Growth, SDH Activity and Microbiological Properties of Aubergine (Solanum melongena L.) crops Irrigated with Treated Wastewater from Casablanca city, Morocco
Article Information
Soukaina Ouansafia*, Fatima Bellalib, Mostafa Kabinea, Hind Maaghlouda, Fahde Abdelilaha
aDepartment of Biology, Health and Environment Laboratory, Hassan II University, Aîn Chock Science Faculty, Casablanca, Morocco
bDepartment of Biology, Biological Engineering Laboratory, Sultan Moulay Slimane University, Faculty of Sciences and Techniques, Beni Mellal, Morocco
*Corresponding Author: Soukaina Ouansafi, Department of Biology, Health and Environment Laboratory, Hassan II University, Aîn Chock Science Faculty, Casablanca, Morocco
Received: 11 January 2021; Accepted: 02 March 2021; Published: 10 March 2021
Citation: Soukaina Ouansafi, Fatima Bellali, Mostafa Kabine, Hind Maaghloud, Fahde Abdelilah. Growth, SDH Activity and Microbiological Properties of Aubergine (Solanum melongena L.) crops Irrigated with Treated Wastewater from Casablanca city, Morocco. International Journal of Plant, Animal and Environmental Sciences 11 (2021): 206-220.
View / Download Pdf Share at FacebookAbstract
Aubergine cultivation experiments were carried out at the Ain Chock Faculty of Science of the Hassan 2 University in Casablanca, to study the effect of potable water (PW), waste water (WW) and treated wastewater (TW) on aubergine crop production and on physico-chemical properties, SDH activity and microbiological properties of aubergine fruits. The aubergine growth was not significantly affected by different treatments. K+, NO3-, lipid, protein and Vitamin C content were insignificant in plant irrigated with TW compared with PW. For the microbiological results, no bacterial colonies of Faecal Streptococcus, Vibrio cholerae or Salmonella were identified in all the fruits studied for any irrigation system and total coliforms and fecal coliforms were below to acceptable limits for plant. The results of SDH activity showed that leaves presented higher activity compared to fruits and roots. Treated wastewater can be a resource for agricultural irrigation.
Keywords
Aubergine; Treated wastewater; Growth; Microbiological properties; Succinate dehydrogenase activity
Aubergine articles; Treated wastewater articles; Growth articles; Microbiological properties articles; Succinate dehydrogenase activity articles
Article Details
Introduction
Aubergine (Solanum melongena L.), otherwise known as eggplant, is an important solanaceous vegetable crop mainly cultivated in tropical and subtropical climates. The most important aubergine-growing countries are China (the world’s largest producer with 57.2% of production), India, Japan, Indonesia, Mediterranean countries such as Turkey, Egypt, Morocco, Italy, Greece, Spain, France, and the U.S.A. [1]. This solanaceous vegetable, as are tomato (Solanum lycopersicon) and pepper (Capsicum annum) and shares similar environmental and cultural requirements as those crops. However, aubergine agriculture is highly dependent on water availability, which remains the main limiting factor in arid and semi-arid regions is especially in Morocco. Apart from conventional waters (i.e. rivers, dams and groundwater), important amounts of wastewater from households and industrial activities are discharged. However, wastewater availability is can be considered alternative water sources saves surface water [2]. It is possible to treat and reuse urban and industrial wastewater for agricultural production in regions with water restrictions [3]. Different technologies have been applied to improve of quality wastewater and meet health and environmental regulations [4]. To our knowledge, there are no published studies in Morocco on the effect of municipal and industrial wastewater treatment on the yield and plant growth of drip-irrigated aubergines. Irrigation studies, intended to optimize use of irrigation water, are necessary to enable the protection of water resources in Morocco. Therefore, the objective of this research we evaluated the use of treated wastewater (TW) on the growth and morphological of eggplants (Solanum melongena L.) in the semi-arid region of Morocco. In addition, the effect of using TW on SDH activity and microbiological properties of eggplant was also evaluated.
Material and Methods
Plant material
The field trial was carried out with the aubergine (Solanum melongena L.) during the growing season of 2017. The aubergine seeds were placed in alveolate trays containing pot at and irrigated daily with drinking water. As soon as seedlings at the 6-leaf stage were obtained, they were transplanted in each plot and irrigated with the three irrigation treatments: irrigation with potable water (PW), irrigation with waste water (WW) and irrigation with treated wastewater (TW). The TW used in this study was taken from the Wastewater Treatment Plant (WWTP) in the province of Mediouna.
