Incidentally Detected Cardiac and Hepatic Hydatid Cyst after Sudden Onset Facial Paralysis: A Case Report
Article Information
Ovidiu Stiru1*, Roxana Carmen Geana1, Laura Antohi1, Leonard David2, Mihai Goicea1, Alina Paunescu1, Ovidiu Chioncel1, Vlad Anton Iliescu1
1Emergency Institute for Cardiovascular Diseases “Prof. Dr. C. C. Iliescu”, Bucharest, Romania
2 General Surgery, Fundeni Institute, Bucharest, Romania
*Corresponding Author: Dr. Ovidiu Stiru, Emergency Institute for Cardiovascular Diseases “Prof. Dr. C. C. Iliescu” Sos. Fundeni 258, sector 2, 022328 Bucharest, Romania
Received: 05 June 2019; Accepted: 25 June 2019; Published: 16 August 2019
Citation: Ovidiu Stiru, Roxana Carmen Geana, Laura Antohi, Leonard David, Mihai Goicea, Alina Paunescu, Ovidiu Chioncel, Vlad Anton Iliescu. Incidentally Detected Cardiac and Hepatic Hydatid Cyst after Sudden Onset Facial Paralysis: A Case Report. Archives of Clinical and Medical Case Reports 3 (2019): 190-198.
View / Download Pdf Share at FacebookAbstract
The simultaneous occurrence of interventricular cardiac septum and hepatic localization for cystic echinococcosis is a very rare form of presentation and it may lead to various complications. We present the case of a 24-year-old man who was admitted to the hospital for evaluation of abrupt onset facial paralysis and incidentally diagnosed with a large hepatic hydatid cyst and a cardiac one located in the interventricular septum. The patient did not have any other symptoms associated with hydatid disease before this event. In this case, we surgically removed both hydatid cysts at the same time, to prevent complications and to establish a better outcome for the patient.
Keywords
Cardiac cyst, Hepatic cyst, Hydatidosis, Facial paralysis
Cardiac cyst articles Cardiac cyst Research articles Cardiac cyst review articles Cardiac cyst PubMed articles Cardiac cyst PubMed Central articles Cardiac cyst 2023 articles Cardiac cyst 2024 articles Cardiac cyst Scopus articles Cardiac cyst impact factor journals Cardiac cyst Scopus journals Cardiac cyst PubMed journals Cardiac cyst medical journals Cardiac cyst free journals Cardiac cyst best journals Cardiac cyst top journals Cardiac cyst free medical journals Cardiac cyst famous journals Cardiac cyst Google Scholar indexed journals Hepatic cyst articles Hepatic cyst Research articles Hepatic cyst review articles Hepatic cyst PubMed articles Hepatic cyst PubMed Central articles Hepatic cyst 2023 articles Hepatic cyst 2024 articles Hepatic cyst Scopus articles Hepatic cyst impact factor journals Hepatic cyst Scopus journals Hepatic cyst PubMed journals Hepatic cyst medical journals Hepatic cyst free journals Hepatic cyst best journals Hepatic cyst top journals Hepatic cyst free medical journals Hepatic cyst famous journals Hepatic cyst Google Scholar indexed journals Hydatidosis articles Hydatidosis Research articles Hydatidosis review articles Hydatidosis PubMed articles Hydatidosis PubMed Central articles Hydatidosis 2023 articles Hydatidosis 2024 articles Hydatidosis Scopus articles Hydatidosis impact factor journals Hydatidosis Scopus journals Hydatidosis PubMed journals Hydatidosis medical journals Hydatidosis free journals Hydatidosis best journals Hydatidosis top journals Hydatidosis free medical journals Hydatidosis famous journals Hydatidosis Google Scholar indexed journals health articles health Research articles health review articles health PubMed articles health PubMed Central articles health 2023 articles health 2024 articles health Scopus articles health impact factor journals health Scopus journals health PubMed journals health medical journals health free journals health best journals health top journals health free medical journals health famous journals health Google Scholar indexed journals Facial paralysis articles Facial paralysis Research