In Vivo Anthelmintic Effect of Ginger (Zingiber Officinale) Powder against Gastointestinal Nematodes of Artificially Infected Pigs
Article Information
Tracey Kiambom1, Marc K Kouam1,2,*, Cedric D Ngangoum1, Bridget Kate1, Alexis Teguia1
1Department of Animal Science, Faculty of Agronomy and Agricultural Sciences, PO BOX 188, Dschang, Cameroon
2Center for Research on Filariases and other Tropical Diseases (CRFilMT), P.O. Box 5797, Yaoundé, Cameroon
*Corresponding Author: Dr. Marc Kouam, Department of Animal Science, Faculty of Agronomy and Agricultural Sciences, PO BOX 188, Dschang, Cameroon
Received: 05 November 2020; Accepted: 12 November 2020; Published: 02 January 2021
Citation: Tracey Kiambom, Marc K Kouam, Cedric D Ngangoum, Bridget Kate, Alexis Teguia. In Vivo Anthelmintic Effect of Ginger (Zingiber Officinale) Powder against Gastointestinal Nematodes of Artificially Infected Pigs. Archives of Veterinary Science and Medicine 4 (2021): 1-12.
View / Download Pdf Share at FacebookAbstract
Anthelminthic resistance remains a major hindrance in controlling gastrointestinal parasites. Hence the need for alternative, more ecofriendly and readily affordable solutions. This paper evaluates the anthelmintic effect of Zingiber officinale (ginger) powder on the parasitic load of pigs experimentally infected with an association of Strongyloides ransomi, Hyostrongylus rubidus, Trichostrongylus axei and Globocephalus urosubulatus. A total of 24 pigs of two months old were randomly allocated to four treatment groups (G1 to G4). All the four groups were infected with 2650 L3 larva. Group 1 (the negative control, T0-) was not treated. Group 2 (the positive control, T0+) was treated with Mebendazole. Group 3(T1) was treated with 12.5g/kg of ginger crude powder and Group 4(T2) was treated with 25g/kg of ginger crude powder. For Strongyloides ransomi eggs, the treatment with 25g/kg of ginger (T2) showed the highest fecal egg count reduction (FECR) of 92.6% followed by treatments with 12.5g/kg of ginger (T1) and treatment with mebendazole (T0+) with a FECR of 83.4% and 72% respectively. Also, treatment with 25g/kg of ginger (T2) showed the highest FECR of 92% followed by treatments with 12.5g/kg of ginger (T1) and treatment with mebendazole (T0+) with FECR of 89.4% and 73.8% respectively for strongyle eggs. The dose of 25g/kg of ginger powder was effective in reducing egg shed and keeping the parasitic load of Strongyloides ransomi and strongyle eggs constantly low for six weeks after treatment. Therefore, 25g/kg of ginger powder could be administered every six weeks to reduce the parasitic load of the studied nematodes.
Keywords
Ginger powder; Strongyloides ransomi; Strongyles; FECR; Pigs
Article Details
1. Introduction
Gastrointestinal nematodes are responsible for substantial loss in the production of swine and other livestock species. They are a major impediment to efficient and profitable livestock production [1, 2], by causing economic losses due to reduced weight gains, poor litter sizes, poor growth rates, visceral organ condemnation at slaughter and deaths [3]. Parasitism is very crucial in animal production but often overlooked due to the fact that clinical signs are not obvious.
For the past years, the control of parasites has been based exclusively on the use of chemotherapeutic drugs. However, the overdue existence and mismanagement of these drugs has led to several limitations one of which is the persistent development of anthelmintic resistance of some parasitic strains to most anthelmintics [4-6]. There has been an alarming evidence of the spread of anthelmintic-resistant parasites in different livestock species (sheep, horses, cattle, pigs) with some farms harboring parasites that are no longer susceptible to the commercially available synthetic anthelmintics [7, 8]. Thus, due to this increasing spread of drug resistance within most common worm populations like the strongyles [9-12] and concerns over drug residues on meat, there is now a growing interest in alternative sources of anthelmintics which are more ecofriendly [13, 14]. Several studies have reported the use of various medicinal plants against gastrointestinal nematodes. However, most of them have only been tested in-vitro. Also most of the plants have been tested only on single species of parasites which is not practical because parasites rarely exist in isolation. Thus it seems more reasonable to proof the efficiency of such plants against a wide range of gastrointestinal parasites instead of a single parasite.
