Influences of Gold King Mine Spill on the Bioaccumulation of Heavy Metals and Metalloids in Zea mays
Article Information
Joshua D Froyum, Jani C Ingram*
Department of Chemistry and Biochemistry, Northern Arizona University, Flagstaff, Arizona, USA
*Corresponding Author: Jani C Ingram, Department of Chemistry and Biochemistry, Northern Arizona University, Flagstaff, Arizona, USA
Received: 21 May 2021; Accepted: 27 May 2021; Published: 11 June 2021
Citation:
Joshua D Froyum, Jani C Ingram. Influences of Gold King Mine Spill on the Bioaccumulation of Heavy Metals and Metalloids in Zea mays. Journal of Environmental Science and Public Health 5 (2021): 342-355.
View / Download Pdf Share at FacebookAbstract
The long-term impacts of heavy metals and metalloids on Navajo Nation agricultural systems has been questioned following the Gold King Mine’s release of over 3 million gallons of acid mine drainage into the San Juan River Watershed in 2015. The present study determined the concentrations of As, Cd, Pb, and U in soil and Zea mays (corn) plants at three test sites and compared them to one control sites in 2017. All soils and corn concentrations for As, Cd, Pb, and U were below set limits. The bioaccumulation factor (BF) of each element revealed that this vegetable is a poor accumulator of As, Cd, and Pb from the soil (BF<1), and good accumulator of uranium (BF>1). To further establish if Zea mays may be considered an accumulator species, examination of the translocation factor (TF) revealed poor translocation from roots to other corn segments for all analytes (TF<1).
Keywords
Zea mays; Bioaccumulation factor; Translocation factor; Soil; Gold King Mine; EPA; FDA
Zea mays articles; Bioaccumulation factor articles; Translocation factor articles; Soil articles; Gold King Mine articles; EPA articles; FDA articles
Zea mays articles Zea mays Research articles Zea mays review articles Zea mays PubMed articles Zea mays PubMed Central articles Zea mays 2023 articles Zea mays 2024 articles Zea mays Scopus articles Zea mays impact factor journals Zea mays Scopus journals Zea mays PubMed journals Zea mays medical journals Zea mays free journals Zea mays best journals Zea mays top journals Zea mays free medical journals Zea mays famous journals Zea mays Google Scholar indexed journals Bioaccumulation factor articles Bioaccumulation factor Research articles Bioaccumulation factor review articles Bioaccumulation factor PubMed articles Bioaccumulation factor PubMed Central articles Bioaccumulation factor 2023 articles Bioaccumulation factor 2024 articles Bioaccumulation factor Scopus articles Bioaccumulation factor impact factor journals Bioaccumulation factor Scopus journals Bioaccumulation factor PubMed journals Bioaccumulation factor medical journals Bioaccumulation factor free journals Bioaccumulation factor best journals Bioaccumulation factor top journals Bioaccumulation factor free medical journals Bioaccumulation factor famous journals Bioaccumulation factor Google Scholar indexed journals Translocation factor articles Translocation factor Research articles Translocation factor review articles Translocation factor PubMed articles Translocation factor PubMed Central articles Translocation factor 2023 articles Translocation factor 2024 articles Translocation factor Scopus articles Translocation factor impact factor journals Translocation factor Scopus journals Translocation factor PubMed journals Translocation factor medical journals Translocation factor free journals Translocation factor best journals Translocation factor top journals Translocation factor free medical journals Translocation factor famous journals Translocation factor Google Scholar indexed journals Soil articles Soil Research articles Soil review articles Soil PubMed articles Soil PubMed Central articles Soil 2023 articles Soil 2024 articles Soil Scopus articles Soil impact factor journals Soil Scopus journals Soil PubMed journals Soil medical journals Soil free journals Soil best journals Soil top journals Soil free medical journals Soil famous journals Soil Google Scholar indexed journals EPA articles EPA Research articles EPA review articles EPA PubMed articles EPA PubMed Central articles EPA 2023 articles EPA 2024 articles EPA Scopus articles EPA impact factor journals EPA Scopus journals EPA PubMed journals EPA medical journals EPA free journals EPA best journals EPA top journals EPA free medical journals EPA famous journals EPA Google Scholar indexed journals FDA articles FDA Research articles FDA review articles FDA PubMed articles FDA PubMed Central articles FDA 2023 articles FDA 2024 articles FDA Scopus articles FDA impact factor journals FDA Scopus journals FDA PubMed journals FDA medical journals FDA free journals FDA best journals FDA top journals FDA free medical journals FDA famous journals FDA Google Scholar indexed journals River articles River Research articles River review articles River PubMed articles River PubMed Central articles River 2023 articles River 2024 articles River Scopus articles River impact factor journals River Scopus journals River PubMed journals River medical journals River free journals River best journals River top journals River free medical journals River famous journals River Google Scholar indexed journals Animals articles Animals Research articles Animals review articles Animals PubMed articles Animals PubMed Central articles Animals 2023 articles Animals 2024 articles Animals Scopus articles Animals impact factor journals Animals Scopus journals Animals PubMed journals Animals medical journals Animals free journals Animals best journals Animals top journals Animals free medical journals Animals famous journals Animals Google Scholar indexed journals agriculture articles agriculture Research articles agriculture review articles agriculture PubMed articles agriculture PubMed Central articles agriculture 2023 articles agriculture 2024 articles agriculture Scopus articles agriculture impact factor journals agriculture Scopus journals agriculture PubMed journals agriculture medical journals agriculture free journals agriculture best journals agriculture top journals agriculture free medical journals agriculture famous journals agriculture Google Scholar indexed journals
Article Details
1. Introduction
Colorado mining in the Animas River Watershed for gold, silver, lead, and zinc started in 1872 following a treaty with the Ute Tribe and continued until the last remaining site, the Sunnyside Mine closed in the 1991 [1, 2]. After the boom or bust mining industry sweep through, an estimated number of over 1500 abandoned mine lands remained in the San Juan Mountains, potentially harming the watershed thru the release of acid mine drainage (AMD) [3]. AMD is water that has become acidic through the oxidation of sulfide-bearing minerals by atmospheric oxygen. The product contains high concentrations of dissolved metals and metalloids commonly associated with the extraction and processing of sulfide-bearing metalliferous ore and sulfide-rich coal deposits [4, 5]. To compound the issue, approximately 8.6 million tons of the total mill tailings production have been released into streams over 120 years of active mining along the Animas River [6].
