<0.01; *p<0.05; L group compared with S; BAC1+L, BAC2+L compared with L by one way ANOVA with Tukey’s post hoc test.
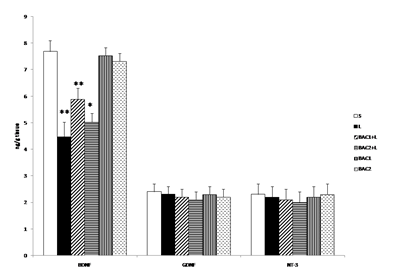
Fig. 3 shows the effect of Bacoside-A on 6-OHDA induced changes in the content of BDNF, GDNF and NT-3 in the striatum of control and experimental rats. The level of BDNF was significantly (p<0.01) decreased in the L group when compared with the control group (S). However treatment with Bacoside-A significantly increased the level of BDNF contents (BAC1+L (p<0.01), BAC2+L (p<0.05)) in the striatum of rat brain when compared with L group. No significant changes observed in Bacoside-A alone administered groups. The contents of GDNF and NT-3 did not show any significant alterations in the 6-OHDA induced group and thereby no changes observed in the Bacoside-A treated groups.
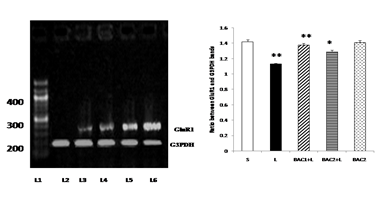
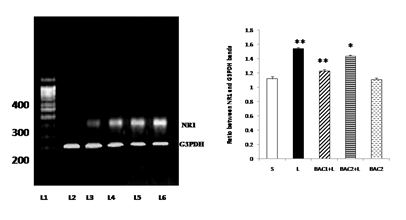
Lane 1: DNA ladder
Lane 2: L - Vehicle treated, lesioned with 6 - hydroxy dopamine on 22nd day.
Lane 3: BAC2 + L - Rats pretreated with Bacoside-A (20mg/kg) orally for 21 days; on 22nd day single dose of 6-hydroxy dopamine (12 µg of 6-OHDA/2µl in 0.1% ascorbic acid-saline) injected into right striatum
Lane 4: BAC1 + L - Rats pretreated with Bacoside-A (10mg/kg) orally for 21 days; on 22nd day single dose of 6-hydroxy dopamine (12 µg of 6-OHDA/2µl in 0.1% ascorbic acid-saline) injected into right striatum
Lane 5: BAC2 alone – Rats administered with Bacoside-A (20mg/kg) alone orally for 21 days, sham – operated.
Lane 6: S - Control, Vehicle treated, sham operated control received 2µl of vehicle (0.1% ascorbic acid-saline) intracranially.
Bacoside - A protected against 6-hydroxydopamine-induced GluR1 down regulation in the striatum of rats as shown in Fig. 4. 6-OHDA lesion resulted in differential regulations on the gene expression of glutamate receptor subunits in the striatal tissues. The results of RT-PCR indicated that there was a reduction in the levels of expression of GluR1 mRNA in the striatum of the 6-OHDA-lesioned rats. Upon Bacoside-A treatment at 10mg/kg b.w, the levels of expression of GluR1 mRNA was found to be significantly increased (p<0.01) than 20mg/kg b.w of Bacoside-A. There were no significant differences in the expression of the GluR1 in the Bacoside-A alone treated rats.
Lane 1: DNA ladder
Lane 2: Control - Vehicle treated, sham operated control received 2µl of vehicle (0.1% ascorbic acid-saline) intracranially.
Lane 3: BAC2 alone - Rats administered with Bacoside-A (20mg/kg) alone orally for 21 days, sham – operated.
Lane 4: L + BAC1 - Rats pretreated with Bacoside-A (10mg/kg) orally for 21 days; on 22nd day single dose of 6-hydroxy dopamine (12 µg of 6-OHDA/2µl in 0.1% ascorbic acid-saline) injected into right striatum
Lane 5: L + BAC2 - Rats pretreated with Bacoside-A (20mg/kg) orally for 21 days; on 22nd day single dose of 6-hydroxy dopamine (12 µg of 6-OHDA/2µl in 0.1% ascorbic acid-saline) injected into right striatum
Lane 6: L - Vehicle treated, lesioned with 6 hydroxy dopamine on 22nd day
Bacoside - A protected against 6-hydroxydopamine-induced NR1 upregulation in the striatum of rats as shown in Fig. 5. The results of RT-PCR indicated that there was an increase in the levels of expression of NR1 mRNA in the striatum of the 6-OHDA-lesioned rats. Upon Bacoside-A treatment at 10mg/kg b.w, the levels of expression of NR1 mRNA was found to be significantly decreased (p<0.01) than 20mg/kg b.w of Bacoside-A. There were no significant differences in the expression of the NR1 in the Bacoside-A alone treated rats.