One of the most commonly used plants which has been tested for its anthelmintic properties is ginger (Zingiber officinale). Some in vitro studies [15-16 ] as well as in vivo [17-20] studies have been carried out to proof the anthelmintic efficacy of Z. officinale rhizome on various parasite species. However, no study has been carried out to evaluate the anthelmintic effects of ginger on the evolution of egg shed by adult worms in pigs which is important in establishing a treatment protocol. The main aim of this study was to evaluate the anthelmintic efficacy of Z. officinale powder on the parasitic load of pigs experimentally infected with an association of Strongyloides ransomi, Hyostrongylus rubidus, Trichostrongylus axei and Globocephalus urosubulatus.
2. Materials and Methods
2.1 Study area
The experiment was carried out at the teaching and research farm of the University of Dschang. Dschang is situated between the latitudes 05°22’58” and 05°30’40” N and longitudes 9°58’55” and 10°7’23” E. It has an average altitude of 1420 m in the West region of Cameroon. This region experiences the rainy season from mid-March to mid-November and the dry season from mid-November to mid-March. Precipitations vary between 1500 and 2000 mm/year and the temperatures vary between 14 and 25°C. Dschang has an average relative humility of 76, 8% [21].
2.2 Plant material
The plant material was made of the ginger rhizome (Zingiber officinale) harvested from the Santa sub division in the North West region of Cameroon. This plant is usually cultivated in the North West and Western regions of Cameroon. The rhizomes of this plant were bought directly from a farmer and then were washed, cleaned and air-dried under a shade for at least 2 weeks. The dry product was then blended with an electric blender to obtain crude powder.
2.3 Animal material
Animals used for this experiment were 24 cross breed pigs, two months old and having an average weight of 20kg. These pigs were purchased from a single local farm. They were hosted in a raised floor piggery built with hard wood. The piggery had four different compartments of 6m² (2m×3m) each corresponding to the four different treatments. Plank feeders with a 50-litres capacity were constructed and placed in each compartment. Well-designed tire rings with a 30-litres capacity were placed in each compartment to serve as water through.
In addition to the proper hygiene and sanitation that was carried out before and during the experiment, vaccination against erysipelas was provided by officials of the Ministry of livestock and fisheries (MINEPIA) in Dschang. Also antiboitics such as combikel and penstrip as well as multivitamins such as stress-vita were given to prevent interference with other diseases.
2.4 Culture of nematode parasitic larvae
Before the experiment proper was conducted, a presurvey was carried out, whereby pig farms in the Dschang locality were visited and fresh faeces was collected and analysed using the simple flotation technique in order to identify and quantify the most prevalent association of parasites which were Strongyloides ransomi and strongyle parasites. Then, a faecal culture was performed to obtain infective larval stages as described by Soulsby [22].
Briefly, the culture was done by placing 5 grams of positive faecal samples of faeces in Petri dishes in layers of 2mm depth. The dishes had loose covers that did not prevent air circulation but deterred flies and reduced desiccation. There were two different batches of the same sample. The first batch was incubated at a temperature of about 27ºC for 48 hours to collect Strongyloides ransomi and Trichostrongylus axei nematodes and the second batch was incubated for 11 days more to obtain Hyostrongylus rubidus and Globocephalus urosubulatus L3 larvae.
After incubation, the larvae were collected using the Baermann technique and identified under the microscope with the help of reference keys [22, 23]. The different L3 larvae were identified to determine the composition of the species involved in the mixed infection which were Strongyloides ransomi, Hyostrongylus rubidus, Trichostrongylus axei and Globocephalus urosubulatus.
