High-Risk Locoregional Renal Cell Cancer: S-TRAC Criteria for the Selection of Adjuvant Treatment Candidates
Article Information
Jennifer Brasero Burgos1*, Victoria Gómez Dos Santos1, Sara Álvarez Rodríguez1, Pablo Gajate Borau2, David Esteban Díaz Pérez1, Javier Lorca Álvaro1, Marta Santiago González1, Víctor Díez Nicolás1, Vital Hevia Palacios1, Francisco Javier Burgos Revilla1
1Urology Department, Ramón y Cajal Hospital, IRYCIS, Madrid, Spain
2Medical Oncology Department, Ramón y Cajal Hospital, IRYCIS, Madrid, Spain
*Corresponding Author: Victoria Gómez Dos Santos, Head of Renal Surgery and Transplantation Unit. Urology Department. Ramón y Cajal Hospital. Alcalá University. IRYCIS. Colmenar Viejo Road, km. 9, 100, 28034, Madrid, Spain
Received: 05 September 2019;Accepted: 20 September 2019;Published: 24 September 2019
Citation: Brasero-Burgos J, Gómez-Dos-Santos V, Álvarez-Rodríguez S, Gajate-Borau P, Díaz-Pérez DE, Lorca-Álvaro J, Santiago-González M, Díez-Nicolás V, Hevia-Palacios V, Burgos-Revilla FJ. High-Risk Locoregional Renal Cell Cancer: S-TRAC Criteria for the Selection of Adjuvant Treatment Candidates. Journal of Surgery and Research 2 (2019): 205-216.
View / Download Pdf Share at FacebookAbstract
Introduction and objective: Clear-cell renal-cell carcinoma (RCCcc) is the genitourinary neoplasm with the highest mortality rate despite primary surgical treatment, which highlights the necessity for adjuvant treatment. To date, only the use of sunitinib in the S-TRAC study (Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy) has shown improvement of disease-free survival (DFS). The aim of the present study is to identify, using a single-center sample of patients with locally advanced RCC, potential candidates for adjuvant treatment by the application of the S-TRAC criteria.
Material and methods: We enrolled patients undergoing oncological nephrectomy from 2009 to 2014. We selected and stratified the patients according to the S-TRAC criteria. DFS and overall survival (OS) were analyzed.
Results: Forty-eight patients out of the 153 (31.4%) previously selected patients met the S-TRAC inclusion criteria. DFS and OS at 5 years were 73.0% and 71.4%, respectively. According to the UISS prognostic model, 85.4%, 4.1% and 6.2% of our patients were assigned to groups A2, B and C, respectively. DFS in these groups was 73%, 100% and 100%, respectively. OS was 76% in group A2 and 67% in group C. No deaths were observed in group B.
Conclusions: At 5 years, a higher proportion of patients in the present study were disease-free (73.0%) compared to the placebo group of the S-TRAC clinical trial (51.3%). Efforts should be focused on the identification of precise prognostic models in order to identify high-risk candidates to receive adjuvant treatment.