Aubergine cultivation experiments were carried out at the Ain Chock Faculty of Science of the Hassan 2 University in Casablanca. In this study, the soil at the experimental site was chosen, because it showed in previous study that it was suitable physicochemical potential and favorable to agricultural activities [5].
The experimental field was arranged according to a randomized plot design with the irrigation treatments (PW, WW and TW), each one replicated three times for a total of 9 plots for the aubergien crops (3 irrigation treatments × 3 replicates). Each plot was 10 m² (2.5 m wide × 4 m long). The main plot treatments consist of irrigation systems drip. A schematic representation of the agriculture system of aubergines crop is shown in Figure 1.
Water physicochemical analysis
All the water samples were analyzed according to standard methods APHA (2005) [6] for the physicochemical parameters. The analysis included the physicochemical parameters of pH, electrical conductivity (ECw; μs cm-1), total sus- solids (TSS; mg.L-1), biological oxygen demand over 5 days (BOD5; mg.L-1), chemical oxygen demand (COD; mg.L-1), nitrate-nitrogen (NO3-N; mg.L-1), nitrite-nitrogen (NO2–N; mg.L-1), total phosphorus (Total P; mg.L-1), orthophosphorus (PO4-P; mg.L-1), nitrogen Kjeldahl total (NTK; mg.L-1), sulphate (SO4-; mg.L-1).
Plant physicochemical analysis
The fruits samples were analyzed for soluble solids content (SSC; %), titratable acidity (TA; %) [7], and Ca2+, Na+, Mg2+, K+ and nitrate NO3−content. Proteins content were estimated by the method of Bradford (1976) [8]. Vitamin C was determined by using 2.6- diclorofenol indofenol dye titration method [9]. Sugar was estimated by the method of Dey (1990) [10]. Lycopene content was determined according to Alda et al., (2009) [11] with few modifications. The lipid content was determined by the method of AOAC (2002) [12].
Plant Growth Measurements
The leaf length and width, stem length and diameter of aubergine plant in each plot were measured. Also, the number of flowers, number of fruits and fresh weight of the fruit were counted.
Bacteriological analysis
Microbiological analysis of the water and aubergine fruit samples from each experimental treatment included the determination of the parameters: Total coliform, Fecal coliform, Escherichia coli, Fecal streptococci, Vibrio cholera and Salmonella sp, which are useful indicators of contamination [13]. Fecal and total coliform counts were performed using tube fermentation method [6]. Escherichia coli were determined using membrane filter procedure and mTEC Agar (Difco 0334) as described by EPA (2002) [14]. Salmonella were identified using MPN technique [15]. Total Vibrios was detected and enumerated by MPN technique according to Koch (1994) [16] and APHA (2005) [6].
Isolation of mitochondria
The mitochondria of aubergine fruit, roots and leaves were isolated using the method of Romani et al., (1969) [17], with slight modifications. Plant material was homogenized in grinding buffer (0.25 M sucrose, 50 mM potassium phosphate (pH 7.2), 6 mM EDTA, 10 mM β-mercaptoethanol, 0.5% soluble PVP, and bovine serum albumin (BSA) at 1 mg·ml–1). The homogenate was filtered through two layers of cheesecloth and centrifuged at 2000× g for 10 min. The supernatant was further centrifuged at 10,000g for 15min. The pellet was resuspended in Suc wash medium (0.25 M sucrose, 50 mM potassium phosphate (pH 7.2), and BSA at 1 mg·ml–1) to give the final mitochondrial fraction.
Measurement of Succinate dehydrogenase (SDH) Activity
The Succinate dehydrogenase (SDH) (EC 1.3.5.1, complex II) activity of the mitochondrial suspension was measured by the methods of Frenkel and Patterson (1973) [18], with some modifications. Isolated mitochondria were used in a 1-mL reaction mixture containing 100 mM potassium phosphate (pH 7.2), 10 mM KCN, 6 mM phenazine methosulfate, 20 mM succinate, and 0.2 mM dichlorophenol indophenol. The reaction was started by the addition of 100 µl of the mitochondrial preparation to the reaction mixture. SDH activity was determined spectrophotometrically at 600 nm.
Statistical analysis
Results were expressed as average ± SEM (Standard Error Mean) and statistically analyzed using Minitab.16. A statistical analysis was performed by using one-way analysis of variance of (ANOVA) followed by Tukey’s Multiple Comparison Test. Significant differences were considered when p< 0.05.