2.5 Experimental design
This experiment consisted of 24 pigs and lasted for 4 months. At the start of the experiment, all the pigs had an average weight of 20kg and were treated against gastrointestinal parasitism using Mebendazole (5mg/kg). The pigs were randomly divided into four comparable groups of 6pigs each (3males, 3 females) and housed per group of treatment. Males and females of each treatment were housed separately. The pigs were fed commercially compounded dry feed supplied by one of the greatest feedstuff Compagnies of the country, Societé des Provenderies du Cameroun (SPC). The pigs were also given tap water to drink ad libitum. The feed was given to the pigs at equal quantities (3kgs per pen) and intervals (7am and 6pm). The pigs were given an adaptation period of one week before inducing the various treatments.
Group 1 which is the negative control (T0-) was infected with 2650L3 larvae and was not treated. Group 2 which is the positive control (T0+) was infected with 2650L3 larvae and treated with Mebendazole. Group 3(T1) and Group 4(T2) were each infected with the same number of larvae (2650 L3) and treated respectively with 250g (12.5g/kg) and 500g(25g/kg) of ginger crude powder. The pigs were inoculated orally using a pipette. Inoculation was done twice. 1400L3 larvae per 5mls was collected with Strongyloides ransomi constituting 60% and Trichostrongylus axei constituting 40% and inoculated on the third day after incubation to the pigs. Then the second batch of 1250L3 was collected per 5mls with Hyostrongylus rubidus constituting 55% and Globocephalus urosubulatus constituting 45% and inoculated 11 days after the first inoculation. Therefore, a total of (2650 L3) mixed infective larvae was inoculated orally to pigs using a pipette [24].
Six weeks after the second inoculation, faecal samples were collected directly from the rectum of all the pigs to determine the presence of eggs and the faecal egg count. Fecal culture was performed again to verify whether the species involved in the infection were effectively those that were used to infest the pigs at the beginning. When all the pigs were confirmed of shedding at least 200 epg, then various treatments were administered. The ginger powder was administered by mixing it in pig feed. After administration of various treatments, faecal samples were collected twice a week for 3 months to evaluate the evolution of egg shed.
2.6 Fecal egg reduction test (FERT)
Plant efficacy was evaluated using the fecal egg reduction test by the following formula:
FERT = [{EPG (pre-treatment) – EPG (14-day post-treatment)} /EPG (pre-treatment] X100 [25].
2.7 Statistical analysis
Data obtained were submitted to logarithmic transformation before analysis. Then, the one-way ANOVA was performed to compare the four different groups. Then the two sample t-test was performed to compare males and females of the same treatment. Significant differences between the means of the different treatments were separated using the Duncan’s Multiple Range Test. The limit of significance was 5%. All the analyses were carried out using SPSS version 20.0.
3. Results
3.1 Plant efficacy
The anthelmintic efficacy of Zingiber officinale on Strongyloides. ransomi is shown in Table 1. Irrespective of sex, treatment T2 showed the highest fecal egg count reduction (FECR) of 92.6% followed by treatments T1 and T0+ with a FECR of 83.4% and 72% respectively.
Table 1: Mean Strongyloides ransomi faecal egg counts per gram of faeces (EPG) and faecal egg count reductions (FECR) with Mebendazole (T0+) and ginger powder (T1, T2) in pigs in Cameroon.