Focusing on the Gold King Mine in the Telluride mining district near Silverton, Colorado that op-erated from 1887 to 1922 before abandonment [7]. Remediation workers contracted by the Environmental Protection Agency (EPA) breached the mine entrance during a leak investigation resulting in AMD pouring out into Cement Creek in August of 2015. The yellow/orange plume of over three million gallons of AMD could be seen heading south into the Animas River, a tributary of the San Juan River that flows through the Navajo Nation [8, 9]. The San Juan River is a major source of water for drinking water, recreation, and agriculture on the Navajo Nation [10]. Investigation of the spill has lead to numerous envi-ronmental investigations, loss of income for farmers, lawsuits, and ultimately the addition of the Gold King Mine area to the Bonita Peak Mining District Superfund site in 2016 [11-14].
The bioaccumulation and translocation of metals and metalloids in Zea mays varies from element to element. For example, a study of translocation demonstrated that Ca, Fe, and Zn are found at highest concentrations in the roots. Based on plant physiology, Ca does not translocate in large amounts beyond the stems and leaves, whereas Fe and Zn move very well into other segments of the plant, relative to Ca [15]. Another study in southeast Turkey noted that corn, mint, eggplant, tomatoes, and peppers all accumulated different, but overall small concentrations of metals in their edible portions after irrigation in wastewater. In general, the translocation factors of trace elements of Cu, Mo, and Zn were higher than Cd, Co, Cr, Ni, and Pb [16]. Higher uptake of certain trace elements is likely based on bioavailable inputs, dominant soil matrix, and plant physiology among many confounding factors.
Besides differences in nutrient selectivity, there are complex interactions between elements ef-fecting plant accumulation and growth. Focusing on Al, treatments at higher concentrations of Al had no effect on the nutrient uptake Ca and Mg, but decreased of P, K, N, Mn, and Fe. The overall effect on the corn plant was reduction of biomass in the roots and shoots indicating toxicity [17]. To alleviate the toxic effects of Al3+ on the inhibition of root elongation, treatments of silicic acid as a source of Si were applied to test plots. Results showed the Al-Si complexes formed limiting the interaction of Al3+ with the corn tissues, increasing root length [18]. In a hydroponic experiment, increasing Cu treatments decreased the corn roots and shoots ability to uptake N, P, and K due to the toxicity of Cu [19]. Both of these case studies demonstrate how changes in inputs can influence accumulation by altering the chemistry. Other confounding factors include organisms that directly or indirectly influence the availability and uptake of specific elements from inputs such as water and soil. For Zea Mays there is a known symbiotic relationship between arbuscular mycorrhizae fungi (AMF) and the corn roots. As mycorrihizal colonization increased so did Cu and Zn root-shoot translocation, but the Cd and Pb decreased [20]. Corn grown with AMF on experimental plots have demonstrated the ability to inhibit Cd, Zn, Cu, Pb, and Mn uptake to some extent to protect the plant, its host.20 In another study maize inoculated with AMF prevented the uptake of As5+ and its reduction to As3+, which protected the plant from oxidative stress [21].
In the present study, the elemental concentrations of As, Cd, Pb, and U were determined by ICP-MS in soil and corn samples along the San Juan River in 2017, approximately two years following the spill. The elements of interest were chosen based on their association with acid mine drainage, except for uranium which was chosen based on historical mining exploitation in the Shiprock area of the Navajo Nation leading to the passing of the Radiation Exposure Compensation Act in 1990 [22-24]. The primary objective was to provide the communities with a regional baseline for heavy metals and metalloids to compare to with an area unaffected area of the San Juan River. The secondary objective of the project was to determine the distinctions in heavy metals and metalloid storage in the soil and corn plant segments. This would allow in-depth look at bioaccumulation factors (BF) and translocation factors (TF) of select elements specifically for corn. Information from the research will be used to advise farmers on potential long-term impacts.