Results and discussion
Quality Characteristics of water samples (PW, WW and TW) used for irrigation of aubergine fruit
The mean values of the different physicochemical parameters evaluated in the three irrigation waters used are provided in Table1. The physicochemical analysis of potable water (PW) revealed that the pH (7.50) is included in the tolerance range for water intended for irrigation of agricultural fields. The results of this study revealed the total absence of TSS, COD, BOD5 and NTK in potable water. The measured electrical conductivity (1242 µs/cm) remains below the critical threshold of 12000 µs/cm. All other components in potable water are PO4-P, SO4-, NO3-, NO2- and F- are present at relatively low levels and below their respective limit values of 2, 250, 30, 5.3 and finally 1 mg/l.
The results for raw waste water (WW) showed that the pH value falls within the range required for water intended for irrigation (6.5-8.4). While the TSS present exceed by more than double the limit values of the Moroccan standards (441 mg/l) against 200 mg/l considered as the maximum tolerated value. The measured EC shows a relatively high value but remains below the critical thresholds (12000 µs/cm). The COD assessed revealed a very high level of 1471.3 mg/l multiplied by 10 the maximum accepted limit in water used for irrigation of agricultural fields. The same is true of BOD5, which proved to be very high compared to the required limit value of 30 mg/l. For PO4-P, SO4- and NO3- elements, their values exceeded their respective limits of 2 mg/l, 250 mg/l and 30 mg/l. For NO2-, F- their contents remained below the required normative values.
As for the evaluation of the physic o-chemical quality of the treated wastewater (TW), it was pointed out that the hydrogen potential of this water is slightly alkaline (7.28) (Table 1). This pH corresponds perfectly to any agricultural activity since its value is included in the interval required by Moroccan standards. Levels of TSS around 30 mg/l are also tolerated by S.E.E.E, which imposes a maximum threshold of 200 mg/l. The chemical oxygen demand value of 81.4 mg/l indicated in the table remains suitable at the threshold set by the S.E.E.E. Finally [19], sulphate concentrations were found to be slightly higher than the reference values (271.4 >250 mg/l).
The levels of the microorganisms assessed in the three irrigation systems used in our experimental study are provided in Table 2. The microbiological assessment of WW revealed the presence of total coliforms with a load of 35 CFU/100 ml and which were the most abundant of all the bacteria studied, followed by faecal coliforms (20 CFU/100 ml) and finally E.coli with a concentration of 11 CFU/100 ml. However, this water is devoid of vibrio cholerae, fecal steptocoques and salmonella. By analysing the microbial quality of the TW, the latter revealed the presence of only three bacterial species total coliforms (12 CFU/100 ml), fecal coliforms with 8 CFU/100 ml and the least abundant are E. coli with 5 CFU/100 ml.