articles Facial paralysis review articles Facial paralysis PubMed articles Facial paralysis PubMed Central articles Facial paralysis 2023 articles Facial paralysis 2024 articles Facial paralysis Scopus articles Facial paralysis impact factor journals Facial paralysis Scopus journals Facial paralysis PubMed journals Facial paralysis medical journals Facial paralysis free journals Facial paralysis best journals Facial paralysis top journals Facial paralysis free medical journals Facial paralysis famous journals Facial paralysis Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals patient articles patient Research articles patient review articles patient PubMed articles patient PubMed Central articles patient 2023 articles patient 2024 articles patient Scopus articles patient impact factor journals patient Scopus journals patient PubMed journals patient medical journals patient free journals patient best journals patient top journals patient free medical journals patient famous journals patient Google Scholar indexed journals disease articles disease Research articles disease review articles disease PubMed articles disease PubMed Central articles disease 2023 articles disease 2024 articles disease Scopus articles disease impact factor journals disease Scopus journals disease PubMed journals disease medical journals disease free journals disease best journals disease top journals disease free medical journals disease famous journals disease Google Scholar indexed journals MRI articles MRI Research articles MRI review articles MRI PubMed articles MRI PubMed Central articles MRI 2023 articles MRI 2024 articles MRI Scopus articles MRI impact factor journals MRI Scopus journals MRI PubMed journals MRI medical journals MRI free journals MRI best journals MRI top journals MRI free medical journals MRI famous journals MRI Google Scholar indexed journals
Article Details
1. Introduction
A flat tapeworm, Echinococcosis granulosus, causes hydatid disease in humans, a parasitic disease that occurs in some endemic regions of the world. The disease is caused by the larval stage of E. granulosus. The life cycle alternates between carnivores and herbivores. The life cycle of this parasite involves dogs as definitive hosts. Eggs are passed in the faeces and eaten by the intermediate hosts, such as sheep, and the larvae encyst in the liver, lungs, and other organs. The natural cycle completes when a dog eats the sheep viscera and ingests the larvae cyst. The protoscolices attach to the small intestinal wall and the worms begin to form proglottids which will be containing the eggs. The eggs pass in the faeces. Human beings act as an intermediate accidental host, and an end point in the parasite’s life cycle. Human infestations develop after accidental ingestion of excreted eggs in animal faeces. Consumption of unwashed and raw contaminated vegetables is the source of the infestation. The embryo develops from the egg and migrates via the intestinal mucosa into the circulation. Any organ can be involved by the cyst formation, most frequently the liver (65%) and lungs (25%) [1, 2]. Cardiac hydatid cysts are found in fewer than 2% of cases of hydatidosis [3]. The most common cardiac locations are the left ventricular wall (60%) followed by the right ventricle (10%), pericardium (7%), left atrium (6-8%), right atrium (4%), and the interventricular septum (4%) [4]. In 50% of such cardiac cases, there is a multiple organ inclusion [4]. Cardiac hydatid cysts grow slowly, the disease may be asymptomatic for a long period of time, is usually detected incidentally on echocardiography taken for other reasons. A definitive diagnosis is made by use of computed tomography (CT), magnetic resonance imaging (MRI), in addition to serologic tests [5]. The disease is treated by a combination of surgery and medical therapy with Albendazole.
