Influence of BRCA Mutations on the Reproductive Potential of Women. A Systematic Review
Article Information
Fabra P1*, Espinòs JJ2, Checa M A1
1University of Barcelona Autonomous, Hospital del Mar-Parc de Salut Mar, Barcelona, Spain
2University of Barcelona Autonomous, Hospital de la Santa Creu in Sant Pau, Barcelona, Spain
*Corresponding author: Fabra P, University of Barcelona Autonomous, Hospital del Mar-Parc de Salut Mar, Paseo Maritimo 25-29, Barcelona, Spain
Received: 23 July 2020; Accepted: 03 August 2020; Published: 21 August 2020
Citation:
Fabra P, Espinòs JJ, Checa M A. Influence of BRCA Mutations on the Reproductive Potential of Women. A Systematic Review. Journal of Women’s Health and Development 3 (2020): 316-329.
View / Download Pdf Share at FacebookAbstract
Breast cancer is the most common cancer in women. About 3% of breast cancers are related to BRCA1 / BRCA2 mutations. It has been suggested that BRCA mutations have a negative impact on the reproductive potential of the carriers, but the clinical evidence is conflicting. The aim of this review is to identify studies that help us to evaluate it. The reproductive potential was evaluated through 1) the ovarian response to pharmacological (gonadotropin) stimulation and 2) measuring Anti-Müllerian hormone (AMH) basal levels. An exhaustive review of the literature has been carried out. All articles focused on this topic were included. Data were extracted and reported in summary tables. The risk of bias of each of the included studies was assessed. The review of the literature does not show differences when evaluating the response of BRCA carriers to an ovarian stimulation protocol. The revision seems to show slight differences in the ovarian reserve. Also, worse results were found in BRCA1 vs. BRCA2 mutation carriers. Further studies are required to verify the results.
Keywords
BRCA, Reproduction, Fertility, Breast cancer
BRCA articles BRCA Research articles BRCA review articles BRCA PubMed articles BRCA PubMed Central articles BRCA 2023 articles BRCA 2024 articles BRCA Scopus articles BRCA impact factor journals BRCA Scopus journals BRCA PubMed journals BRCA medical journals BRCA free journals BRCA best journals BRCA top journals BRCA free medical journals BRCA famous journals BRCA Google Scholar indexed journals Reproduction articles Reproduction Research articles Reproduction review articles Reproduction PubMed articles Reproduction PubMed Central articles Reproduction 2023 articles Reproduction 2024 articles Reproduction Scopus articles Reproduction impact factor journals Reproduction Scopus journals Reproduction PubMed journals Reproduction medical journals Reproduction free journals Reproduction best journals Reproduction top journals Reproduction free medical journals Reproduction famous journals Reproduction Google Scholar indexed journals Fertility articles Fertility Research articles Fertility review articles Fertility PubMed articles Fertility PubMed Central articles Fertility 2023 articles Fertility 2024 articles Fertility Scopus articles Fertility impact factor journals Fertility Scopus journals Fertility PubMed journals Fertility medical journals Fertility free journals Fertility best journals Fertility top journals Fertility free medical journals Fertility famous journals Fertility Google Scholar indexed journals "Breast cancer articles Breast cancer Research articles Breast cancer review articles Breast cancer PubMed articles Breast cancer PubMed Central articles Breast cancer 2023 articles Breast cancer 2024 articles Breast cancer Scopus articles Breast cancer impact factor journals Breast cancer Scopus journals Breast cancer PubMed journals Breast cancer medical journals Breast cancer free journals Breast cancer best journals Breast cancer top journals Breast cancer free medical journals Breast cancer famous journals Breast cancer Google Scholar indexed journals " electrochemiluminescence articles electrochemiluminescence Research articles electrochemiluminescence review articles electrochemiluminescence PubMed articles electrochemiluminescence PubMed Central articles electrochemiluminescence 2023 articles electrochemiluminescence 2024 articles electrochemiluminescence Scopus articles electrochemiluminescence impact factor journals electrochemiluminescence Scopus journals electrochemiluminescence PubMed journals electrochemiluminescence medical journals electrochemiluminescence free journals electrochemiluminescence best journals electrochemiluminescence top journals electrochemiluminescence free medical journals electrochemiluminescence famous journals electrochemiluminescence Google Scholar indexed journals AMH group articles AMH group Research articles AMH group review articles AMH group PubMed articles AMH group PubMed Central articles AMH group 2023 articles AMH group 2024 articles AMH group Scopus articles AMH group impact factor journals AMH