Table1: Physicochemical analysis in water samples
|
Physicochemical parameters |
PW |
WW |
TW |
Limit values |
|
pH |
7.50 ± 0.05a |
6.94±0.055 c |
7.28±0.7b |
6.5- 8.4* |
|
TSS (mg/l) |
0± 0 a |
441±8.09 c |
27.4± 3.11 b |
200* |
|
EC(µs /cm) |
1242 ±21.25a |
5188 ±47 c |
3920.2± 53.78 b |
12000* |
|
COD (mg/l) |
0±0 a |
1471.3±10.30 c |
81.4± 7.43 b |
90* |
|
BOD5 (mg/l) |
0±0 a |
517.2±9.09 c |
18.17± 6.19 b |
30* |
|
NTK (mg/l) |
0±0 a |
88.5±4.02 c |
19.02± 1.39 b |
- |
|
P Total (mg/l) |
0.0091±0.0002a |
15.8±2.26 c |
9.46± 0.74 b |
- |
|
PO4-P (mg/l) |
0.0089±0.001 a |
9.77 ±0.85 |
4.9± 0.6 b |
2** |
|
SO4- (mg/l) |
90± 4.19 a |
357.8 ±5.21 c |
271.4± 0.47 b |
250* |
|
NO3- (mg/l) |
3.31± 0.15 a |
37.48± 0.77 c |
19.6± 2.03 b |
30* |
|
NO2- (mg/l) |
0.002±0.01 a |
0.09± 0.01 b |
0.03± 0.025 c |
5*, 3** |
|
F- (mg/l) |
0.003± 0.0006 a |
0.57±0.05 c |
0.25± 0.14 b |
1* |
The data are presented as the average ± S.D; different letters indicate significant differences by the Tukey multiple comparison test (P < 0.05). * : (S.E.E.E; 2007) [19]. ** : (FAO; 1992) [1]
Table 2: Microbiological examination of water samples
|
Type of micro-organisms |
PW |
WW |
TW |
Limit values |
|
Total Coliforms (CFU/100 ml) |
0a±0.0 |
35b±0.3 |
12c±0.8 |
1000/100 ml * |
|
Faecal coliforms (CFU/100 ml) |
0a±0.0 |
20b±0.1 |
8c±0.9 |
1000/100 ml * |
|
Escherichia coli (CFU/100 ml) |
0a±0.0 |
11b±0.3 |
5c±0.2 |
- * |
|
Fecal Streptococci (CFU/100 ml) |
0±0.0 |
0±0.0 |
0±0.0 |
- * |
|
Vibrio cholerae (CFU/450 ml) |
0±0.0 |
0±0.0 |
0±0.0 |
Zero per 450 ml * |
|
Salmonella sp (CFU/5000 ml) |
0±0.0 |
0±0.0 |
0±0.0 |
Zero by 5 l * |
The data are presented as the means ± S.D; different letters indicates significant differences from pure water according to Tukey’s test (P < 0.05). * :(WHO; 1989) [20]. ** : Moroccan limit standards [21]
Fruit development and yield
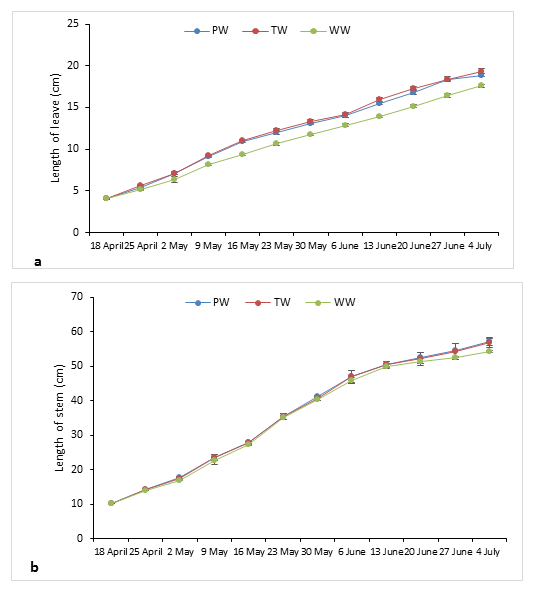
The growth of the aubergine plants studied was represented in Figures 2 and 3. The evolution of the size of the stem showed that the two curves corresponding to the TW and the PW were almost confused not showing any significant difference opposite the curve representing the WW which was slightly below the other two curves; this divergence was all the more perceptible (F= 7.19 and P=0.026) towards the end of the experiment and more precisely from 20 June for a final height of 54.33±1.53 cm for the UW against 56.97±1.74 cm and 57.00±3.00 cm respectively for the TW and the PW.
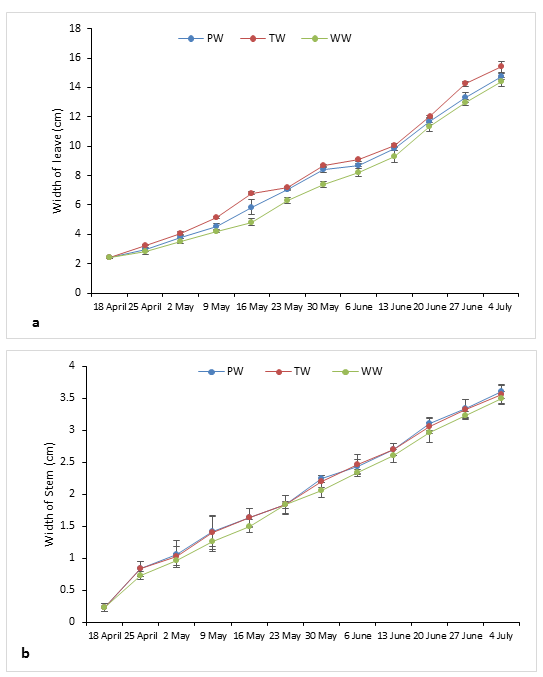
The same observations were made with respect to width as shown in Figure 3 where we notice that the three curves are almost mixed up mainly those of the PW and the TW and the existing difference between the three irrigation waters is of the order of a few millimetres therefore considered statistically insignificant (F= 0.54 and P=0.609).