2. Case Report
A 24-year-old male was referred to our hospital from a neurology clinic where he had been evaluated for chief complaint of abrupt onset facial paralysis and mild headaches. Physical examination in our clinic, one week after the onset of the symptoms, was unremarkable, with exception of the neurological examination that showed a mild facial paralysis. Past medical and surgical history were unremarkable. Social history revealed that the patient has been in close contact with animals such as sheep and canines by the nature of his occupation. His initial vital signs were normal, the temperature was normal. Laboratory test findings only showed neutrophilia (WBC count at 14.710/μL, neutrophil count at 8.470/μL) and ALT of 55 u/L (normal range 10-50 u/L). The electrocardiogram (EKG) showed sinus rhythm at 80/min, QRS axis at +45°, negative T waves in DIII lead, no other ST-T abnormality. Cerebral tomography done at the referring hospital a week prior to admission revealed subcortical, thalamic, mesencephalic and hypothalamic small ischemic lesions, some of them with hemorrhagic transformation. Transthoracic echocardiography (TTE) showed a cystic structure measuring 23/19 mm located at the junction between the apical and the middle third of the interventricular septum toward its anterior part (Figure 1).
At this level, a mobile mass measuring approximately 3/1,5 cm was attached to the hyperechogenic wall of the cystic structure, highly suggestive for thrombus. A new head CT was obtained at our clinic. Hemorrhagic cerebral lesions were stable as compared to the prior head CT. The neurological evaluation raised the suspicion of facial paralysis a frigore, and in the context of the mobile mass attached to the cystic cardiac wall, it is considered appropriate to start anticoagulant treatment. Continuous infusion of unfractionated heparin was started. A full-body CT-scan was performed and confirmed the presence of the cardiac cyst (Figure 2). Consequently, a second larger cystic formation was discovered located in the hepatic parenchyma, measuring 62/47 mm (Figure 3). Serologic tests for Echinococcus granulosus were sent as clinical suspicion was very high for hydatid disease. Empiric Albendazole (400 mg twice daily) treatment was thus initiated. Serologically, specific Echinococcus granulosus (E. granulosus) and Echinococcus multilocularis (E. multilocularis) antibodies were investigated by commercial Enzyme-Linked Immunosorbent Assay (ELISA) test. E. multilocularis antibodies were positive by ELISA. Hydatid cyst antibodies had positive 1/320 titer in the Hemagglutination Inhibition Test (HAI). As expected, ELISA were highly positive: 5,37 (normal range <0,9) for echinococcus antibodies. With Albendazole treatment, leukocytosis resolved. Overall status remained very good. Since the patient was 24 years old and did not have any risk factors for coronary atherosclerosis, further investigations were not performed.
Surgery was scheduled after cerebral CT examination showed stabilization of the hemorrhagic lesions in order to minimize the cerebral hemorrhage risk during CPB. We decided to remove first the cardiac hydatid cyst. The patient underwent median sternotomy and was placed on standard aorto-bicaval cardiopulmonary bypass. After aorta was cross-clamped, for cardiac arrest we used cold potassium-enriched blood cardioplegic solution. The left atrium was vented through the right upper pulmonary vein. The pericardial cavity was wrapped with sponges moistened with 20% hypertonic saline solution and povidone iodine 10% solution (larvicidal solution). The incision to excise the cyst was made at the apical left lateral wall ventricle parallel to the left anterior descending coronary artery, to avoid damaging that vessel. A 2-3 cm well-incapsulated hydatid cyst was found in the proximal third of the interventricular septum (Figure 4). We found the cyst not to be covered by the thrombus. A syringe with 50 ml of larvicidal solution was introduced into the cystic sac. After 5 minutes the cavity was aspirated with the same syringe and the cyst cavity was opened and the protoscoleces (fertile cysts) derived from the germinal layer was carefully removed completely. The edge of the cyst was excised and the cavity in ventricular septum was washed with a larvicidal solution and left open. The left ventriculotomy was closed with 3-0 polypropylene continuous sutures over Teflon felt. At the end, the pericardial cavity was washed with a larvicidal solution. The patient was weaned from cardiopulmonary bypass, intraoperative TEE examination showed normal biventricular function without any traces of intraseptal cardiac hydatid cysts. In the same surgical procedure we also removed the hepatic cyst. Before closing the chest, laparotomy was performed with exposure of the surface of encapsulated hepatic hydatid cyst. In similar fashion, the hepatic cyst was wrapped with sponges moistened with larvicidal solution. The cyst was punctured with a wide needle and 100 cm3 of the cystic content was aspirated, then the cyst content was sterilized by mixing the remaining contents with the larvicide solution. After a few minutes, the cyst was completely aspirated and opened. The cyst content, endocyst and remaining daughter cysts should be enucleated from the pericyst with a sharp spoon and the residual cavity was washed again with a larvicidal solution (Figure 5). The patient had an uneventful postoperative course, with a minimal intentesive care unit stay and was discharged in the 7th day postoperatively with 400 mg albendazole twice a day for the next six weeks postoperatively. One year follow-up showed no pathological findings.