group Scopus journals AMH group PubMed journals AMH group medical journals AMH group free journals AMH group best journals AMH group top journals AMH group free medical journals AMH group famous journals AMH group Google Scholar indexed journals oocytes articles oocytes Research articles oocytes review articles oocytes PubMed articles oocytes PubMed Central articles oocytes 2023 articles oocytes 2024 articles oocytes Scopus articles oocytes impact factor journals oocytes Scopus journals oocytes PubMed journals oocytes medical journals oocytes free journals oocytes best journals oocytes top journals oocytes free medical journals oocytes famous journals oocytes Google Scholar indexed journals BRCA mutation articles BRCA mutation Research articles BRCA mutation review articles BRCA mutation PubMed articles BRCA mutation PubMed Central articles BRCA mutation 2023 articles BRCA mutation 2024 articles BRCA mutation Scopus articles BRCA mutation impact factor journals BRCA mutation Scopus journals BRCA mutation PubMed journals BRCA mutation medical journals BRCA mutation free journals BRCA mutation best journals BRCA mutation top journals BRCA mutation free medical journals BRCA mutation famous journals BRCA mutation Google Scholar indexed journals protein articles protein Research articles protein review articles protein PubMed articles protein PubMed Central articles protein 2023 articles protein 2024 articles protein Scopus articles protein impact factor journals protein Scopus journals protein PubMed journals protein medical journals protein free journals protein best journals protein top journals protein free medical journals protein famous journals protein Google Scholar indexed journals meta-analyses articles meta-analyses Research articles meta-analyses review articles meta-analyses PubMed articles meta-analyses PubMed Central articles meta-analyses 2023 articles meta-analyses 2024 articles meta-analyses Scopus articles meta-analyses impact factor journals meta-analyses Scopus journals meta-analyses PubMed journals meta-analyses medical journals meta-analyses free journals meta-analyses best journals meta-analyses top journals meta-analyses free medical journals meta-analyses famous journals meta-analyses Google Scholar indexed journals
Article Details
1. Introduction
Breast cancer is the most common cancer in women. With an incidence of more than 2 million new cases diagnosed worldwide in 2018 it accounts for almost one in four cancer cases among women [1, 2]. Although most cases of breast cancer are sporadic, roughly 8-10% are associated with a heritable gene mutation. About 30% of these hereditary cancers are due to a mutation in the BRCA1 and BRCA2 genes. Therefore, 3% of total breast cancers are due to BRCA1/ BRCA2 mutations [3-7]. The germline mutations on these genes are inherited in an autosomal dominant way, so women who carry them have an increased lifetime risk for developing breast and ovarian cancer. The penetrance is incomplete, being the lifetime risks of breast and ovarian cancers of about 72-65% and 45-40% respectively for BRCA1, and 69-45% and 25-17% respectively for BRCA2 [3, 6, 7]. The germline mutations do not only increase the risk of developing cancer, but also the risk of developing it before 50, that is, earlier than the average of the general population [5]. It has been suggested in several studies that, in addition to increased cancer risk, BRCA mutations have a negative impact on the reproductive potential of the carriers. This hypothesis has biological plausibility. BRCA1 and BRCA2 are tumour suppressor genes acting to ensure the integrity of the genome through repair of DNA double stranded breaks [8, 9] and through maintaining the length of the telomeres [10, 11, 12]. These mechanisms of genetic damage are involved in both oocyte damage and carcinogenic transformations. Nevertheless, despite a strong biologic rationale supported by preclinical data, not all the clinical studies find a relationship between decreased fertility and BRCA mutations. Depending on the studies and the outcomes evaluated (age of menopause, parity, ovarian reserve, etc.) the conclusions are different [4, 13-21]. Therefore, this hypothesis is controversial. Given these discrepancies, the aim of this work is to summarize the evidence accumulated in the literature focused on this subject. In order to clarify the impact of BRCA 1 and BRCA 2 mutations on female fertility one main objective and two secondary objectives were defined. The main one was to evaluate the impact of a BRCA mutation on the ovarian response to a controlled ovarian stimulation measured in recovered oocytes. The secondary objectives were 1) to evaluate if there are differences in the ovarian reserve markers using blood levels of Anti-Müllerian hormone (AMH), and 2) to elucidate if there are differences between the effects of the BRCA1 and BRCA2 mutations.