Concerning the length of the leaves of aubergine, it has known almost the same evolution as that of the length of the stem showing an almost perfect similarity between the two curves corresponding to the irrigations through the PW and the TW. A significant difference
(F= 44.78 and P= 0.000) was observed with respect to the leaves of plants fed with UW whose length increased over time more slowly reaching a final length of 17.6 cm only, unlike the TW and PW, whose values were 19.37 cm and 18.87 cm respectively. Also, at the leaf blade width, significant differences were found between the three leaf blade types with a slight overshoot of the curve representing the TW as the mode of irrigation throughout this crop year compared to the other two curves for WW and PW (F= 8.83 and P=0.016). The foliar area calculated from the last two parameters (leaf length and leaf width) showed slightly higher values (Table 3) in aubergine plants irrigated with TW 55.93±2.10 cm² than those fed with PW 52.50± 0.44 cm² or by UW 51.67±1.37cm². Moreover, the number of leaves produced was on average 91.33±1.53 in the plot irrigated with PW. This number was not very distinct from that obtained in the plot irrigated with TW 90.33±2.31 and even the UW did not influence this parameter because the leaves obtained were almost identical to the other two water treatments with a value of 88.00±1.00 (Table 3).
The other morphological parameters relating to the subterranean vegetative system revealed that the root system generally had a good development regardless of the mode of irrigation used with values of 43.67±3.21 cm equivalent to PW, 43.20±3.65 cm for the TW and 41.67±3.21 cm for the use of the UW.
The yield of the aubergine plants was monitored by measuring the number of flowers, number of fruits, size of the fruit, its biomass and its length. The number of flowers from plants of this species was identical using PW and TW with values of 12.33±0.58 and 12.00±1.00 respectively. This number was significantly smaller when using the WW 10.33±0.58 (Table 3). During fructification, the results reported showed that the number of fruits obtained with the three water treatments was almost identical with the amounts of 8.67±.58, 8.33±1.15 and 7.67±0.58 corresponding respectively to PW, the TW and the WW, based on statistical analysis using the Tukey test. Concerning the fruit sizes, also no significant difference was observed between the three irrigation modes since the existing gap did not exceed 2 mm at the maximum (Table 3). For the last two parameters: biomass and fruit length, no significant differences were recorded when applying the Tukey statistical test. Although the most important biomasses characterized the plants irrigated with PW (209.50± 5.22) g, the fruits obtained with TW as a means of irrigation had lost on average only 2 grams which is considered as negligible and not significant loss (Table 3). In addition, the use of WW, which is the most contaminated of the three irrigation waters, did not influence the yield of this species since the fresh weight of aubergine fruit collected at the time of harvest was 202.33± 5.13 g. With regard to the length of the fruit, the three water-based treatments resulted in a good size of fruit with a difference of one centimetre in length between the three types of fruit, which were not very significant.
Another recent Algerian study [22] which was interested in evaluating the effect of certain salts on the development of eggplant and which showed in the presence of increased saline stress the plant had an adaptive capacity to cope with this imbalance by partially reducing the dimensions of some parts of its vegetative apparatus in order to save maximum energy and guarantee its survival.
Some plants resist salinity by regulating the potential osmotic of cells as a defense strategy. They may accumulate higher concentrations of low molecular weight organic solutes, such as proline, betaine, soluble sugars or certain amino acids, which are generally low in concentration when plants are not under stress. Accumulation of proline under saline stress would protect the cell by balancing the osmotic pressure of the cytosol with the external environment. A positive correlation between proline accumulation and salt tolerance was suggested according to Kumar et al (2003) [23].
Indeed, when plants are confronted with abiotic stress situations (drought or high salinity) and therefore the water potential of the soil becomes too low or subject to biotic stress affecting the root system such as the presence of large quantities of nematodes or bacterial germs, plants try to resist these different stresses and adjust their water potential in order to preserve a good metabolic function. Among the strategies adopted by these plants are: adjusting their intracellular osmotic pressure to maintain good water absorption by accumulating a maximum of osmolytes such as salts and certain organic molecules at the cytosol level which explains the accumulation of Na+, k+, carbohydrates and even proteins at the level of fruits irrigated with TW and even more with the use of WW.