Keywords
Renal cancer, Adjuvant treatment, Sunitinib, Risk prognostic models
Renal cancer articles, Adjuvant treatment articles, Sunitinib articles, Risk prognostic models articles
Renal cancer articles Renal cancer Research articles Renal cancer review articles Renal cancer PubMed articles Renal cancer PubMed Central articles Renal cancer 2023 articles Renal cancer 2024 articles Renal cancer Scopus articles Renal cancer impact factor journals Renal cancer Scopus journals Renal cancer PubMed journals Renal cancer medical journals Renal cancer free journals Renal cancer best journals Renal cancer top journals Renal cancer free medical journals Renal cancer famous journals Renal cancer Google Scholar indexed journals Adjuvant treatment articles Adjuvant treatment Research articles Adjuvant treatment review articles Adjuvant treatment PubMed articles Adjuvant treatment PubMed Central articles Adjuvant treatment 2023 articles Adjuvant treatment 2024 articles Adjuvant treatment Scopus articles Adjuvant treatment impact factor journals Adjuvant treatment Scopus journals Adjuvant treatment PubMed journals Adjuvant treatment medical journals Adjuvant treatment free journals Adjuvant treatment best journals Adjuvant treatment top journals Adjuvant treatment free medical journals Adjuvant treatment famous journals Adjuvant treatment Google Scholar indexed journals Sunitinib articles Sunitinib Research articles Sunitinib review articles Sunitinib PubMed articles Sunitinib PubMed Central articles Sunitinib 2023 articles Sunitinib 2024 articles Sunitinib Scopus articles Sunitinib impact factor journals Sunitinib Scopus journals Sunitinib PubMed journals Sunitinib medical journals Sunitinib free journals Sunitinib best journals Sunitinib top journals Sunitinib free medical journals Sunitinib famous journals Sunitinib Google Scholar indexed journals Risk prognostic models articles Risk prognostic models Research articles Risk prognostic models review articles Risk prognostic models PubMed articles Risk prognostic models PubMed Central articles Risk prognostic models 2023 articles Risk prognostic models 2024 articles Risk prognostic models Scopus articles Risk prognostic models impact factor journals Risk prognostic models Scopus journals Risk prognostic models PubMed journals Risk prognostic models medical journals Risk prognostic models free journals Risk prognostic models best journals Risk prognostic models top journals Risk prognostic models free medical journals Risk prognostic models famous journals Risk prognostic models Google Scholar indexed journals adjuvant treatment articles adjuvant treatment Research articles adjuvant treatment review articles adjuvant treatment PubMed articles adjuvant treatment PubMed Central articles adjuvant treatment 2023 articles adjuvant treatment 2024 articles adjuvant treatment Scopus articles adjuvant treatment impact factor journals adjuvant treatment Scopus journals adjuvant treatment PubMed journals adjuvant treatment medical journals adjuvant treatment free journals adjuvant treatment best journals adjuvant treatment top journals adjuvant treatment free medical journals adjuvant treatment famous journals adjuvant treatment Google Scholar indexed journals cancer survival rates articles cancer survival rates Research articles cancer survival rates review articles cancer survival rates PubMed articles cancer survival rates PubMed Central articles cancer survival rates 2023 articles cancer survival rates 2024 articles cancer survival rates Scopus articles cancer survival rates impact factor journals cancer survival rates Scopus journals cancer survival rates PubMed journals cancer survival rates medical journals cancer survival rates free journals cancer survival rates best journals cancer survival rates top journals cancer survival rates free medical journals cancer survival rates famous journals cancer survival rates Google Scholar indexed journals Renal cell carcinoma articles Renal cell carcinoma Research articles Renal cell carcinoma review articles Renal cell carcinoma PubMed articles Renal cell carcinoma PubMed Central articles Renal cell carcinoma 2023 articles Renal cell carcinoma 2024 articles Renal cell carcinoma Scopus articles Renal cell carcinoma impact factor journals Renal cell carcinoma Scopus journals Renal cell carcinoma PubMed journals Renal cell carcinoma medical journals Renal cell carcinoma free journals Renal cell carcinoma best journals Renal cell carcinoma top journals Renal cell carcinoma free medical journals Renal cell carcinoma famous journals Renal cell carcinoma Google Scholar indexed journals cancer-specific survival articles cancer-specific survival Research articles cancer-specific survival review articles cancer-specific survival PubMed articles cancer-specific survival PubMed Central articles cancer-specific survival 2023 articles