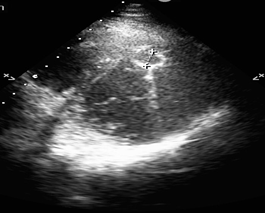
Figure 1: Echocardiographic aspect of the hydatid cyst located in the interventricular septum.
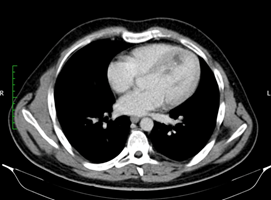
Figure 2: CT showing a cystic lesion on the interventricular septum.
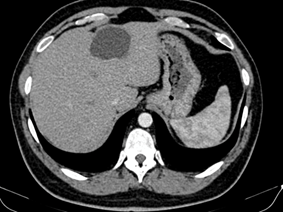
Figure 3: CT showing a cystic lesion of the liver.
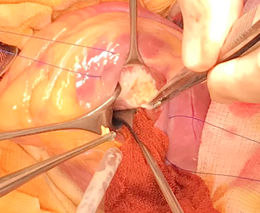
Figure 4: Intraoperative view of the cardiac cyst.
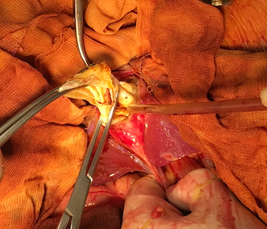
Figure 5: Intraoperative view of the remaining cavity after the inactivation of the cardiac cyst.
3. Discussion
Hydatidosis is a parasitic infection caused by Echinococcus granulosus. After infestation, the embryo usually reaches the myocardium via the coronary circulation from the left side of the heart and reaches the liver via intestinal mucosa into the portal circulation. Enlargement in the cyst volume requires a period of one to five years [6] and can compromise adjacent structures, thus causing symptoms. During this time it may calcify and die, or grow in situ. Due to slow disease progression, patients suffering from hepatic and cardiac echinococcosis may be initially asymptomatic for a long time. The clinical manifestation may vary according to the location, size and age of the cysts and the extent of calcification. When symptomatic, patients generally present with palpitation, dyspnea, atypical angina pectoris, cough, haemoptysis, compression with acute coronary syndrome, valve obstruction and biventricular systolic dysfunction, cardiac syncope, arrhythmias and conduction disorders, acute pericarditis, or cyst rupture with cardiac tamponade [7], anaphylactic reaction with profound circulatory collapse and cerebral or peripheral arterial embolism [8], pulmonary embolism with pulmonary hypertension [7] or even sudden death [9]. The most frequently involved cardiac region is the left ventricle while the right ventricle and the interventricular septum are the less affected regions, respectively [10].
Our patient was asymptomatic for years and did not have any complaints associated with hydatid disease before the sudden occurrence of the facial paralysis accident with which the cardiac cyst has been diagnosed. The first problem was diagnosed the etiology of facial paralysis. Repeat cerebral CT revealed small ischemic lesions, stationary in evolution. TTE showed a cystic structure on the left side of the interventricular septum with a mobile mass attached, highly suggestive for thrombus. We considered the small cerebral ischemic lesions detected on cerebral CT to be the result of the thrombus embolising from the mobile mass attached to the wall of the cardiac hydatid cyst. Since facial paralysis was not accompanied by any other neurological sign and the ischemic lesions were small and disseminated, we suspected that it was rather a paralysis a frigore than the result of ischemic stroke. The second diagnostic problem after ultrasound identification of a cardiac tumor mass was the possible confusion with the simultaneous development of left ventricular myxoma or papillary fibroelastomas with concomitant hepatic hydatid cyst [11].