2. Methods
We conducted a systematic review according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement principles [22]. All the following items were defined a priori in the design phase of this systematic review. A summary of the methodology can be seen in Table 1.
2.1 Types of studies
We have included all observational studies that report data about al least one of the selected outcome measures (retrieved oocytes and AMH). No restrictions on language, date or publication status were imposed.
2.2 Types of participants
Women who had undergone a fertility treatment with a controlled ovarian stimulation and / or an ovarian reserve test (AMH). They have been classified according to positive exposure (BRCA carriers) or negative exposure (non-BRCA carriers).
2.3 Types of exposure
The included studies are observational due to the characteristics of the data to be analyzed. Therefore, we evaluated exposures, that is, presence or not of BRCA mutations. Ideally, only BRCA mutations proven to be pathogenic because they have a verified significant disturbing effect on protein translation should be included. Information about the specific mutations affecting BRCA is part of the risk of bias in the evaluation of the studies. In the same way, the quality of the information about the protocols of controlled ovarian stimulation is considered in the evaluation.
2.4 Types of comparator
The results of the exposed population (BRCA mutation carriers) are compared with the results of the unexposed population (BRCA non-mutation). The assessment of the genetics of the non-exposed population is taken into account in the classification of the risk of bias.
2.5 Types of outcome measures
The primary outcome was the total number or the number of mature oocytes (metaphase recovered per patient after controlled ovarian stimulation. The secondary outcome was AMH blood level. AMH measurements should be made with a fully automated electrochemiluminescence immunoassay platform and results be given by ng/mL. These parameters have been chosen to prove the impairment of the reproductive capacity of BRCA mutation carriers because they are objective/numeric data. Oocyte retrieval was chosen as the main objective since it is a clinical parameter. AMH values were left as a secondary objective as they are only representing a biochemical marker. Although it was not part of the original protocol, an overview of studies that give different results between BRCA 1 and BRCA 2 has also been included. The purpose of showing these “post-hoc” data is to give a broader idea of what is being reported in the literature.
2.6 Search methods for the identification of studies to be included
A literature search was performed in parallel on MEDLINE and on SCOPUS. All references were introduced into the EndNote reference manager, where duplicated publications were identified. In addition, reverse citation was used to find relevant studies.
2.7 Selection of studies
Two independent reviewers (P.F. and J.E.) assessed the studies for inclusion in our review with a standardized procedure using a list containing the inclusion criteria, interventions and outcomes to be analyzed. Disagreements among reviewers were discussed and settled by consensus between both authors.
2.8 Data collection and analysis
A data extraction sheet was developed. It includes the following variables: name of the study, first author name, country, year of publication, type of study, study period, characteristics of the study population, number of patients (both total and BRCA mutation carriers), outcomes (AMH or mature yield oocytes). In addition, we reported if there were different results between BRCA1 and BRCA2.
2.9 Assessment of the risk of bias in the included studies
The relationship between reduced fertility and the BRCA mutation cannot be subjected to experimental studies. Therefore, all of the studies included in this review were observational ones and a high risk of bias should be expected. Even so, the review was submitted to a tool for assessing risk of bias for observational studies: ROBINS-E [23].
2.10 Registration
Our protocol has been registered in PROSPERO (International prospective register of systematic reviews).
Table 1. Summary of the methodology of this systematic review.