Lane 1: DNA ladder
Lane 2: L - Vehicle treated, lesioned with 6 hydroxy dopamine on 22nd day.
Lane 3: L + BAC2 - Rats pretreated with Bacoside-A (20mg/kg) orally for 21 days; on 22nd day single dose of 6-hydroxy dopamine (12 µg of 6-OHDA/2µl in 0.1% ascorbic acid-saline) injected into right striatum
Lane 4: L + BAC1 - Rats pretreated with Bacoside-A (10mg/kg) orally for 21 days; on 22nd day single dose of 6-hydroxy dopamine (12 µg of 6-OHDA/2µl in 0.1% ascorbic acid-saline) injected into right striatum
Lane 5: BAC2 alone – Rats administered with Bacoside-A (20mg/kg) alone orally for 21 days, sham – operated.
Lane 6: Control - Vehicle treated, sham operated control received 2µl of vehicle (0.1% ascorbic acid-saline) intracranially.
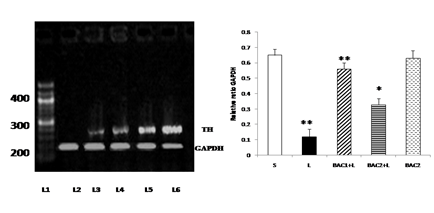
Bacoside - A protected against 6-hydroxy dopamine-induced tyrosine hydroxylase mRNA down regulation in the substantia nigra of rats as shown in Fig. 6. 6-OHDA microinjection caused marked down regulation of tyrosine hydroxylase mRNA level in 6-OHDA induced rats. This effect was restored significantly (p<0.01) by the 10mg/kg b.w of Bacoside – A treatment than 20mg/kg b.w. of Bacoside-A dosage. No changes were observed in the Bacoside - A alone treated group.
Discussion
In the pathophysiology of PD, different components have been proposed for the nigrostriatal degeneration of dopaminergic neurons. Among these components, glial cell actuation inferred oxidative pressure and irritation stays as one of the chief causes which includes enactment of microglial cells and receptive astrocytes, harmed blood mind boundary (BBB) and invaded fringe resistant cells (Block and Hong, 2005; Choi et al., 2005). Microglial reaction to dynamic dopaminergic neurodegeneration has been concentrated in rodent model of PD instigated by intrastriatal one-sided 6-OHDA infusion.
The aftereffects of this examination give proves that the declaration of three distinctive neurotrophic factors inside the mesostriatal framework are influenced by the neurotoxic injury of the nigrostriatal pathway. The declaration of three neurotrophic factors BDNF, GDNF and NT-3 in the denervated striatum was investigated in the 6-OHDA prompted rodents. BDNF was seen as diminished while GDNF and NT-3 didn't show any huge changes. Neurotrophins, specifically BDNF are key particles in mind work (McAllister et al., 1999; Aid et al., 2007) and are profoundly communicated in the mesostriatal framework, assuming a significant job in the separation, development, upkeep and fix of DA neurons (Baquet et al., 2005). Glial cell line-determined neurotrophic factor is a far off individual from the TGF-β group of neurotrophic factors and is communicated in the substantia nigra and striatum, just as other mind areas, in both the creating and grown-up cerebrum of rodents and people (Pochon et al., 1997; Schaar et al., 1993; Springer et al., 1994). The utilitarian receptor for GDNF is a two-part receptor complex that comprises of a ligand restricting GDNF family receptor, GDNFR-α1 or GDNFR-α2, and the receptor protein kinase ret (Durbec et al., 1996; Jing et al., 1996; Treanor et al., 1996; Trupp et al., 1997). In rodents, dopamine neurons express both GDNFR-α mRNA and ret mRNA during advancement and all through adulthood while just GDNFR-α mRNA is communicated in ventral striatum during improvement (Nosrat et al., 1997). Along these lines the useful receptor of GDNF seems, by all accounts, to be available in dopamine neurons all through the lifetime of rodents. Injury to dopamine neurons or the striatum can evoke changes in the statement of GDNF or its receptor. In the current examination, we see that GDNF indicated no noteworthy changes in the striatum of rodents.