cancer-specific survival 2024 articles cancer-specific survival Scopus articles cancer-specific survival impact factor journals cancer-specific survival Scopus journals cancer-specific survival PubMed journals cancer-specific survival medical journals cancer-specific survival free journals cancer-specific survival best journals cancer-specific survival top journals cancer-specific survival free medical journals cancer-specific survival famous journals cancer-specific survival Google Scholar indexed journals tumor suppressor articles tumor suppressor Research articles tumor suppressor review articles tumor suppressor PubMed articles tumor suppressor PubMed Central articles tumor suppressor 2023 articles tumor suppressor 2024 articles tumor suppressor Scopus articles tumor suppressor impact factor journals tumor suppressor Scopus journals tumor suppressor PubMed journals tumor suppressor medical journals tumor suppressor free journals tumor suppressor best journals tumor suppressor top journals tumor suppressor free medical journals tumor suppressor famous journals tumor suppressor Google Scholar indexed journals tumor histology articles tumor histology Research articles tumor histology review articles tumor histology PubMed articles tumor histology PubMed Central articles tumor histology 2023 articles tumor histology 2024 articles tumor histology Scopus articles tumor histology impact factor journals tumor histology Scopus journals tumor histology PubMed journals tumor histology medical journals tumor histology free journals tumor histology best journals tumor histology top journals tumor histology free medical journals tumor histology famous journals tumor histology Google Scholar indexed journals
Article Details
1. Introduction
Renal cell carcinoma (RCC) accounts for 2-3% of all neoplasms [1]. An increase in incidence has been seen during the last decade because of a greater incidental diagnosis in imaging tests performed for another reason [2]. Risk factors have been identified, which include smoking, hypertension and obesity [3]. The three main types of RCC are clear cell (RCCcc), papillary type I and II (RCCp) and chromophobe (RCCch) [4]. RCCcc is the most frequent type and has the worst prognosis [5]. According to the TNM classification, the specific cancer survival rates for RCCcc are 91%, 74%, 67% and 32% for stages I, II, III and IV, respectively [6]. Thus, locally advanced RCCcc is one of the most lethal genitourinary malignancies, and surgery is its only curative treatment [7]. However, the risk of recurrence after nephrectomy reaches 40%, with OS at 5 years of 30% at stage T4 and 50% in the case of lymphatic involvement [3, 8].
To improve these results, the role of adjuvant treatments after surgery is being investigated to eliminate possible foci of clinically undetectable micrometastases [7]. Several adjuvant strategies, including cytokine therapy, radiotherapy, and hormone therapy, have been explored to decrease the rate of relapse, but none have been successful. The proven efficacy of antiangiogenic therapies, including the vascular endothelial growth factor (VEGF) pathway inhibitors in patients with metastatic renal-cell carcinoma (mRCC) supports the evaluation of these drugs as adjuvant therapy [7].
In 2016, the S-TRAC study demonstrated an improvement in DFS in patients with locally advanced RCCcc who were treated with adjuvant sunitinib compared to those treated with placebo for 1 year after nephrectomy. This study observed only a moderate worsening in the quality of life in the patients who received sunitinib [9]. However, the other studies with VEGF inhibitors have obtained negative results, so the use of sunitinib continues to be controversial. The aim of the present study was to identify patients at high risk of recurrence according to S-TRAC criteria and select potential candidates with locally advanced RCC for adjuvant treatment in a single-center sample.
2. Materials and Methods
We retrospectively reviewed hospital surgical and clinical records from January 2009 to December 2014. The study was approved by the hospital ethics committee and was conducted in accordance with International Conference on Harmonisation Good Clinical Practice guidelines and applicable local regulatory requirements and laws. Eligible patients were at least 18 years of age and had received a diagnosis of locoregional RCC (tumor stage 3 or higher, regional lymph-node metastasis, or both). Other eligibility criteria included histologic confirmation of RCCcc, no previous systemic treatment and a score of no more than 2 on the Eastern Cooperative Oncology Group (ECOG) scale before nephrectomy. Patients were stratified into risk groups according to the 2002 UCLA Integrated System (UISS) prognostic model:
- Group A: Stage 3 (T3), no or undetermined nodal involvement (N0/Nx), no metastasis (M0).