Table 3: The morphological parameters of the vegetative and reproductive system evaluated at the level of aubergine plants subjected to three different water regimes
|
Morphological parameters |
PW |
TW |
WW |
|
|
Vegetative system |
Leaf area (cm²) |
52.50 ± 0.44b |
55.93 ± 2.10a |
51.67 ± 1.37b |
|
Number of leaves |
91.33 ± 1.53a |
90.33 ± 2.31a |
88.00 ± 1.00a |
|
|
Root length (cm) |
43.67 ± 3.21a |
43.20 ± 3.65a |
41.67 ± 3.21a |
|
|
Plant height (cm) |
57.00 ± 3.00a |
56.97 ± 1.74a |
54.33 ± 1.53a |
|
|
Reproductive system |
Number of flowers |
12.33 ± 0.58a |
12.00 ± 1.00a |
10.33 ± 0.58b |
|
Number of fruits |
8.67 ± 0.58a |
8.33 ± 1.15a |
7.67 ± 0.58a |
|
|
Fruit size (cm) |
6.87 ± 0.15a |
6.77 ± 0.25a |
6.67 ± 0.15a |
|
|
Fruit biomass (g) |
209.50 ± 5.22a |
207.40 ± 7.37a |
202.33 ± 5.13a |
|
|
Fruit length (cm) |
16.60 ± 0.17a |
15.83 ± 0.76ab |
15.50 ± 0.50b |
The data are presented as the average± S.D; different letters indicate significant differences by the Tukey multiple comparison test (P < 0.05)
Quality parameters of aubergine fruit from following irrigation with PW, WW and TW
The constitutional aspect of aubergine fruit was based on the determination of the contents of different elements considered as the main indicators of its good quality. The results of this organoleptic composition were reported in Table (4). The majority of its constituents increased with the use of TW and especially with UW, such is the case of soluble solids with a content of 4.45% with control water (PW), and increased slightly with TW (5.87%) and exceeded this rate with the employment of the WW (10.41%). These increases also affected carbohydrates with a concentration of 2 g/100 g with the use of WW compared to PW. Even the lipid content almost doubled when using this raw used water from 0.26g/100g to 0.4g/100 g. Same observations for K+, Na+, Ca2+, Sulfur and Vitamin C, whose loads increased discretely with the TW and increased sharply with the use of the WW. These increases were significant for the following: pH, titrable acidity, soluble solids, carbohydrates, Na+, Ca2+, and sulphur. Other constituents have seen their contents decrease compared to fruit irrigated with PW such as NO3- and Mg2+ which began to gradually decline with TW and WW.
The use of TW, some constituents showed no significant differences following the application of the Turkey multiple comparison test compared to PW, such is the case of lipids, K+, NO3- and Vitamin C.
The five bacteriological parameters assessed at the level of aubergine fruit under three different water regimes were represented in Table 5. A total absence of any bacterial germ was found in fruits belonging to plants irrigated with PW. Using TW, two microbiological genera were found at these organs with the following amounts 2.2±0.2 log CFU/g and 1.66±0.22 log CFU/g corresponding to total and faecal coliforms respectively. As we moved towards contaminated water (WW), the fruits contained more bacteria with levels of 12.7±0.2 log CFU/g, 7.33±0.2 log CFU/g and 1±0.0 log CFU/g respectively for total coliforms, fecal coliforms and Escherichia coli. Differences in bacterial content between the three fruit types were significant as noted in the Table 5. No bacterial colonies of Faecal Streptococcus, Vibrio cholerae or Salmonella were identified in all the fruits studied for any irrigation system.
In a study by Hussain (2020) [24], tomato and aubergine crops used have been shown to have low total coliform contamination and below critical thresholds, unlike radish and spinach species that have shown high microbial loads. These results are perfectly consistent with those obtained during our experimental approach in which the lowest levels of the bacterial communities evaluated were found in aubergine and followed by tomato.