Past examinations have built up that the neurotrophins BDNF and NT-3 are communicated inside the mesostriatal framework during advancement and all through adulthood (Seroogy et al., 1994; Escandon et al., 1994).The bountiful articulation of these neurotrophins in the ventral midbrain during improvement proposes that these two neurotrophins may assume a significant job in separation, development and target innervation of dopamine neurons. The continued articulation of these neurotrophins proposes a job for the upkeep and fix of the mesostriatal framework all through the lifetime of the living being. In this investigation, we watched essentially lower levels of BDNF and no checked changes in the degrees of NT-3 in the lesioned striatum and along these lines the degree of BDNF was seen as expanded after treatment with Bacoside-A.
Neuro-irritation and oxidative pressure has been accounted for to assume a pivotal job in the pathogenesis of PD just as 6-OHDA initiated Parkinson resembles indications (Broom et al., 2011). Numerous in-vivo and in-vitro contemplates have likewise affirmed the job of receptive oxygen species (ROS), oxidative pressure and exhausted cell reinforcement pool initiated by 6-OHDA (Naveilhan et al., 1994; Khan et al., 2010). 6-OHDA is a neurotoxin having auxiliary closeness with dopamine and norepinephrine. Upon its organization, 6-OHDA is specifically taken up by dopaminergic neurons by means of plasma film transporters of catecholamine, where it is promptly oxidized to hydrogen peroxide and paraquinone (Heikkila and Cohen, 1971; Saner and Thoenen, 1971). 6-OHDA organization has been accounted for to expand TNF-α and IL-6 levels both in substantia nigra and striatum (Mogi et al., 2000). 6-OHDA is likewise a powerful inhibitor of mitochondrial respiratory compounds; produces metabolic deficiency, vitality exhaustion and oxidative pressure prompting neurodegeneration (Glinka et al., 1997). Poisonous impacts of 6-OHDA to some extent are additionally intervened by initiation of microglia (Whitton, 1997). Further, raised degrees of TNF-α have been seen in the cerebrospinal liquid and after death minds of PD patients (Boka et al., 1994). In concurrence with the announced investigations, in the current examination, 6-OHDA organization altogether caused expanded degrees of incendiary cytokines, TNF-α and IL-6 in the striatal district. In concurrence with detailed examinations, in the current investigation, 6-OHDA organization essentially caused expanded degrees of fiery cytokines in the striatal area, though Bacoside-A treatment fundamentally constricted the raised degrees of provocative middle people proposing their inhibitory impact on the neuro-incendiary course which prompts dopaminergic neurodegeneration.
Cell demise prompted by 6-OHDA has been proposed to be interceded by ROS got from 6-OHDA autooxidation and inhibitory impact on the mitochondrial respiratory chain particularly mind boggling – I (NADH dehydrogenase) and complex – III (Rodriguez-Pallares et al., 2007). Hindrance of mitochondrial complex prompts restraint of electron transport chain, hence prompting a condition of metabolic deficiency (Vila et al., 2008). During mitochondrial restraint, the creation of ROS, (for example, superoxide anions) increments with the ensuing harm to the phone proteins prompts upregulation and interpretation of ace incendiary markers, for example, NF-κB and enlistment of apoptotic proteins, for example, caspase-3 which at last prompts neuronal passing (Vila and Przedborski, 2003; Vila et al., 2008). As per the above examinations, the present investigation likewise exhibited critical decrease in the mind boggling I movement following 6-OHDA organization. Treatment with Bacoside-An essentially lightened the mitochondrial dysfunctions prompted by 6-OHDA. In this manner, it might be conceivable that cancer prevention agent capability of Bacoside-An applied critical cytoprotective action and lessened the mitochondrial brokenness. The decrease of MTT [3-(4,5 – Dimethylthiazol – 2 – yl)- 2,5 – diphenyltetrazolium bromide, a yellow tetrazole] to purple formazan in living cells (Mosmann, 1983) occur just when reductase chemicals are dynamic, and in this manner change is frequently utilized as a proportion of reasonable (living) cells. In the current investigation, 6-OHDA organization altogether caused decrease in mitochondrial redox limit, while, Bacoside-A treatment essentially lessened the Complex-III restraint brought about by 6-OHDA demonstrating the neuroprotection because of increment in the quantity of suitable neuronal cells.