- A1: Low risk. Includes any Fuhrman grade and an ECOG score of 0 or Fuhrman grade 1 and an ECOG ≥ 1.
- A2: High risk. Includes Fuhrman grade 2 or higher and an ECOG score ≥ 1
- Group B: Stage 4 tumor (T4), N0/Nx, M0.
- Group C: Any tumor stage, locoregional nodal involvement (N1), M0.
Follow-up after nephrectomy was performed in accordance with the European Clinical Guidelines on RCC and included thoracoabdominal-pelvic CT with a periodicity according to the risk group. In the case of high-risk RCC, CT was performed at 6 months after surgery and then annually for up to 5 years. After 5 years, CT was performed every 2 years. A descriptive study of patients’ baseline and oncological characteristics was performed. Continuous variables were expressed as the mean and confidence interval or median and range, as required, and categorical variables as the number and percentage. DFS and OS were assessed using the Kaplan-Meir method.
3. Results
We collected data about 153 patients subjected to nephrectomy for RCC between 2009 and 2014. 48 patients out of 153 (31.4%) met S-TRAC eligibility criteria and were subsequently selected and classified according to UISS categories. Two patients were not classifiable due to lack of complete data. Radical surgery was performed in 40 patients (81.6%) while partial nephrectomy was performed in the remaining 8 patients (16.3%). Table 1 shows study patients’ baseline and oncological characteristics and their risk stratification according to the UISS classification. Additionally, Table 1 includes placebo- and sunitinib-treated patients’ characteristics from the S-TRAC clinical trial.
Our present study includes patients older than S-TRAC, with a similar sex distribution and with an ECOG score that includes a higher percentage of the ≥ 2 group. Regarding risk profile, the percentage of patients assigned to the highest risk group (Group C) was slightly lower than in that in the placebo- and sunitinib-treated populations in the S-TRAC trial. The mean time of follow-up was 4.6 (SD 2.42) years, which was shorter than the median duration of DFS in the sunitinib and placebo groups (6.8 years and 5.6 years, respectively) in the S-TRAC clinical trial. The median time of follow-up for DFS and OS were not reached in the present study (Figure 1 and 2).
|
Characteristics n (%) |
Sample of our |
S-TRAC |
|
|
Placebo (n=306) |
Sunitinib (n=309) |
||
|
Age |
|||
|
Mean (min-max) |
64 (30-89) |
58 (21-82) |
57 (25-83) |
|
18-64 (%) |
23 (47.9) |
224 (73.2) |
233 (75.4) |
|
≥ 65 (%) |
25 (52) |
82 (26.8) |
76 (24.6) |
|
Sex (%) |
|||
|
Male |
38 (77.6) |
229 (74.8) |
222 (71.8) |
|
Female |
10 (20.4) |
77 (25.2) |
87 (28.2) |
|
ECOG (%) |
|||
|
0 |
30 (62.5) |
220 (71.9) |
228 (73.8) |
|
1 |
13 (27.1) |
84 (27.5) |
79 (25.6) |
|
≥2 |
2 (4.2) |
0 |
1 (0.3) |
|
Unknown |
3 (6.2) |
2 (0.7) |
1 (0.3) |
|
UISS Classification (%) |
|||
|
A |
41 (85.4) |
278 (90.8) |
280 (90.6) |
|
. A1 |
. 0 |
. 112 (36.6) |
. 115 (37.2) |
|
. A2 |
. 41 (85.4)* |
. 166 (54.2) |
. 165 (53.4) |
|
B |
2 (4.1)** |
4 (1.3) |
4 (1.3) |
|
C |
3 (6.2)*** |
24 (7.8) |
25 (8.1) |
|
Not classifiable |
2 (4.1) |
0 |
0 |
*Consisting of 34 (83%) patients pT3aN0, 5 (12%) pT3bN0 and 2 (5%) pT3c according to the TNM classification; **Consisting of 2 (100%) T4N0 patients according to the TNM classification; *** Consisting of 2 (67%) patients pT1aN1 and 1 (33%) pT3bN2 according to the TNM classification
Table 1: Baseline characteristics.