3. Results
The first search in the different databases yielded 383 potential records. 195 were duplicates and were subsequently removed. A secondary screening was performed with the information retrieved from the title and abstract of the remaining 188 items. 19 studies remained because they met the selection criteria of population, exposure population, comparation population and outcomes. A further full-text analysis was carried on these 19 studies 3 were excluded because their contents were not relevant for our purposes. Finally, in the full-text analysis we identified 1 additional important work through reverse citation (Figure 1). So, the total number of studies included in our review was 17.
3.1 Relevant studies and their results
The remaining 17 articles were classified into 3 groups according to their outcomes. Some articles that give valid
results are present in more than one group. The results for each group have been:
3.1.1 Recovered oocytes group: It includes those articles that relate the BRCA mutation to recovered oocytes through a follicular puncture after controlled ovarian stimulation. The results are shown in Table 2. It contains a total of 8 articles comparing the results between BRCA carriers and non- carriers in terms of recovered oocytes: 5 cohort studies and 3 case-control studies. There mutation carriers and non-carriers, and 4 articles (including 98 carriers) where they are significant. The number of MII oocytes obtained was used as the measure for those articles reporting it. When no MII data are shown, we have reported the number of oocytes retrieved, regardless of maturity.
3.1.2 AMH group: It includes those studies that relate the BRCA mutation to the ovarian reserve using AMH levels as a measure of it. The results are shown in Table 3. It contains a total of 12 articles comparing the AMH levels between BRCA carriers and non-carriers. A significant relationship between a decreased ovarian reserve and the fact of being a BRCA mutation carrier can be found in 8 of the 12 studies reporting ovarian reserve data. These 8 articles, include a total of 745 subjects with BRCA mutations vs. a total of 232 BRCA mutation carriers in studies where no differences are found.
3.1.3 BRCA1 vs BRCA2 group: Includes those articles that differentiate between BRCA 1 and BRCA 2, regardless of their methodology. The results are shown in Table 4. Of the 17 articles included in this revision, 12 articles show separated results for BRCA1 and BRCA2, while 5 do not differentiate between them. Out of the former ones, 3 report no difference between groups, 8 observed worse results for BRCA1 mutation carriers and 1 article reports worse results for BRCA2 ones.
3.2 Risk of bias
An assessment of the risk of bias of all the studies was performed using the ROBINS-E system (Table 5).
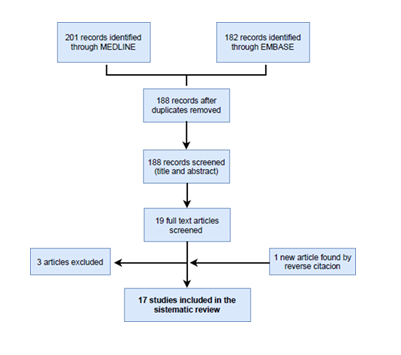
Figure 1: Flow chart of the selection process.
Table 2: Group 1: articles relating BRCA mutations with reduced recovered oocytes.
Table 3: Group 2: articles relating BRCA mutations with low ovarian reserve (AMH).
Table 4: Group 3: articles included that differentiate between BRCA 1 and BRCA 2 mutation carriers.
Table 5: Risk of bias of the included studies.