The reduction in GluR1 proteins is seen basically in neurons that bear qualities of striatal neurons (checked on by Bolam and Bennett, 1995; Gerfen and Wilson, 1996; Smith et al., 1998). The current discoveries in this way show there are differential impacts of dopamine denervation on GluR1 articulation in various subpopulations of striatal neurons. Medium spiked neurons are the chief yield neurons of the neostriatum that structure the two significant pathways of the basal ganglia, for example the immediate and roundabout pathways (checked on by Gerfen and Wilson, 1996; Smith et al., 1998; Bolam et al., 2000). These two significant pathways intervene excitatory signs that principally emerge from the cortex and the thalamus and glutamate is used as the synapse (Gerfen and Wilson, 1996; Smith et al., 1998; Bolam et al., 2000). The decrease in GluR1 receptor articulation in neurons may along these lines influence glutamate neurotransmission through the two significant pathways in the basal ganglia. It is entrenched that the sharp neurons are the significant focuses of the dopaminergic synaptic contributions from the SNpc (evaluated by Smith and Bolam, 1990; Gerfen and Wilson, 1996; Smith et al., 1998; Bolam et al., 2000). Past investigations have shown that the quantity of spines of assumed medium sharp neurons just as the quantity of deviated neurotransmitters is altogether diminished in the neostriatum after dopamine denervation (Ingham et al., 1993, 1998; Arbuthnott et al., 2000). A huge extent of GluR1 receptor immunoreactivity is found in spines and furthermore confined in the postsynaptic film of deviated neurotransmitters at the subcellular level (Bernard and Bolam, 1998). Glutamatergic pathways are known to be over-dynamic in Parkinsonian condition and these pathways incorporate the corticostriatal pathway and the pathway started from the subthalamic core (Calabresi et al., 1993; Greenamyre, 1993, 2000; Blandini et al., 1996a, b, 1997; Chase et al., 1998; Rodriguez et al., 1998; Chase and Oh, 2000). The over-action of glutamatergic pathways may consequently additionally contribute in the adjustment of the GluR1 articulation in the barbed neurons. The decrease of GluR1 receptor articulation might be a reaction to the over-movement of glutamatergic pathways.
Another intriguing finding of the current examination is that there is an up-guideline of NR1 mRNA in the striatal tissues after the 6-OHDA sore. The current discoveries do give some insight that an expansion in mRNA articulation of NR1 may not influence the general accessibility of NR1 proteins in striatal neurons. In any case, it isn't known how an expansion in NR1 mRNA articulation just could influence the NMDA divert properties in striatal neurons after dopamine denervation (Tremblay et al., 1995). Strikingly, a past report has demonstrated that in spite of the fact that the general wealth of NR subunits doesn't change in striatal tissues, NR1 and NR2B proteins are seen as diminished in the film parts of striatal tissues and these outcomes show an adjustment in turnover of NR subunits (Dunah et al., 2000). Likewise, the phosphorylated NR1 and tyrosine phosphorylated NR2B are found to diminish after dopamine denervation (Dunah et al., 2000). The phosphorylation of NR subunits are likewise known to prompt tweak of the particle channel conductance and the initial properties of the NMDA channels (Grosshans and Browning, 2001; Scott et al., 2001; Vissel et al., 2001). These demonstrate that the physiological conditions of NMDA receptors may change after dopamine denervation. As referenced above, dopamine denervation has brought about critical decrease of spines just as awry neurotransmitters in the neostriatum (Ingham et al., 1993, 1998; Arbuthnott et al., 2000). In this manner, the expansion in NR1 mRNA articulation might be a pay instrument to battle the impact of spine decreases after dopamine denervation.
Bacoside – A treatment potentiates a helpful impact by turning around the adjustments in quality articulation for GluR1 and NR1 receptor that happen during 6-OHDA prompted Parkinson's infection, bringing about diminished glutamate-intervened excitotoxity in the over animated cerebrum districts. Along these lines, it is apparent that Bacoside-A treatment to 6-OHDA initiated rodents renders security against glutamate related excitotoxicity, related with engine and subjective deficiencies which will have helpful importance in the administration of Parkinson's sickness.