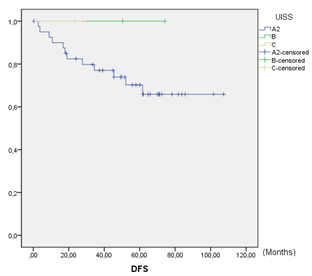
Figure 1: Disease-free survival.
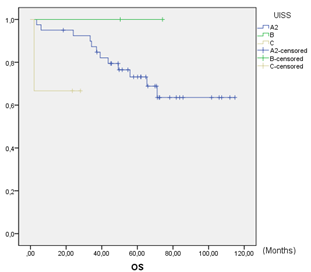
Figure 2: Overall survival.
DFS at 5 years was 73.0% (Figure 1). In the global series, 27% (13/48) of patient relapses were observed. Of the relapses, 23.1% (3/13) were local relapses, 61.5% (8/13) were distance relapses and finally, 15.4% (2/13) presented both local and distance recurrences. DFS in UISS groups A2, B and C were 73%, 100% and 100%, respectively. Global OS at 5 years was 71.4%. OS was 76% in group A2 and 67% in group C. No deaths were observed in group B. No statistically significant differences were observed among the UISS groups (Figure 2). A total of 28.6% (14/48) of patients died during follow-up, and 35.7% (5/14) of the deaths were due to recurrence and progression of the RCC. The remaining 64.3% (9/14) deaths were due to other non-oncological causes. Both oncological and non-oncological deaths were similarly distributed over time. The median cancer-specific survival (CSS) was 3.25 (SD 0.67) years.
4. Discussion
Despite progress in the systemic treatment of advanced RCC over the last decade, it remains one of the most lethal genitourinary malignancies. OS rates at 5 years for patients with locally advanced or lymph-node positive disease remain at 53%, and this OS rate drops to 8% for those with T4 stage disease or with distant metastases. Surgery is the primary treatment for locally advanced RCC. To improve on these outcomes, effective adjuvant therapy is needed for high-risk RCC [7, 10]. In our series, only 27% of the patients with nonmetastatic RCC, considered at high risk according to the UISS prognostic model, presented local or distant recurrence. This percentage was clearly lower than the recurrence rate in the placebo populations in published TKIs clinical trials on advanced RCC. The therapeutic landscape for RCC has changed dramatically with the introduction of targeted molecular therapies. Much of this progress is attributed to characterization of the von Hippel-Lindau (VHL) tumor suppressor and its role in the carcinogenesis of ccRCC. Bi-allelic loss of VHL leads to unmitigated activation of hypoxia-inducible factor (HIF)-1α, which serves as a transcription factor for pro-angiogenic genes in a tumor cell, including for VEGF and platelet-derived growth factor. This sequence promotes angiogenesis to facilitate tumor growth and progression. Overproduction of HIF-1α can also occur due to hyperactive signaling via the mammalian target of rapamycin (mTOR) pathway, which may occur independently of VHL loss [7].
Drugs exploiting these two mechanistically unique pathways, referred to as targeted therapy, are available and have been successfully used in treating mRCC. Targeted agents that are currently Food and Drug Administration (FDA)-approved for mRCC include six inhibitors of the VEGF receptor (sunitinib, sorafenib, pazopinib, axitinib, cabozantinib and lenvatinib), two mTOR inhibitors (everolimus and temsirolimus), and one VEGF inhibitor (bevacizumab, in combination with interferon-α [IFN-a]). Several clinical trials have been conducted to test most of these drugs in the context of loco-regional advanced RCC after surgery [7]. The identification of patients who are at increased risk of relapse is key for the development of rational adjuvant strategies. A number of predictive models have been developed to accomplish this goal. These models all incorporate widely available, easily obtainable, clinicopathological variables that are associated with prognosis following surgery (Table 2) [11, 12]. Variables that showed the greatest predictive capacity in DFS and CSS were TNM stage, Furhman nuclear grade and tumor size. We selected the UISS model because it has been one of the most commonly used models, and more specifically, in the S-TRAC clinical trial the UISS model was unique with a positive result regarding DFS for adjuvant treatment.