4. Discussion
The review of the data in the published literature seems to point out to no differences in terms of recovered oocytes after ovarian stimulation in women carrying the BRCA mutation. However, when we look at ovarian reserve the data seem to show a consistent trend of worse indicators. The review of the published data in the literature also seems to report worse reproductive outcomes in women who carry mutations in BRCA1 compared to BRCA2 mutation carriers. The different results obtained between AMH values-based and response to ovarian stimulation-based studies can be explained in terms of clinical relevance. Some articles find statistically significant differences in the value of the AMH, but they are small and not clinically relevant [32, 33, 35], especially when AMH values are high [8, 29, 30]. They may not be reported in the stimulation results when levels of AMH are high. Additionally, in the result of an ovarian stimulation, external factors like the stimulation protocol or follicular puncture technique could also interfere. If studies had been conducted at the time in life when small differences in AMH levels have great clinical repercussions (patients older than 35 years), the findings would be more conclusive. Probably we would see more differences in terms of recovery of mature oocytes, as reported by Giodano et al. [33]. Additionally, it has been suggested that the BRCA mutation effect on ovarian reserve manifests itself more prominently toward the end of the reproductive lifespan [14] as a result of the accumulation of DNA damage and lack of repair. Furthermore, we must not forget that AMH is an ovarian reserve marker without a clear relationship to the likelihood of spontaneous pregnancy. Some articles show that there was no difference in spontaneous parity/fertility between carriers and no-carriers [4, 19, 20, 21]. Although it could be assumed that BRCA carriers had worse reproductive outcomes, a review of the current literature shows that BRCA carriers only experience a decrease in one analytical parameter. Most probably in the daily clinic the BRCA mutation would not have a significant effect on the probability of pregnancy of our patients. Finally, the worse results in BRCA1 mutations (vs BRCA2 ones) has biological plausibility. BRCA2 has a more limited role in repairing double-stranded DNA breaks. Accordingly, BRCA2 mutations tend to develop fewer cancers and at a later age, compared to BRCA1 ones [3]. Therefore, it could be inferred that any effect derived from a mutation would be stronger in BRCA1-carriers. Mouse models would support this hypothesis [37].
The limitations of this review arise from different issues.
First, there is a lack of concordance between all the articles included. In some studies, the outcomes are considered statistically significant while in others this is not the case. The main reasons for these differences are the different types of patients included and different confounding factors intervention assessed in the multivariate analysis. As for the population, some studies use cancer patients, others women with a high risk of cancer and, others, general populations. In the first case the presence of a malignant disease can reduce the reproductive potential by itself [40] in an independent way, and in the BRCA-carriers group they would only be considering those individuals who have a higher penetrance of the mutation (they have already developed cancer). If BRCA mutation negative women at high risk of cancer are used (family history of cancer as a reason to test for BRCA), other mechanisms that reduce ovarian reserve may be acting. Finally, in the case of the general population, it is wrongly assumed that controls without a family history are all BRCA-negative. Regarding confounding factors that would have a critical effect on the risk of bias of observational studies, each article considers different ones (age, body mass index, ovarian surgeries, etc.), with some studies being stricter than others. These and other factors (reported in the risk of bias in Table 4) may be influencing the internal validity of the studies. Second, the lack of quality of evidence from included studies. Actually, the biggest limitation of the studies (and not included in the ROBBINS-E – Table 4) is, probably, their small size and, especially, the small number of carriers included. This affects their external validity and leads to different outcomes. Studies with the largest number of patients reported in this review were not prospective. To resolve some of these limitations, our group attempted to do a meta-analysis from the data gathered in this review in order to increase the sample size, obtain more precise data and better statistical power. The previously commented heterogeneity between the groups and the different ways of measuring the effects made it impossible. For example, some articles reported data in means / standard deviation, while others used in median/ interquartile range. Thus, the data could not be combined. Owing to these limitations the results should be taken with caution. The review does not show differences in the clinical results (oocyte recovery) of BRCA carriers, but we must keep in mind that, as we mentioned before, these could appear at the end of the reproductive life of women. Despite the fact that the decrease in fertility of BRCA mutation carriers does not appear to be clinically relevant, it is still important to give the right reproductive advice to these women. It must be kept in mind that they have a diminished reproductive window (early oncological pathology contraindicating pregnancy, gonadotoxic treatments and early onco-prophylactic surgery) in a society that has been already made it tighter, because of the present way of life [2, 41]. Thereby, early oocyte cryopreservation should play a role for these women regardless of their ovarian reserve: absence of need of ovarian stimulation if cancer appears, more embryos for preimplantation genetic testing and, prophylactic salpingectomy regardless of whether reproductive desire has been fulfilled. Although, apparently there are no clinical implications on the reproductive potential of BRCA patients, the results of the review are still inconclusive. A prospective multicenter study would be able to clarify the situation. Fertility is a very important aspect in women’s lives [42], including those with BRCA mutations and knowing how this condition may be affecting it should be a priority.