|
Treatments |
Pig sex |
Mean EPG±SD (pre- treatment) |
Mean EPG±SD (post -treatment) |
FECR |
|
T0+ |
♂ |
2378 ± 236.76 |
1025 ± 1097.60 |
56.9% |
|
♀ |
2128 ± 243.94 |
235 ± 27.83 |
89% |
|
|
♂♀ |
2253 ± 254.90 |
630 ± 818.18 |
72% |
|
|
T1 |
♂ |
2390 ± 126.78 |
268 ± 85.44 |
88% |
|
♀ |
2522 ± 120.86 |
545 ± 149.77 |
78.4% |
|
|
♂♀ |
2456 ± 132.18 |
407 ± 186.69 |
83.4% |
|
|
T2 |
♂ |
2433 ± 72.85 |
207 ± 36.17 |
91.5% |
|
♀ |
2312 ± 269.45 |
143 ± 67.14 |
93.8% |
|
|
♂♀ |
2373 ± 188.69 |
175 ± 59.41 |
92.6% |
T0+: positive control (animals treated with Mebendazole)
T1: animals treated with a dose of 12.5g/kg of ginger powder
T2: animals treated with a dose 25g/kg of ginger powder
The FECR for strongyles is presented in Table 2. Regardless of sex, treatment T2 had the highest FECR of 92% followed by treatments T1 and T0+ with a FECR of 89.4% and 73.8% respectively.
Table 2: Mean strongylid parasites faecal egg counts per gram of faeces (EPG) and faecal egg count reductions (FECR) with Mebendazole (T0+) and ginger powder (T1, T2) in pigs in Cameroon.
|
Treatments |
Pig sex |
Mean EPG±SD (pre- treatment) |
Mean EPG±SD (post -treatment) |
FECR |
|
T0+ |
♂ |
4785 ± 202.97 |
2058 ± 2760.18 |
57% |
|
♀ |
4245 ± 594.30 |
2301 ± 11.54 |
45.8% |
|
|
♂♀ |
4515 ± 495.21 |
1180 ± 1993.30 |
73.8% |
|
|
T1 |
♂ |
4963 ± 137.50 |
420 ± 95 .01 |
91.5% |
|
♀ |
4815 ± 786.19 |
621 ±100.66 |
87.1% |
|
|
♂♀ |
4889 ± 511.27 |
521 ± 140.94 |
89.4% |
|
|
T2 |
♂ |
5113 ± 105.03 |
475 ± 115 .00 |
90.7% |
|
♀ |
5037 ± 308.59 |
333 ± 50.08 |
93.4% |
|
|
♂♀ |
5075 ± 210.40 |
404 ± 110.96 |
92.0% |
T0+: positive control (animals treated with Mebendazole)
T1: animals treated with a dose of 12.5g/kg of ginger powder
T2: animals treated with a dose 25g/kg of ginger powder.
3.2 Effects of ginger powder on the evolution of egg shed by Strongyloides ransomi
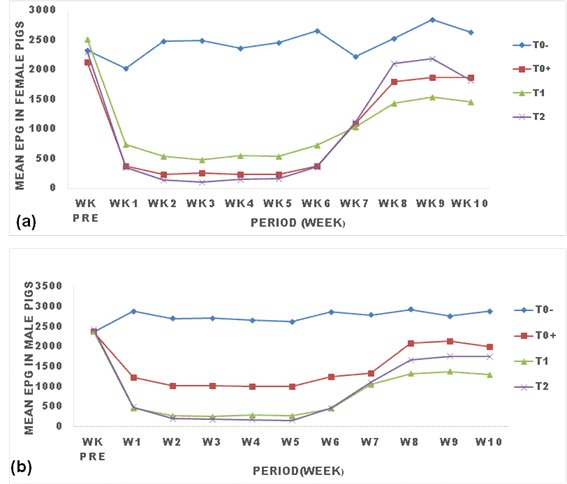
The effect of ginger powder on the evolution of egg shed by Strongyloides ransomi is presented in Figure 1. There was a clear effect of treatments (T0+, T1 and T2) on the evolution of S. ransomi eggs shed. All treatments led to the reduction of the parasitic load of this nematode from week one post treatment to week 6 where the parasitic load remained constantly low till the 7th week where it then rose up and remained constantly high throughout the experiment but never reached the load in the negative control (T0-). Treatment with Mebendazole and ginger powder led to a rapid drop in EPG from the first week post-treatment. Eggs in treated pigs decreased and remained significantly low (p<0.05) compared with untreated pigs for all treatments (T0+, T1, T2). Treatment with 25g/kg (T2) of ginger powder significantly (p<0.05) caused the lowest EPG in males and females pigs, followed by treatment with 12.5g/kg, then treatment with Mebendazole (Figure 1).