In view of the above discoveries, Bacoside-A seems to have potential neuroprotective impact in lightening the cell changes related with 6-OHDA organization. The weakening of these modifications affirms their remedial potential which was conceivable due to their neuropharmacological action and rebuilding of mitochondrial compound complex movement. Further examinations to comprehend the arrangement of occasions would merit researching.
Acknowledgement
The author is thankful to University Grants Commission, New Delhi, India for the financial assistance rendered for this study in the form of Project Fellow. The author thank Central Research Facility, Sri Ramachandra Medical University for their timely help in doing RT-PCR studies.
Declaration of Interest
The authors declare that there is no conflict of interests.
References
- Aid T, Kazantseva A, Piirsoo M, et al. Mouse and rat BDNF gene structure and expression revisited. Journal of neuroscience research 85 (2007): 525-535.
- Anbarasi K, Kathirvel G, Vani G, et al. Cigarette smoking induces heat shock protein 70 kDa expression and apoptosis in rat brain: modulation by bacoside A. Neuroscience 138 (2006): 1127-1135.
- Arbuthnott GW, Ingham CA, Wickens JR. Dopamine and synaptic plasticity in the neostriatum. The Journal of Anatomy 196 (2000): 587-596.
- Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. Journal of Neuroscience 25 (2005): 6251-6259.
- Beal MF. Excitotoxicity and nitric oxide in Parkinson's disease pathogenesis. Annals of neurology 44 (1998): S110-S114.
- Bernard V, Bolam JP. Subcellular and subsynaptic distribution of the NR1 subunit of the NMDA receptor in the neostriatum and globus pallidus of the rat: co-localization at synapses with the GluR2/3 subunit of the AMPA receptor. European Journal of Neuroscience 10 (1998): 3721-3736.
- Blandini F, Garcia-Osuna M, Greenamyre JT. Subthalamic ablation reverses changes in basal ganglia oxidative metabolism and motor response to apomorphine induced by nigrostriatal lesion in rats. European Journal of Neuroscience 9 (1997): 1407-1413.
- Blandini F, Greenamyre JT, Nappi G. The role of glutamate in the pathophysiology of Parkinson's disease. Functional Neurology 11 (1996): 3-15.
- Blandini F, Porter RH, Greenamyre JT. Glutamate and Parkinson’s disease. Molecular Neurobiology 12 (1996): 73-94.
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Progress in Neurobiology 76 (2005): 77-98.
- Boka G, Anglade P, Wallach D, et al. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neuroscience Letters 172 (1994): 151-154.
- Bolam JP. Microcircuitry of the neostriatum. Molecular and Cellular Mechanisms of Neostriatal Function (1995): 1-9.
- Bolam JP, Hanley JJ, Booth PA, et al. Synaptic organisation of the basal ganglia. The Journal of Anatomy 196 (2000): 527-542.
- Broom L, Marinova-Mutafchieva L, Sadeghian M, et al. Neuroprotection by the selective iNOS inhibitor GW274150 in a model of Parkinson disease. Free Radical Biology and Medicine 50 (2011): 633-640.
- Calabresi P, Mercuri NB, Sancesario G, et al. Electrophysiology of dopamine-denervated striatal neurons. Implications for Parkinson's disease. Brain: a Journal of Neurology 116 (1993): 433-452.
- Carman LS, Gage FH, Shults CW. Partial lesion of the substantia nigra: relation between extent of lesion and rotational behavior. Brain Research 553 (1991): 275-283.
- Chakravarty AK, Garai S, Masuda K, et al. Bacopasides III—V: Three new triterpenoid glycosides from Bacopa monniera. Chemical and Pharmaceutical Bulletin 51 (2003): 215-217.
- Chakravarty AK, Sarkar T, Masuda K, et al. Bacopaside I and II: two pseudojujubogenin glycosides from Bacopa monniera. Phytochemistry 58 (2001): 553-556.
- Chase TN, Oh JD. Striatal mechanisms and pathogenesis of parkinsonian signs and motor complications. Annals of Neurology 47 (2000): S122-S129.