However, the literature describes left ventricular myxoma only in case of recurrence of the disease [12, 13]. Specific diagnosis of hydatid cyst in general is based on clinical presentation, serology tests and non-invasive imaging techniques such as TEE, CT and MRI. The ELISA is one of the most specific serologic tests that can be used and a positive result for Echinococcus antibodies confirms the diagnosis [14]. Serologic tests can be false-negative in 10% to 20% of patients with hepatic hydatid cysts and 50% with cardiac cysts; this is most likely linked with an insufficient immune response [15]. Echocardiography remains the most efficient method in the diagnosis, which provides important findings: size and number of cysts, cyst locations and relationship with adjacent structures [1]. In our case, the positive serological tests confirmed the presence of hydatidosis. Due to the high risk of the spontaneous rupture of cysts or systemic embolisms, the treatment of choice, even in asymptomatic cases, is the complete removal of cysts with early cardiac surgery under cardiopulmonary bypass. From the current literature, an estimation of prognosis for patients that received non-surgical treatment is not possible. For cardiac location, there is a case report of surgical treatment on beating heart when the cyst is located on the epicardium [16]. In our case, both CT and echocardiographic findings were indicating intraseptal involvement so the cardiopulmonary bypass by conventional technique was used. When a patient presents with hydatid cysts in cardiac and alternate location, it is possible to elect to combine or not the cardiac surgery with laparotomy.
The general principle is to give priority to the cysts before complications occur. In our case, the emergency was to remove the cardiac cyst that was responsible for stroke. The incision to excise the intraseptal cyst must offer direct access and can be made from the atrium, ventricle or apex cordis. In our case, we prefer an incision in the left apical lateral ventricle. However, there are also reports with sudden cardiac death after resection of the cardiac hydatid cysts [17]. The contents of the cyst must be entirely aspirated and the germinative membrane removed. Although many larvicidal agents have been introduced in order to prevent dissemination and recurrence is widely used, none is 100% effective and safe for intraoperative use. We used a relatively nontoxic protoscolicidal agent, a combination between 20% hypertonic saline solution and 10% povidone iodine solution. This combination destroys protoscolices by creating a significant osmotic gradient [18]. We have also used this solution to isolated surrounding areas to prevent the risk of development of secondary cysts as a result of leakage from the primary cyst during the surgical procedure and to wash out the remaining cavity.
Along with the surgical treatment, currently the use of the synthetics benzimidazole-derivative anthelmintic has been shown to be effective in the treatment of hydatid disease and to prevent recurrence of cysts after operation [19]. Medical treatment should be started a few weeks prior to surgery, yet for cardiac cysts that therapy may lead to a higher risk of cyst wall destruction and rupture [20]. Surgical resection should be followed by therapy with albendazole (10–15mg/kg body weight per day) or mebendazole (40–50 mg/kg body weight per day). In our case, the patient was treated preoperatively for 15 days with albendazole 400 mg twice per day and was discharged with the same dose for the next six weeks postoperatively. About 10% of all hydatid cysts tend to recur after surgery, but this rate may decrease with albendazole therapy. However, albendazole therapy is contraindicated for large cysts with the risk of rupture and also for inactive or calcified cysts [21].
4.Conclusions
Simultaneous cardiac and hepatic hydatid cysts are rare and often asymptomatic in their early stages. Clinical suspicion is important in reaching an early diagnosis. In patients with cardiac masses the possibility of cardiac hydatid disease should be taken into consideration, even outside an endemic area. Serology tests, TEE, CT and MRI are useful for diagnosis and establishing the location of hydatid cysts. Due to the high risk of their associated complications, hydatid cysts should be removed surgically, even in asymptomatic patients.