TO: negative control (untreated animals); TO+: positive control (animals treated with Mebendazole); T1: animals treated with 250 g (12.5g/kg) of ginger powder; T2: animals treated with 500 g (25g/kg) of ginger powder, epg: egg per gram of feces, Wk pre: week before treatment.
The trend in the evolution of egg load was similar between male and female pigs, even though the epg with Mebendazole treatment in female tend to be lower than in male pigs (p>0.05).
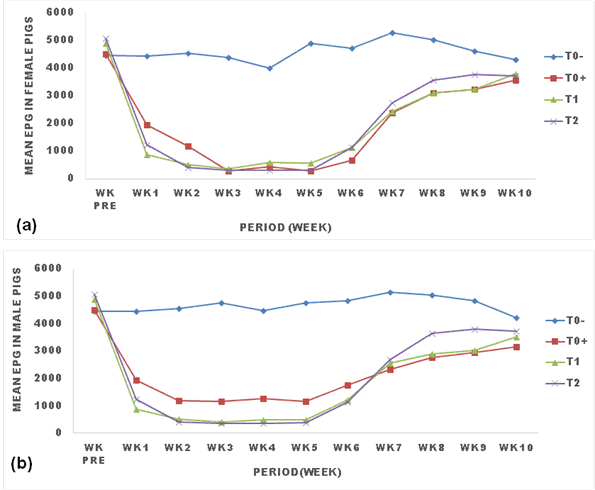
3.3 Effects of ginger powder on the evolution of strongyle eggs shed in pigs
Figure 2 presents the effect of ginger powder on the evolution of strongyle eggs shedding in pigs. All treatments reduced the egg load of strongyles from week one to week 6 post treatment where the parasitic load remained constantly low. The egg load then rose from week 7 to week 8 and remained constant from there to the end of the experiment. Eggs in treated pigs decreased and remained significantly low(p<0.05) compared with untreated pigs for all treatments (T0+, T1, T2). Treatment with 25g/kg(T2) of ginger powder significantly (p<0.05) caused the lowest EPG in males and females pigs (Figure 2).
TO: negative control (untreated animals); TO+: positive control (animals treated with Mebendazole); T1: animals treated with 250 g (12.5g/kg) of ginger powder; T2: animals treated with 500 g (25g/kg) of ginger powder, epg: egg per gram of feces, Wk pre: week before treatment.
The trend in the evolution of egg load was different between male and female pigs as the treatment with Mebendazole(T0+) significantly(p<0.05) caused a lower EPG in females than males throughout the experiment.
4. Discussion
For Strongyloides ransomi eggs, the treatment with 25g/kg of ginger (T2) showed the highest FECR of 92.6% followed by the treatments with 12.5g/kg of ginger(T1) and treatment with mebendazole(T0+) with a FECR of 83.4% and 72% respectively. Also, the treatment with 25g/kg of ginger (T2) showed the highest FECR of 92% followed by the treatments with 12.5g/kg of ginger(T1) and treatment with mebendazole (T0+) with FECR of 89.4% and 73.8% respectively for strongyle eggs. According to the World Association for the Advancement of Veterinary Parasitology guideline [26], when an anthelmintic has a FECR >90%, it is considered effective. When the reduction efficacy is between 80%-90%, it is considered equivocal and needs to be repeated for confirmation. Then when the FECR is <80% this means that the worms are resistant to this anthelmintic and such anthelmintic should never be used on the farm again. Therefore, the treatment with 25g/kg of ginger (T2) was effective in the treatment of both Strongyloides ransomi and strongyle eggs meanwhile these same parasites were resistant to the positive control (T0+). This result corroborates that of Adeniji et al., [27] who reported ginger to have a 100% reduction efficacy on mixed species of helminthes in yankasa lambs. This results also confirms that of Hayajneh et al., [28] who reported that ginger (FECR=91%) recorded a higher anthelmintic efficacy than ivermectin (FECR=84%) and albendazole (FECR=60%) against a mixed infection of Trichostrongylus, Oestertagia and Haemonchous in awassi sheep. Similar results were also reported by Lin et al [15] who analysed the effect of isolated compounds of ginger against Angiostrongylus cantonensis and Anisakis simplex parasites and reported that gingerol showed higher anthelmintic activity than mebendazole and albendazole. However, the results of this study disagrees with the findings of Iqbal et al., [18] who reported ginger efficacy (FECR=66.6%) to be less effective than the positive