- Chase TN, Oh JD, Blanchet PJ. Neostriatal mechanisms in Parkinson's disease. Neurology 51 (1998): S30-S35.
- Choi DK, Pennathur S, Perier C, et al. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson's disease in mice. Journal of Neuroscience 25 (2005): 6594-6600.
- Deepak M, Sangli GK, Arun PC, et al. Quantitative determination of the major saponin mixture bacoside A in Bacopa monnieri by HPLC. Phytochemical Analysis: An International Journal of Plant Chemical and Biochemical Techniques 16 (2005): 24-29.
- Dunah AW, Wang Y, Yasuda RP, et al. Alterations in subunit expression, composition, and phosphorylation of striataln-methyl-d-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson's disease. Molecular Pharmacology 57 (2000): 342-352.
- Durbec P, Marcos-Gutierrez CV, Kilkenny C, et al. GDNF signalling through the Ret receptor tyrosine kinase. Nature 381 (1996): 789-793.
- Escandon E, Soppet D, Rosenthal A, et al. Regulation of neurotrophin receptor expression during embryonic and postnatal development. Journal of Neuroscience 14 (1994): 2054-2068.
- Garai S, Mahato SB, Ohtani K, et al. Dammarane-type triterpenoid saponins from Bacopa monniera. Phytochemistry 42 (1996): 815-820.
- Garai S, Mahato SB, Ohtani K, et al. Bacopasaponin DA pseudojujubogenin glycoside from Bacopa monniera. Phytochemistry 43 (1996): 447-449.
- Gasic GP, Hollmann M. Molecular neurobiology of glutamate receptors. Annual Review of Physiology 54 (1992): 507-536.
- Gerfen, C. R., and C. J. Wilson. "The basal ganglia: In: Swanson LW, Bjorklund A and Hokfelt T, eds. Handbook of Chemical Neuroanatomy, Vol 12: Integrated Systems of CNS, Part III." (1996): 371-468.
- Glinka Y, Gassen M, Youdim MB. Mechanism of 6-hydroxydopamine neurotoxicity. InAdvances in Research on Neurodegeneration (1997): 55-66.
- Greenamyre JT. Glutamate-dopamine interactions in the basal ganglia: relationship to Parkinson's disease. Journal of Neural Transmission/General Section JNT 91 (1993): 255-269.
- Greenamyre JT. New targets for therapy in Parkinson's disease: Pathogenesis and pathophysiology. Functional Neurology 15 (2000): 67-80.
- Grosshans DR, Browning MD. Protein kinase C activation induces tyrosine phosphorylation of the NR2A and NR2B subunits of the NMDA receptor. Journal of Neurochemistry 76 (2001): 737-744.
- Håkansson A, Westberg L, Nilsson S, et al. Interaction of polymorphisms in the genes encoding interleukin-6 and estrogen receptor beta on the susceptibility to Parkinson's disease. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 133 (2005): 88-92.
- Heikiila R, Cohen G. Inhibition of biogenic amine uptake by hydrogen peroxide: a mechanism for toxic effects of 6-hydroxydopamine. Science 172 (1971): 1257-1258.
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annual Review of Neuroscience 17 (1994): 31-108.
- Hornykiewicz O, Kish S. Biochemical pathology of Parkinson’s disease. Adv Neurol 45 (1986):19–34
- Hunter RL, Dragicevic N, Seifert K, et al. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. Journal of Neurochemistry 100 (2007): 1375-1386.
- Ingham CA, Hood SH, Taggart P, et al. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. Journal of Neuroscience 18 (1998): 4732-4743.
- Ingham CA, Hood SH, Van Maldegem B, et al. Morphological changes in the rat neostriatum after unilateral 6-hydroxydopamine injections into the nigrostriatal pathway. Experimental Brain Research 93 (1993): 17-27.
- Jing S, Wen D, Yu Y, et al. GDNF–induced activation of the ret protein tyrosine kinase is mediated by GDNFR-α, a novel receptor for GDNF. Cell 85 (1996): 1113-1124.
- Kang HC, Lee YM, Kim HD, et al. Safe and effective use of the ketogenic diet in children with epilepsy and mitochondrial respiratory chain complex defects. Epilepsia 48 (2007): 82-88.
- Khan MM, Ahmad A, Ishrat T, et al. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson's disease. Brain Research 1328 (2010): 139-151.