Table 4: Quality parameters of harvested tomato fruit following irrigation with PW, WW and TW
|
PW |
TW |
WW |
|
|
pH |
5.2 ± 0.1a |
5 ± 0.1b |
4.7 ± 0.1c |
|
EC (dS/ m) |
4.2 ± 0.02b |
4.31 ± 0.02ab |
4.4 ± 0.1a |
|
Titratable acidity (%) |
0.172 ± 0.003c |
0.188 ± 0.002b |
0.197 ± 0.002a |
|
Soluble solids (%) |
4.45 ± 0.05c |
5.87 ± 0.04b |
10.41 ± 0.03a |
|
Glucides (g/100g) |
4.03 ± 0.03c |
4.94 ± 0.01b |
6.05 ± 0.01a |
|
Lipids (g /100g) |
0.26 ± 0.01b |
0.31 ± 0.03b |
0.4 ± 0.04a |
|
Proteins (g /100g) |
1.22 ± 0.03ab |
1.26 ± 0.04a |
1.17 ± 0.02b |
|
K+ (mg/100 g) |
121 ± 1b |
123 ± 2b |
129 ± 2a |
|
Na+ (mg/100 g) |
6.8 ± 0.2c |
7.3 ± 0.2b |
8.1 ± 0.1a |
|
Ca2+ (mg/100 g) |
21.3 ± 0.3c |
23.2 ± 0.1b |
25.9 ± 0.1a |
|
NO3- (mg/100 g) |
17.6 ± 0.2a |
17.3 ± 0.1a |
16.8 ± 0.2b |
|
Mg2+ (mg/100 g) |
14,7 ± 0,05a |
14,2 ± 0,1b |
13,84 ± 0,01c |
|
Sulfur (mg/100 g) |
9 ± 0.03c |
9.12 ± 0.01b |
9.17 ± 0.01a |
|
Vitamine C (mg/100 g) |
1.28 ± 0.01b |
1.31 ± 0.01b |
1.6 ± 0.05a |
The data are presented as the average± S.D; different letters indicate significant differences by the Tukey multiple comparison test (P < 0.05)
Table 5: Bacteriological parameters in aubergine fruit samples according to the pure water (PW), treated wastewater (TW) and waste water (WW) irrigation
|
Bacteriological parameters (log CFU/g) |
Irrigation Treatment |
||
|
PW |
TW |
WW |
|
|
Total coliform |
00±0.0c |
2.2±0.2b |
12.7±0.2a |
|
Fecal coliform |
00±0.0c |
1.66±0.22b |
7.33±0.25a |
|
Escherichia coli |
00±0.0c |
00±0.0b |
1±0.0a |
|
Fecal Streptococcus |
00±0.0 |
00±0.0 |
00±0.0 |
|
Vibrio cholerae |
00±0.0 |
00±0.0 |
00±00 |
|
Salmonella sp |
Absent |
Absent |
Absent |
The data are presented as the average± S.D; different letters indicate significant differences by the Tukey multiple comparison test (P < 0.05)
Enzymatic activity of SDH in roots, leaves and fruits
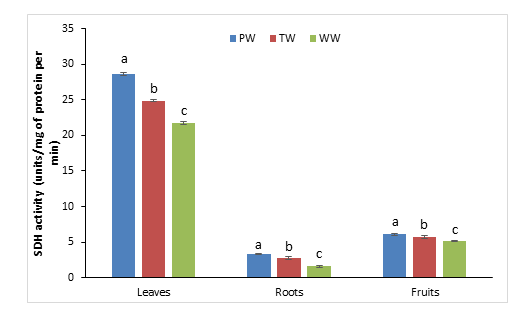
The Succinate dehydrogenase (SDH) activity was measured at the level of the fruit, the subterranean vegetative apparatus (roots) and in the foliar system with the use of three different waters PW, TW and WW and the results of this biochemical analysis are shown in Figure 4. Slight fluctuations of SDH activity in fruit and roots were observed during the change in water treatment and were considered statistically significant following the application of the Fisher test. At the leaf level, these differences are even more significant with enzymatic activities ranging from 21.8 with WW to 28.6 (unit/mg of protein per min) in the presence of PW. In the case of aubergine as in the other species the strongest enzymatic activities concerned the leaves and the weakest of them were observed in the root system.
A recent study on aubergine under saline stress [25] revealed that the measured levels of soluble sugars in this species are too low to have a relevant osmotic effect, and they only increase slightly with increased salinity. This same study showed that the levels of malonedialdehyde (MDA) which is a product of lipid membrane peroxidation and thus a reliable marker of oxidative stress [26] did not increase at all in aubergine in response to salt treatments, a slight change occurred only in the presence of 300 mM of NaCl. Even the H2O2 levels of the leaves showed no variation in response to salt treatment. This proves that these experimental conditions did not generate oxidative stress in this salt-treated species and this is probably due to the relatively high tolerance of this species and in particular its genotype which is predisposed to withstand this kind of stress.
Different letters indicate significant differences by the Tukey multiple comparison test (P < 0.05)
Conclusion
Our results show clearly that treated wastewater can be used as crop water supply of aubergine. The aubergine yield using irrigation with TW was found important when compared to yields from crops using PW and WW in addition, the microbiological quality of the aubergine was generally maintained. The observed peaks of microbial contamination were recorded on harvested plants irrigated with WW. The aubergine fruit irrigated with TW retained high mitochondrial SDH activity as compared with WW.
Conflicts of Interest
The authors declare no conflict of interest.
References
- InstitutionalAuthorName, FA. FAO Production Yearbook Food and Agriculture Organization Rome (1998).