control levamisole (FECR=99.2). Also the findings of Aurora et al., [29] contrasts the results of this study as he reported ginger to be less effective (FECR=87%) than mebendazole (FECR=100%) against Ascaris suum. These differences could be attributed to the differences in parasite species involved as some parasites could be more susceptible to ginger treatment than others. Differences in the treatment doses could be the reason of the contrast in results; in fact, according to some authors [18, 16, 30], the effectiveness of ginger treatment increases in a dose dependent manner, i e the higher the dose, the more effective the treatment. In our study too, the higher the dose, the more effective the dose; treatment with 25g/kg of ginger powder reduced the epg the most in both S. ransomi and strongyles, with FECR of 92.6% and 92% respectively, compared with 12.5g/kg of ginger powder with FECR of 83.4% and 89.4% respectively.
Apart from the negative control (T0-) all the treatments started to reduce the parasitic load of Strongyloides ransomi and strongyle eggs from week one to week 6 post treatment where the parasitic load remained constantly low. The treatment with 25g/kg of ginger (T2) was the most effective followed by the treatment with 12.5g/kg of ginger (T1) and mebendazole(T0+) respectively. These findings suggest that ginger treatment is effective in reducing egg load for six weeks after treatment thus proposing a treatment interval. Indeed, 25g/kg (T2) of ginger powder could be administered as an anthelmintic against the above nematodes every six weeks. According to Quian & Liu [31], the mechanism of action of ginger is both central and peripheral. In fact, ginger exhibits gastrointestinal prokinetic activity via activation of cholinergic receptors. Thus, the cholinergic components of ginger crude powder which are mainly gingerols, gingerdiole, gingerdione and shogoals activated the neuromuscular junctions of the studied nematode worms causing a paralysis that either prevented them from shedding eggs or led to their expulsion via faeces. Given that no study has been carried out to evaluate the effects of ginger on the evolution of egg shedding so as to establish a treatment protocol and interval with ginger, more research on various parasite groups should be encouraged.
Our study is the first to establish that 25g/kg of ginger powder should be used as an antihelmintic against Strongyloides ransomi and strongylid parasites every six weeks to keep the pigs more productive. As regards the time of action of the various treatments, there was a highly significant difference in the effect of treatment on the parasitic load of Strongyloides ransomi eggs from week 1 post treatment to week 6 post-treatment. Treatment with 25g/kg of ginger crude powder (T2) was very effective in reducing the parasitic load of the above nematodes within one week to six weeks-post treatment. This result contrast the findings of Sanderson [32] who reported that there was no significant difference in the epg of Schistosoma mansoni between the treated (ginger extract) and control groups until week 8 post treatment in laboratory mice. Our result is comparable with that of Abdel-hafeez et al., [33] who studied the anti-Gardia lamblia activity of ginger extract and confirmed that ginger treatment significantly lowered the number of parasites after 48hours, with a reduction rate of 94,4%, compared with the positive control(nitazoxanide) with a lower reduction rate of 92.93%. This difference could be attributed to the differences in parasite species studied as some species could be more susceptible to ginger treatment than others.