- King TE, Howard RL. [52] Preparations and properties of soluble NADH dehydrogenases from cardiac muscle. InMethods in Enzymology 10 (1967): 275-294.
- Konitsiotis S, Blanchet PJ, Verhagen L, et al. AMPA receptor blockade improves levodopa-induced dyskinesia in MPTP monkeys. Neurology 54 (2000): 1589-1595.
- Lai SK, Wong CK, Yang MS, et al. Changes in expression of N-methyl-D-aspartate receptor subunits in the rat neostriatum after a single dose of antisense oligonucleotide specific for N-methyl-D-aspartate receptor 1 subunit. Neuroscience 98 (2000): 493-500.
- Lee CS, Sauer H, Björklund A. Dopaminergic neuronal degeneration and motor impairments following axon terminal lesion by intrastriatal 6-hydroxydopamine in the rat. Neuroscience 72 (1996): 641-653.
- Mahato SB, Garai S, Chakravarty AK. Bacopasaponins E and F: two jujubogenin bisdesmosides from Bacopa monniera. Phytochemistry 53 (2000): 711-714.
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annual Review of Neuroscience 22 (1999): 295-318.
- Mogi M, Togari A, Kondo T, et al. Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from parkinsonian brain. Journal of Neural Transmission 107 (2000): 335-341.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65 (1983): 55-63.
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science 258 (1992): 597-603.
- Nakanishi S, Nakajima Y, Masu M, et al. Glutamate receptors: brain function and signal transduction. Brain Research Reviews 26 (1998): 230-235.
- Naveilhan P, Neveu I, Jehan F, et al. Reactive oxygen species influence nerve growth factor synthesis in primary rat astrocytes. Journal of Neurochemistry 62 (1994): 2178-2186.
- Nosrat CA, Tomac A, Hoffer BJ, et al. Cellular and developmental patterns of expression of Ret and glial cell line-derived neurotrophic factor receptor alpha mRNAs. Experimental Brain Research 115 (1997): 410-422.
- Olney JW, Zorumski CF, Stewart GR, et al. Excitotoxicity of L-dopa and 6-OH-dopa: implications for Parkinson's and Huntington's diseases. Experimental Neurology 108 (1990): 269-272.
- Orr CF, Rowe DB, Halliday GM. An inflammatory review of Parkinson’s disease. Progress in Neurobiology 68 (2002): 325-340.
- Pochon NM, Menoud A, Tseng JL, et al. Neuronal GDNF expression in the adult rat nervous system identified by in situ hybridization. European Journal of Neuroscience 9 (1997): 463-471.
- Pycock CJ. Turning behaviour in animals. InCommentaries in the Neurosciences (1980): 461-512.
- Rastogi RP, Mehrotra BN. Compendium of Indian medicinal plant (vol. I, 1960-1969). CDRI Lucknow and PDI, New Delhi (1991).
- Rastogi RP, Mehrotra BN. Compendium of Indian medicinal plants, vol II (1970–1979). Central Drug Institute, Lucknow, India and Publication and Information Directorate, CSIR, Dr. KS Krishnan Marg, New Delhi, India (1993): 169-70..
- Rastogi RP, Mehrotra BN, Sinha S, et al. Compendium of Indian Medicinal Plants Vol. 4 (1985–1989). Central Drug Research Institute, Lucknow and National Institute of Science Communication, New Delhi (1995).
- Rastogi RP, Mehrotra BN. Compendium of Indian Medicinal Plants, Vol. 5.1990^ 94. Central Drug Research Institute (1998).
- Rodriguez MC, Obeso JA, Olanow CW. Subthalamic nucleus-mediated excitotoxicity in Parkinson's disease: a target for neuroprotection. Annals of Neurology 44 (1998): S175-S188.
- Rodriguez-Pallares J, Parga JA, Munoz A, et al. Mechanism of 6-hydroxydopamine neurotoxicity: the role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. Journal of Neurochemistry 103 (2007): 145-156.
- Sairam K, Rao CV, Babu MD, et al. Prophylactic and curative effects of Bacopa monniera in gastric ulcer models. Phytomedicine 8 (2001): 423-430.
- Saner A, Thoenen H. Model experiments on the molecular mechanism of action of 6-hydroxydopamine. Molecular Pharmacology 7 (1971): 147-154.