References
- Global cancer observatory. International Agency for research on cancer. World Health Organization (2018).
- Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio I T, et Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 30 (2019): 1194-1220.
- Antoniou AC, Cunningham AP, Peto J, Evans DG, Lalloo F, Narod SA, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and Br J Cancer 98 (2008): 1457-1466.
- Collins IM, Milne RL, McLachlan SA, Friedlander M, Hickey M, Weideman PC, et Do BRCA1 and BRCA2 mutation carriers have earlier natural menopause than their non-carrier relatives? Results from the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer. J Clin Oncol 31 (2013): 3920-3925.
- Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 25 (2007): 1329-1333.
- Karoline B Kuchenbaecker, John L Hopper, Daniel R Barnes, Kelly-Anne Phillips, Thea M Mooij, Marie-José Roos-Blom, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation JAMA 317 (2017): 2402-2416.
- Virginia A Moyer, U.S. Preventive Services Task Force. Risk assessment, genetic counselling, and genetic testing for BRCA-related cancer in women: S. Preventive Services Task Force recommendation statement. Ann Intern Med 160 (2014): 271-281.
- Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci 95 (2004): 866-871.
- Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108 (2002): 171-182.
- Ballal RD, Saha T, Fan S, Haddad BR, Rosen EM. BRCA1 localization to the telomere and its loss from the telomere in response to DNA J Biol Chem 284 (2009): 36083-36098.
- French JD, Dunn J, Smart CE, Manning N, Brown MA. Disruption of BRCA1 function results in telomere lengthening and increased anaphase bridge formation in immortalized cell lines. Genes Chromosomes Cancer 45 (2006): 277-289.
- McPherson JP, Hande MP, Poonepalli A, Lemmers B, Zablocki E, Migon E, et A role for Brca1 in chromosome end maintenance. Hum Mol Genet 15 (2006): 831-838.
- Oktay K, Turan V, Titus S, Stobezki R, Liu L. BRCA mutations, DNA repair deficiency, and ovarian aging. Biol Reprod 93 (2015): 67.
- Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer J Clin Oncol 28 (2010): 4664.
- Lin WT, Beattie M, Chen LM, Oktay K, Crawford SL, Gold EB, et Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern California. Cancer-Am Cancer Soc 119 (2013): 1652-1659.
- Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol 17 (1999): 2365-2370.
- Rzepka-Górska I, Tarnowski B, Chudecka-Glaz A, Górski B, Zielinska D, Toloczko-Grabarek A. Premature menopause in patients with BRCA1 gene mutation. Breast Cancer Res Treat 100 (2006): 59-63.
- Gal I, Sadetzki S, Gershoni-Baruch R, Oberman B, Carp H, Papa MZ, et Offspring gender ratio and the rate of recurrent spontaneous miscarriages in Jewish women at high risk for breast/ovarian cancer. Am J Hum Genet 74 (2004): 1270-1275.
- Pal T, Keefe D, Sun P, Narod SA, Hereditary Breast Cancer Clinical Study Group. Fertility in women with BRCA mutations: a case-control study. Fertil Steril 93 (2010): 1805-1808.
- Moslehi R, Singh R, Lessner L, Friedman JM. Impact of BRCA mutations on female fertility and offspring sex ratio. Am J Hum Biol 22 (2010): 201-205.
- Smith KR, Hanson HA, Mineau GP, Buys SS. Effects of BRCA1 and BRCA2 mutations on female fertility. Proc Biol Sci 279 (2012): 1389-1395.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339 (2009): b2700
- Bero L, Chartres N, Diong J, Fabbri A, Ghersi D, Lam J, et al. The risk of bias in observational studies of exposures (ROBINS-E) tool: concerns arising from application to observational studies of exposures. Syst Rev 7 (2018): 242.
- Porcu E, Cillo GM, Cipriani L, Sacilotto F, Notarangelo L, Damiano G, et al. Impact of BRCA1 and BRCA2 mutations on ovarian reserve and fertility preservation outcomes in young women with breast cancer. J Assist Reprod Genet (2019).