The UISS model includes two tumor-specific features, namely, TNM stage and Fuhrman grade, together with a patient specific feature, namely, the ECOG performance status. This combination of features stratifies patients into low-risk, intermediate-risk and high-risk prognostic categories. In patients with non-metastatic disease, the application of the UISS model correctly predicted 2-year and 5-year survival values regardless of tumor histology in 76.5-86.3% of patients [13]. In recent years, several trials utilizing different VEGF TKIs have been completed and reported conflicting results regarding clinical benefit and patient safety. Table 3 summarizes the main characteristics of these trials [9, 14, 15].
*0.8 abdominal and bone metastases; 0.82 thoracic metastases
Table 2: Postoperative predictive nomograms of recurrence in nonmetastatic RCC.
*44% sunitinib group and 45% sorafenib group had to stop treatment because of toxicity; 55% of patients had to reduce doses due to adverse effects grade ≥ 3; ** 28% had to interrupt treatment because of toxicity; 34% of patients had to reduce doses due to adverse effects grade ≥ 3; *** The dose of the study had to be modified to 600 mg due to high toxicity with 800 mg
Table 3: ASSURE, S-TRAC, and PROTECT characteristics.
Overall, antiangiogenics did not improve DFS (HR 0.92, 95% CI 0.78-1.07) or OS (HR 0.99, 95% CI 0.79-1.25) when compared to placebo in postnephrectomy patients with nonmetastatic RCC. Similarly, DFS was comparable between the two groups (HR 0.89, 95% CI 0.78-1.02) when examining the effect of VEGF TKIs in the subsets of patients with the highest risk of relapse as reported by the individual trials PROTECT, ASSURE, or S-TRAC (PROTECT: pT2 G3-4 N0, pT3-T4 G any N0, or pT any G any N1; ASSURE 2017: pT3, pT4 or node-positive disease: and S-TRAC 2017: T3, no or undetermined nodal involvement, Fuhrman grade 2, and ECOG performance score 1 or T4 and/or nodal involvement) [7, 9, 14, 15].
Finally, in 2018, the Axitinib Versus Placebo in Patients at High Risk of Recurrent Renal Cell Carcinoma (ATLAS) study was stopped owing to futility at a preplanned interim analysis. This study included patients with RCCcc ≥ pT2 and/or N+, Gany and ECOG 0-1. The available data showed no significant difference in DFS according to the independent review committee (IRC) assessment (HR 0.870, 95% CI 0.660-1.147; p=0.3211). In the highest-risk subpopulation, a 36% and 27% reduction in risk of a DFS event with axitinib was observed in the investigator assessment (HR 0.641, 95% CI 0.468–0.879; p=0.0051) and IRC assessment (HR 0.735, 95% CI 0.525-1.028; p=0.0704), respectively. The incidence of adverse effects was similar in both groups, although the toxicity ≥ 3 degree was greater in the axitinib group (61% vs. 30%). The OS data were not mature [16].