- Scharr DG, Sieber BA, Dreyfus CF, et al. Regional and cell-specific expression of GDNF in rat brain. Experimental Neurology 124 (1993): 368-371.
- Scott DB, Blanpied TA, Swanson GT, et al. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. Journal of Neuroscience 21 (2001): 3063-3072.
- Seroogy KB, Lundgren KH, Tran TM, et al. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. Journal of Comparative Neurology 342 (1994): 321-334.
- Shobana C, Sumathi T. Studies on behavioral, biochemical, immunohistochemical and quantification of dopamine and its metabolites in the striatum of 6-hydroxy dopamine induced Parkinsonism in rats - Attenuation by Bacoside-A, a major phytoconstituent of Bacopa monniera. International Journal of Applied Biology and Pharmaceutical Technology 4 (2013): 120-142
- Skaper SD. The brain as a target for inflammatory processes and neuroprotective strategies. Annals of the New York Academy of Sciences 1122 (2007): 23-34.
- Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends in Neurosciences 13 (1990): 259-265.
- Smith Y, Bevan MD, Shink E, et al. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience 86 (1998): 353-387.
- Springer JE, Mu X, Bergmann LW, et al. Expression of GDNF mRNA in rat and human nervous tissue. Experimental Neurology 127 (1994): 167-170.
- Steece-Collier K, Chambers LK, Jaw-Tsai SS, et al. Antiparkinsonian actions of CP-101,606, an antagonist of NR2B subunit-containing N-methyl-d-aspartate receptors. Experimental Neurology 163 (2000): 239-243.
- Sze SC, Wong CK, Yung KK. Modulation of the gene expression of N-methyl-D-aspartate receptor NR2B subunit in the rat neostriatum by a single dose of specific antisense oligodeoxynucleotide. Neurochemistry International 39 (2001): 319-327.
- The Wealth of India A Dictionary of Indian Raw materials and Industrial products; Raw materials Publications and Information Directorate 2 (1988): 2-3
- Treanor JJ, Goodman L, de Sauvage F, et al. Characterization of a multicomponent receptor for GDNF. Nature 382 (1996): 80-83.
- Tremblay M, Salin P, Soghomonian JJ. Effect of 6-OHDA lesions on striatal mRNA levels encoding for glutamate receptor subunits. Neuroreport 6 (1995): 2225-2229.
- Trupp M, Belluardo N, Funakoshi H, et al. Complementary and overlapping expression of GDNF, c-RET proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J. Neurosci 17 (1997): 3554-3567.
- Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiologica Scandinavica 82 (1971): 69-93.
- Vijitruth R, Liu M, Choi DY, et al. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson's disease. Journal of Neuroinflammation 3 (2006): 6.
- Vila M, Przedborski S. Targeting programmed cell death in neurodegenerative diseases. Nature Reviews Neuroscience 4 (2003): 365-375.
- Vila M, Ramonet D, Perier C. Mitochondrial alterations in Parkinson’s disease: new clues. Journal of Neurochemistry 107 (2008): 317-328.
- Vissel B, Krupp JJ, Heinemann SF, et al. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nature Neuroscience 4 (2001): 587-596.
- Varrier PK, Nambiar VP, Ramankutty C. Tylophora indica Indian medicinal plants-a compendium of 500 species. Orient Longman, New Delhi (1994): 66-68.
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson's disease. British Journal of Pharmacology 150 (2007): 963-976.
- Wong H, Anderson WD, Cheng T, et al. Monitoring mRNA expression by polymerase chain reaction: the" primer-dropping" method. Analytical Biochemistry 223 (1994): 251-258.
- Yoshioka A, Ikegaki N, Williams M, et al. Expression of N-methyl-D-aspartate (NMDA) and non-NMDA glutamate receptor genes in neuroblastoma, medulloblastoma, and other cell lines. Journal of Neuroscience Research 46 (1996): 164-172.
- Yurek DM, Fletcher-Turner A. Lesion-induced increase of BDNF is greater in the striatum of young versus old rat brain. Experimental Neurology 161 (2000): 392-396.
- Zhou J, Pliego-Rivero B, Bradford HF, et al. The BDNF content of postnatal and adult rat brain: the effects of 6-hydroxydopamine lesions in adult brain. Developmental Brain Research 97 (1996): 297-303.


 Impact Factor: * 3.0
Impact Factor: * 3.0 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