- Turan V, Bedoschi G, Emirdar V, Moy F, Oktay K. Ovarian Stimulation in Patients With Cancer: Impact of Letrozole and BRCA Mutations on Fertility Preservation Cycle Outcomes. Reprod Sci (2018): 26-32.
- Derks-Smeets IAP, van Tilborg TC, van Montfoort A, Smits L, Torrance HL, Meijer-Hoogeveen M, et al. BRCA1 mutation carriers have a lower number of mature oocytes after ovarian stimulation for IVF/PGD. J Assist Reprod Genet 34 (2017): 1475-1482.
- Gunnala V, Fields J, Irani M, D'Angelo D, Xu K, Schattman G, et al. BRCA carriers have similar reproductive potential at baseline to noncarriers: comparisons in cancer and cancer-free cohorts undergoing fertility preservation. Fertil Steril 111 (2019): 363-371.
- Lambertini M, Goldrat O, Ferreira AR, Dechene J, Azim HA Jr, Desir J, et al. Reproductive potential and performance of fertility preservation strategies in BRCA- mutated breast cancer patients. Ann Oncol 29 (2018): 237-243.
- Shapira M, Raanani H, Feldman B, Srebnik N, Dereck-Haim S, Manela D, et al . BRCA mutation carriers show normal ovarian response in in vitro fertilization cycles. Fertil Steril 104 (2015): 1162-1167.
- Shapira M, Raanani H, Meirow D. IVF for fertility preservation in breast cancer patients--efficacy and safety J Assist Reprod Genet 32 (2015): 1171-1178.
- Kyung-A Son, Dong-Yun Lee, DooSeok Choi. Association of BRCA Mutations and Anti-müllerian Hormone Level in Young Breast Cancer Patients Front Endocrinol (Lausanne) 10 (2019):
- Johnson L, Sammel MD, Domchek S, Schanne A, Prewitt M, Gracia C. Antimüllerian hormone levels are lower in BRCA2 mutation Fertil Steril 107 (2017): 1256-1265.
- Giordano S, Garrett-Mayer E, Mittal N, Smith K, Shulman L, Passaglia C, et al. Association of BRCA1 Mutations with Impaired Ovarian Reserve: Connection Between Infertility and Breast/Ovarian Cancer Risk. J Adolesc Young Adult Oncol 5 (2016): 337-343.
- Phillips KA, Collins IM, Milne RL, McLachlan SA, Friedlander M, Hickey M, et al. Anti-Müllerian hormone serum concentrations of women with germline BRCA1 or BRCA2 mutations. Hum Reprod 31 (2016): 1126-1132.
- Wang ET, Pisarska MD, Bresee C, Chen YD, Lester J, Afshar Y, et al. BRCA1 germline mutations may be associated with reduced ovarian reserve. Fertil Steril 102 (2014): 1723-1728.
- Pavonea ME, Mittala N, Smitha K, Barnato Giordanob S. AMH values in reproductive aged women with and without the BRCA1 mutation. Fertil Steril. September 102 (2014): e156.
- Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med 5 (2013): 172ra21.
- Michaelson-Cohen R, Mor P, Srebnik N, Beller U, Levy-Lahad E, Eldar-Geva T. BRCA mutation carriers do not have compromised ovarian reserve. Int J Gynecol Cancer 24 (2014): 233-237.
- Van Tilborg TC, Derks-Smeets IA, Bos AM, Oosterwijk JC, van Golde RJ, de Die- Smulders CE, et al. Serum AMH levels in healthy women from BRCA1/2 mutated families: are they reduced? Hum Reprod 31 (2016): 2651-2659.
- Agarwal A, Said TM. Implications of systemic malignancies on human fertility. Reprod Biomed Online 9 (2004): 673-679.
- Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, et al. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 36 (2018): 1994-2001.
- Shani Paluch-Shimon, Olivia Pagani, Ann H Partridge, Omalkhair Abulkhair, Maria-João Cardoso, Rebecca Alexandra Dent, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast 35 (2017): 203-217.


 Impact Factor: * 1.1
Impact Factor: * 1.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 