Conflict of Interest
The authors declare that they have no competing interests.
Funding Source
None.
References
- Miralles A, Bracamonte L, Pavie A, et al. Cardiac echinococcosis. Surgical treatment and results. J Thorac Cardiovasc Surg 107 (1994): 184-190.
- Iyigun O, Uysal S, Sancak R, et al. Multiple organ involvement hydatid cysts in a 2 year-old boy. J Trop Pediatr 50 (2004): 374-376.
- Dighiero J, Canabal E J, Aguirre CV, et al. Echinococcus disease of the heart. Circulation 1958;17:127–32.
- Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 17 (2004): 107-135.
- King CH, et al. Principles and Practice of Infectious Diseases, Mandell GL, Bennett JE and Dolin R eds, 6th Ed, Elsevier Churchill Livingstone, Philadelphia (2005): 3290-3292.
- Tuncer E, Tas SG, Mataraci I, et al. Surgical treatment of cardiac hydatid disease in 13 patients. Tex Heart Inst J 37 (2010): 189-93.
- Perez-Gomez F, Duran H, Tamames S, et al. Blanes Cardiac echinococcosis:clinical picture and complications British Heart Journal 35 (I973): I326-I33I.
- Özer N, et al. Hydatid cyst of the heart as a rare cause of embolization: report of 5 cases and review of published reports. J Am Soc Echocardiogr 14 (2001): 299-302.
- Laglera S, Garcia-Enquita MA, Martinez-Gutierrrez F, et al. A case of cardiac hydatidosis. Br J Anaesth 79 (1997): 671-673.
- Yaliniz H, Tokcan A, Salih OK, et al. Surgical treatment of cardiac hydatid disease: a report of 7 cases. Tex Heart Inst J 33 (2006): 333-339.
- Saxena P, Shehatha J, Naran A, et al. Papillary Fibroelastoma of the Interventricular Septum Mimicking a Cardiac Myxoma Tex Heart Inst J 37 (2010): 119-120.
- Macarie C, Stoica E, Chioncel O, et al. Recurrent atrial myxoma. Rom J Intern Med 42 (2004): 625-634.
- Iliescu VA, Dorobantu LF, Stiru O, et al. Second recurrence of cardiac myxoma 7 years after the initial operation Chirurgia (Bucur) 103 (2008): 239-241.
- Saglican Y, Yalçin O, Kaygusuz E. Cystic Echinococcosis: one entity, two unusual locations. Turkiye Parazitol Derg 40 (2016): 51-53.
- Ammann RW, Eckert J. Cestodes. Echinococcus published erratum appears in Gastroenterol Clin North Am 1996. Gastroenterol Clin North Am 25 (1996): 655-689.
- Rossouw GJ, Knott-Craig CJ, Erasmus PE. Cardiac echinococcosis: cyst removal in a beating heart. Ann Thorac Surg 53 (1992): 328-329.
- Murat V, Qian Z, Guo S, et al. Cardiac and pericardial echinococcosis: report of 15 cases. Asian Cardiovasc Thorac Ann 15 (2007): 278-279.
- Guidelines for treatment of cystic and alveolar echinococcosis in humans. WHO Informal Working Group on Echinococcosis. Bull World Health Organ 74 (1996): 231-242.
- Djoshibaev S, Kudaiberdiev T, Maralov A, et al. Surgical treatment of isolated cardiac echinococciasis: report of five cases. Anadolu Kardiyol Derg 3 (2003): 137-143.
- Giorgadze O, Nadareishivili A, Goziridze M, et al. Unusual recurrence of hydatid cysts of the heart: report of two cases and review of the clinical and surgical aspects of the disease. J Card Surg 15 (2000): 223-228.
- Falagas ME, Bliziotis IA. Albendazole for the treatment of human echinococcosis: a review of comparative clinical trials. Am J Med Sci 334 (2007): 171-179.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