Only the S-TRAC trial showed a clinical and statistically significant reduction of 24% in the occurrence of recurrence events in comparison to placebo [9, 10]. In light of the positive results of S-TRAC, the Food and Drug Administration (FDA) approved sunitinib as adjuvant therapy in high-risk RCC in November 2017 [7]. However, the European Medicines Agency (EMA) did not view the results in the same way [17], and controversy regarding the benefit of sunitinib in the adjuvant setting continues. Two studies evaluated the efficacy of sunitinib versus placebo in RCC patients. In a meta-analysis of these two trials (ASSURE 2016 and S-TRAC 2016), sunitinib did not show any improvement in the overall cohort for either DFS (HR 0.89, 95% CI 0.67-1.19) or OS (HR 1.11, 95% CI 0.90-1.37) [18, 19]. However, it is important to note the high heterogeneity, which is most likely secondary to the differences in design and study populations between ASSURE and S-TRAC, mainly the inclusion of fewer high-risk patients in ASSURE (T1b) compared to S-TRAC (>T3). In addition, approximately 20% of the patients included in ASSURE had non-clear cell histology compared to mainly clear-cell RCC in S-TRAC (Table 3).
To evaluate if S-TRAC results could be applied to our actual RCC population and, consequently, to consider sunitinib adjuvant treatment after surgery for high-risk patients with loco-regional, advanced, nonmetastatic disease, we performed a risk assessment by means of a UISS prognostic model, and DFS and OS were assessed. DFS at 5 years in our series was 73%, which was greater than in the previously described clinical trial where DFS in the placebo population varied from 51.3 to 56.4% and 64.0% in the S-TRAC, ASSURE and PROTECT trials, respectively [9, 14, 15]. One possible explanation could be the smaller number of patients included in the present study. Nevertheless, we are obliged to identify in our RCC population the very high-risk patients who would benefit from adjuvant treatment or inclusion in a clinical trial and who would probably correspond to those with lymph node metastases.
Among the possible reasons for failure of VEGF TKIs in the adjuvant setting, poor tolerability and risk of non-adherence to treatment or treatment withdrawal are major issues in potentially cured patients that could result in an excess number of dose reductions and treatment pauses and ultimately lead to a suboptimal dose intensity of the adjuvant treatment. A systematic review comparing the ASSURE, S-TRAC and PROTECT data showed an association to a higher risk of grade 3-4 toxicity compared to placebo (64.3% vs. 22.7%, HR 2.74, 95% CI 2.49-3.03). Treatment suspension rates varies among the different clinical trials from 23% (ATLAS) to 45% (ASSURE). The most frequent VEGF TKIs adverse events were hand-foot syndrome, diarrhea, hypertension, increased transaminases and fatigue [21, 22].
Beyond comparing the results of the studies presented and that of our sample, what remains as the main problem in the context of the targeted therapies is the proper selection of patients. It is crucial to identify patients who are truly going to benefit from adjuvant treatment and to achieve balance between clinical benefit and the avoidance of overtreatment and unnecessary adverse effects. This premise has led our center to a policy of action guided by a multidisciplinary team composed of urologists and medical oncologists (with joint consultation and their own agendas) and supported by the clinical trials unit. The strategy of our group, as recommended by the different clinical guidelines, is to prioritize the inclusion of patients in clinical trials and relegate the use of sunitinib to very high-risk patients (groups B and/or C of the UISS) with a comorbidity-favorable profile and in the absence of available trials. Two ongoing post-nephrectomy RCTs are evaluating the efficacy of adjuvant sorafenib therapy (SORCE study) and everolimus therapy (EVEREST). However, given the disappointing findings discussed above, positive results seem unlikely [22].
4. Conclusion
The management of RCCcc, especially in advanced stages, is a challenge because it represents a disease with a high risk of recurrence and mortality. To date, only the use of sunitinib as an adjuvant treatment has been approved by the FDA after the results of the S-TRAC study. In our study, only 27% of the selected patients based on the UISS model had tumor recurrences. Future studies should potentially focus on identifying patients at higher risk of relapse on the basis of clinicopathological and molecular biomarkers that improve classification accuracy of actual prognostic models. Taking into consideration the controversial results of clinical trials and the significant toxicity of VEGF TKIs, there is currently insufficient evidence for the use of VEGF TKIs in the adjuvant setting in patients with advanced RCC after nephrectomy. Novel immune checkpoint inhibitors hold promise for the adjuvant therapy of RCC. However, improved patient selection and stratification, use of active, biology-driven treatments and improved management of therapy are required to prevent failure of these and other novel agents in the future. Finally, multidisciplinary management of all patients with RCC is mandatory.