Ginger crude powder was chosen for this work because crude ginger powder has been demonstrated to be more effective in reducing egg shedding than ginger extracts. According to Iqbal et al. [18], the effect of crude powder and aqueous extract of ginger in sheep naturally infected with mixed species of gastrointestinal nematodes including Trichostrongylus colubriformis, Haemonchus contortus, Oesophagostomum columbianum, Trichostrongylus axei, Trichuris ovis and Strongyloides papillosus is not different, with ginger crude powder showing a higher FECR of 66.6% compared with the aqueous extract having a lower FECR of 25.6%. Also Hayajneh et al. [28], evaluated the resistance of various anthelmintics (albendazole and ivermectin ) in comparison to ginger powder and showed that parasites had a lower resistance to ginger powder as compared to albendazole and ivermectin which showed higher anthelmintic resistance. Another reason justifying our choice was that some metabolites and bioactive compounds can be lost or not fully extracted when doing and extract. Moreover, ginger crude powder can be easily prepared and used than extracts by most farmers.
In conclusion, this study showed that mixing 25g/kg of ginger powder in pig feed is more effective in reducing the parasitic load of Strongyloides ransomi and strongylid parasites when compared to Mebendazole. This dose is effective in reducing egg shed and keeping the parasitic load of Strongyloides ransomi and strongyle eggs constantly low for six weeks after treatment. Thus, 25g/kg of ginger powder could be administered every six weeks to reduce the parasitic load of Strongyloides ransomi and strongyles. Though ginger crude powder was proven to be effective as an anthelmintic in pigs, further research is necessary to describe the effects of ginger crude powder on growth, blood and reproduction- related parameters which are all determinants of the proper health and productivity of an animal.
Acknowledgements
The authors thank the Nwalimu Nyerere Scholorship scheme under the African Union for their financial support in terms of tuition fees and monthly stipend for the first author.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.
References
- Boes JA, Shi F, Xuguang L, et al. Prevalence and distribution of pig helminths in the Dongting Lake Region (Hunan Province) of the People's Republic of China. Journal of Helminthology 74 (2000): 45-52.
- Joachim AN, Dülmer A. Daugschies, et al. Occurrence of helminths in pig fattening units with different management systems in Northern Germany. Veterinary Parasitology 96 (2001): 135-146.
- Stewart BT, Hoyt PG. Internal parasites of swine: in Diseases of Swine (2006): 901910.
- Brunet S, Frank J, Herve H. Effects of sainfoin (Onobrychis viciifolia) extract and monomers of condensed tannins on the association of abomasal nematode larvae with fundic explants. International Journal for Parasitology 38 (2008): 783-790.
- Cheng G, Haihong H, Shuyu X, et al. Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Frontiers in microbiology 5 (2014): 217.
- Hoste H, Frank J, Spiridoula A, et al. The effects of tannin-rich plants on parasitic nematodes in ruminants.Trends in parasitology 22 (2006): 253-261.
- Behnke JM, David JB, Gillian S, et al. Developing novel anthelmintics from plant cysteine proteinases. Parasites and vectors 1 (2008): 29.
- Jabbar A, Zafar I, Dominique K, et al. Anthelmintic resistance: the state of play revisited. Life sciences 79 (2006): 24132431.
- Maingi N. Observations on changes in resistance to Levamisole in Haemonchus contortus after passage in sheep and calves (1991).
- Sallé G, Jacques C, Isoline B, et al. Risk factor analysis of equine strongyle resistance to anthelmintics. International Journal for Parasitology: Drugs and Drug Resistance 7 (2017): 407-415.
- Sanna GA, Pipiac C, Tamponi R, et al. Anthelmintics efficacy against intestinal strongyles in horses of Sardinia, Italy. Parasite epidemiology and control 1 (2016): 15-19.
- Waller, Peter J. Sustainable helminth control of ruminants in developing countries. Veterinary Parasitology 71 (1997): 195-207.
- Matthee SF, Dreyer WA Hoffmann, Van N. An introductory survey of helminth control practices in South Africa and anthelmintic resistance on Thoroughbred stud farms in the Western Cape Province. Journal of the South African Veterinary Association 73 (2002): 195-200.
- Wolstenholme, Adrian J, Ian F, et al. Drug resistance in veterinary helminths. Trends in parasitology 20 (2004): 469-476.