- Kalavrouziotis IK, Kokkinos P, Oron G, et al. Current status in wastewater treatment, reuse and research in some Mediterranean countries. Desalination and Water Treatment 53 (2015): 2015-2030.
- Ilias A, Panoras A, Angelakis A. Wastewater recycling in Greece: The case of Thessaloniki. Sustainability 6 (2014): 2876-2892.
- Chen W, Bai Y, Zhang W, et al. Perceptions of different stakeholders on reclaimed water reuse: the case of Beijing, China. Sustainability 7 (2015): 9696-9710.
- Ouansafi S, Abdelilah F, Kabine M, et al. The effects of soil proprieties on the yield and the growth of tomato plants and fruits irrigated by treated wastewater. AIMS Agriculture and Food 4 (2019): 921-938.
- Eaton AD, Clesceri LS, Rice EW, et al. APHA: standard methods for the examination of water and wastewater. Centennial Edition., APHA, AWWA, WEF, Washington, DC (2005).
- AOAC (Association of Official Analytical Chemists). Official methods of analysis of AOAC international.16th Edn (1995).
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72 (1976): 248-254.
- Ranganna S. Handbook of analysis and quality control for fruit and vegetable products. Tata McGraw-Hill Education (1986).
- Dey PM. Oligosaccharides. InMethods in Plant Biochemistry 2 (1990): 189-218.
- Alda LM, Gogoasa I, Bordean DM, et al. Lycopene content of tomatoes and tomato products. Journal of Agroalimentary Processes and Technologies 15 (2009): 540-542.
- AOAC (Association of Official Analytical Chemists) Official methods of analysis of AOAC international. In: Horwitz W (ed.), 17th Edn., (2002) Gaithersburg, MD: AOAC International, USA, 2200.
- Tallon P, Magajna B, Lofranco C, et al. Microbial indicators of faecal contamination in water: a current perspective. Water, Air, and Soil Pollution 166 (2005): 139-166.
- EPA (Environmental Protection Agency) Method 1103.1: Escherichia coli (E. coli) in water by membrane filtration using membrane-thermotolerant Escherichia coli agar (mTEC). Report EPA 821-R-02-023. Office of Water Environmental Protection, Washington DC (2002).
- El-Lathy AM, El-Taweel GE, El-Sonosy MW, et al. Determination of pathogenic bacteria in wastewater using conventional and PCR techniques. Environmental Biotechnology 5 (2009): 73-80.
- Koch A. Growth measurement. Methods for General and Molecular Bacteriology (1994): 248-277.
- Romani RJ, Ida KY, Fisher LK. Isolation of tightly coupled mitochondria from acidic plant tissues. Plant Physiology 44 (1969): 311.
- Frenkel C, Patterson ME. Effect of carbon dioxide on activity of succinic dehydrogenase in ‘Bartlett’pears during cold storage. HortScience 8 (1973).
- E.E.E (State Secretariat at the Ministry of Energy, Mines, Water and Environment). Dirctorate of Research and Water Planning, Water quality standards for water irrigation, Rabat (2007).
- WHO( World Health Organization).Health guidelines for the use of wastewater in agriculture and aquaculture: report of a WHO scientific group (1989) [meeting held in Geneva from 18 to 23 November 1987].
- Moroccan limit standards for water used to irrigation.
- Ahmed ZO, Ines ZA, Ahmed SS. Évaluation De L’effet De Deux Sels Nocifs (Nacl Et Na2so4) Sur Quelques Paramètres Morpho-Physiologique De L’aubergine Cultivée En Hors Sol. Agrobiologia 9 (2019): 1405-1414.
- Kumar SG, Reddy AM, Sudhakar C. NaCl effects on proline metabolism in two high yielding genotypes of mulberry (Morus alba L.) with contrasting salt tolerance. Plant Science 165 (2003): 1245-1251.
- Hussain MI, Qureshi AS. Health risks of heavy metal exposure and microbial contamination through consumption of vegetables irrigated with treated wastewater at Dubai, UAE. Environmental Science and Pollution Research 27 (2020): 11213-11226.
- Brenes M, Pérez J, González-Orenga S, et al. Comparative studies on the physiological and biochemical responses to salt stress of eggplant (Solanum melongena) and its rootstock S. torvum. Agriculture 10 (2020): 328.
- Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutrition, Metabolism and Cardiovascular Diseases 15 (2005): 316-328.
- Pescod MB. Wastewater treatment and use in agriculture (1992).

 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 