Acknowledgements
The authors acknowledge the kidney, transplant and research section of our hospital for their help and support.
Conflict of Interests
There is no conflict of interest to declare.
References
- European Network of Cancer Registries: Eurocim version 4.0. Lyon, France (2001).
- Lindblad P. Epidemiology of renal cell carcinoma. Scand J Surg 93 (2004): 88.
- Janowitz T, Welsh SJ, Zaki K, et al. Adjuvant therapy in renal cell carcinoma-past, present, and future. SeminOncol 40 (2013): 482-449.
- Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. EurUrol 70 (2016): 93.
- Beck SD, Patel MI, Snyder ME, et al. Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann SurgOncol 11 (2004): 71.
- Tsui KH, Shvarts O, Smith RB, et al. Prognostic indicators for renal cell carcinoma: a multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol 163 (2000): 1090.
- Meissner MA, McCormick BZ, Karam JA, et al. Adjuvant therapy for advanced renal cell carcinoma. Expert Review of Anticancer Therapy 18 (2018): 663-671.
- Bazzi WM, Sjoberg DD, Feuerstein MA, et al. Long-term survival rates after resection for locally advanced kidney cancer: Memorial Sloan Kettering Cancer Center 1989 to 2012 experience. The Journal of urology 193 (2015): 1911-1916.
- Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Enl J Med M 375 (2016): 23.
- Staehler M, Motzer RJ, George DJ, et al. Adjuvant sunitinib in patients with high-risk renal cell carcinoma: safety, therapy management, and patient-reported outcomes in the S-TRAC trial. European Socity for Medical Oncology 29 (2018): 2098-2104.
- Klatte T, Rossi SH, Stewart GD. Prognostic factor and prognostic models for renal cell carcinoma: a literatura review. World Journal of Urology 36 (2018): 1943-1952.
- Leibovich BC, Blute ML, Cheville JC, et al. Prediction of Progression after Radical Nephrectomy for Patients with Clear Cell Renal Cell Carcinoma. A Stratification Tool for Prospective Clinical Trials. American Cancer Society 97 (2003): 7.
- Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol 19 (2001): 1649-1657.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 387 (2016): 2008-2016.
- Motzer RJ, Haas NB, Donskov F, et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J Clin Oncol 35 (2017): 3916-3923.
- Gross-Goupil M, Kwon TG, Eto M, et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Annals of Oncology 29 (2018): 2371-2378.
- European Urology Guidelines of EAU. Oncology guidelines: renal cell carcinoma (2019).
- Motzer RJ, Ravaud A, Patard JJ, et al. Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: subgroup analyses and updated overall survival results. Eur Urol 73 (2018): 62-68.
- Haas NB, Manola J, Dutcher JP, et al. Adjuvant treatment for high risk clear cell renal cancer: updated results of a high-risk subset of the ASSURE randomized trial. JAMA Oncol 3 (2017): 1249-1252.
- Lenis AT, Donin NM, Johnson DC, et al. Adjuvant therapy for high-risk localized kidney cancer- emerging evidence and future clinical trials. The Journal of Urology 199 (2018): 43-52.
- Sun M, Marconi L, Eisen T, et al. Adjuvant Vascular Endothelial Growth Factor–targeted Therapy in Renal Cell Carcinoma: A Systematic Review and Pooled Analysis. European Urology 74 (2018): 611-620.
- Sonbol MB, Firwana B, Hilal T, et al. Adjuvant Antiangiogenic Agents in Post-nephrectomy Renal Cell Carcinoma: A Systematic Review and Meta-analysis. European Urology Oncology (2018): 101-108


 Impact Factor: * 4.2
Impact Factor: * 4.2 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 