- Lin, Rong-Jyh, Chung-Y C, et al. Larvicidal activities of ginger (Zingiber officinale) against Angiostrongylus cantonensis. Acta Tropica 115 (2010): 69-76.
- Moazeni, Mohammad, Ali Nazer. In vitro lethal effect of Zingiber officinale on protoscolices of hydatid cyst from sheep liver. Microbiology Research 2 (2011): e25e25.
- Kiambom T, Kouam MK, Teguia A. In vivo lavicidal effects of ginger (Zingiber officinale) powder on pigs artificially infected with gastointestinal nematode larvae. Scientific Journal of Veterinary Advances 9 (2020): 299-308.
- Iqbal, Zafar, Muhammad L, et al. In vivo anthelmintic activity of ginger against gastrointestinal nematodes of sheep. Journal of ethnopharmacology 106 (2006): 285-287.
- Matthews KK, O’Brien NC, Whitley JM, et al. Investigation of possible pumpkin seeds and ginger effects on gastrointestinal nematode infection indicators in meat goat kids and lambs. Small Ruminant Research 136 (2016): 1-6.
- Mostafa, Osama MS, Ali AS, et al. Assessment of the antischistosomal activity of ginger (Zingiber officinale) against Schistosoma mansoni harbored in C57BL/6 Mice. J Drug Res Egypt 33 (2012): 25-33.
- Pamo TE, Boukil FA, Fonteh F, et al., Composition chimique et effet de la supplémentation avec Calliandra calothyrsus et Leucaena leucocephala sur la production laitière et la croissance des chevreaux nains de Guinée. Livestock Research for Rural Development 17 (2005).
- Soulsby EJL, Helminths. Arthropods and Protozoa of domesticated animals 291 (1982).
- Van W, Jan A, Estelle M. Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: A practical lab guide. Onderstepoort Journal of Veterinary Research 80 (2013): 00-00.
- Kiambom T, Kouam MK, Teguia A. In vivo lavicidal effects of ginger (Zingiber officinale) powder on pigs artificially infected with gastointestinal nematode larvae. Scientific Journal of Veterinary Advances 9 (2020): 299-308.
- Roepstorff, Allan, Peter Na. Epidemiology, diagnosis and control of helminth parasites of swine. Rome: Fao 3 (1998).
- Coles GC, Bauer FM, Borgsteede SG, et al. World Association for the Advancement of Veterinary Parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance.Veterinary parasitology 44 (1992): 35-44.
- Adeniji SA, Adediran TO, Ososanya, et al. Antihelminthic and anticoccidial effects of Zingiber officinale Roscoe fortified diets fed to Yankasa rams. Livestock Research for Rural Development 29 (2017): 7.
- Hayajneh, Firas M, Hosam HT, et al. Evaluation of Anthelmintics Resistance Against Gastrointestinal Parasites Infection in Awassi Sheep in Jordan and The use of Alternative Herbal Anthelmentics (2019).
- Aurora M, Lisa V, Hayre H, et al. The antihelminthic effect of 50% zingiber officinale (Ginger) rhizome decoction on Ascaris suum. College of Medicine - Cebu Doctor's University. (2006), (Full dissertation).
- El-Sayed NM, Safar EH. A brief insight on anti-Toxoplasma gondii activity of some medicinal plants. Aperito J Bacteriol Virol Parasitol 1 (2014): 107.
- Qian DS, Liu Z. Pharmacologic studies of antimotion sickness actions of ginger. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi= Chinese journal of integrated traditional and Western medicine 12 (1992): 95-98.
- Sanderson LA. Bartlett, Whitfield. In vitro and in vivo studies on the bioactivity of a ginger (Zingiber officinale) extract towards adult schistosomes and their egg production. Journal of helminthology 76 (2002): 241-247.
- Abdel-Hafeez, Ekhlas HA, Azza K, et al. Anti-Giardia lamblia activity of ginger (Zingiber officinale) extract in an improved modified axenic culture. Parasitologists United Journal 9 (2016): 7.

 Impact Factor: * 1.1
Impact Factor: * 1.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 