2. Material and Methods
2.1 Site description
Three farm plots were selected near Shiprock and Upper Fruitland, NM on the Navajo Nation that used San Juan River irrigation water downstream of the Animas River connection. This is part of the potentially contaminated or test zone. In addition, one farm plot was selected near Bloomfield, NM that used San Juan River irrigation water prior to the Animas River connection.
|
Site ID |
Location |
Group |
|
C1 |
Bloomfield |
Control |
|
T1 |
Upper Fruitland |
Test |
|
T2 |
Shiprock |
Test |
|
T3 |
Shiprock |
Test |
Table 1: Key for control and test site locations along the San Juan River.
2.2 Sample collection
Upon arrival at each site a Garmin Trek10 GPS handheld unit was used to record the GPS coordinates in decimal meters. Important site characteristics for soil and corn were recorded on data sheets and photos of the undisturbed site were taken. A dead-blow hammer was used to drive five 2-foot PVC pipe segments with a 1.5in diameter into the agricultural field soil. The PVC pipes were driven 1-foot into the ground before extraction. The pipe ends were covered with plastic wrap and reinforced with duct tape. Five mature, Zea mays plants and their associated roots were extracted from the plots with shovels and wrapped in plastic wrap to avoid cross-contamination. This process was repeated at each site and samples were returned to the lab for analysis.
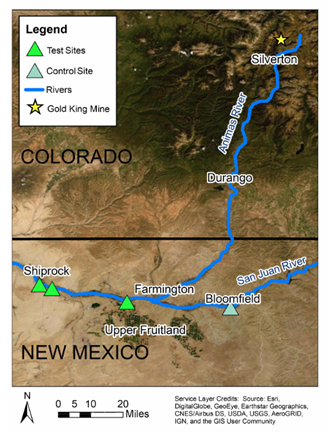
Figure 1. Topographic map showing the distribution of sampling sites along the San Juan River relative to the Gold King Mine spill in the Animas River tributary.
2.3 Soil pre-digestion
Soil cores were removed from the PVC pipes immediately following field collection by removing the plastic wrap and duct tape. Then, the core was placed into a vice grip where a clean plastic-lined shorter pipe was used to apply hand pressure or rubber mallet pressure to force to the core out of the pipe.
The top and bottom inch of the cores were segmented from the rest of the core for further digestion. The top and bottom inch were dried for 48hrs at room temperature and 2mm sieved for homogenization of each sample.
2.4 Corn pre-digestion
Corn plants were dried out on butcher paper for one week to avoid mi-crobial growth before segmentation into the following parts: kernels, cobs, husks, stems/leaves, and roots. The roots were additionally rinsed with nanopure water to remove soil. Each segment was grinded into a fine powder in a stainless steel spice grinder. Grinders were cleaned with metal-free soap (citranox) and rinsed three times with nanopure between each sample. The homogenized powders were dried for 24hrs. Approximately 20g samples of corn segments were weighed into porcelain crucibles and dry ashed in a Thermo Lindberg/Blue muffle furnace to burn off organics. The temperature ramp was an increase 15°C/min between each temperature hold. The holds were at 200°C for 2hrs, 400°C for 2hrs, and 600°C for the remainder of 48hrs time frame. The result was white/grey ash.
2.5 Soil sample digestion
Samples of 0.5000g of sifted soil and 10mL of HNO3 (67-70% essay VWR Aristar Ultrapure) were digested in the MARS6 microwave digestion system loaded with 55mL MARSXpress vessels. The EPA 3051a surface leach digestion was carried out with a 5.5min ramp to 175°C and hold time of 4.5min. Along with each soil batch NIST SRM 2709a soil sample (digestion and analysis check) and a blank sample (contamination check) was digested. Samples were filtered via Whatman 0.45μm PVDF w/PP filters and 30mL Norm-jet luer-lock PP/PE syringes into centrifuge tubes.
2.6 Corn sample digestion
Exactly 0.2000g of ashed corn, 9mL of HNO3 (67-70% essay VWR Aristar Ultrapure), and 3mL of HF (47-51% essay VWR Aristar Ultrapure) in 55mL MARSXPress vessels was digested in the MARS6. The EPA 3052 total decomposition digestion was carried out with a 5.5min ramp to 185°C and hold time of 9.5 min. Then, 1.35g of H3BO3 was added to each sample and the boric acid HF neutralization program was run on the MARS6. The program is carried out with a 20min ramp to 170°C and hold time of 10min. This neutralizes the free fluorine ions in solution. Along with each corn batch NIST SRM 2709a soil sample and a blank was digested. Samples were filtered via Whatman 0.45μm PVDF w/PP filters and 30mL Norm-jet luer-lock PP/PE syringes into clean centrifuge tubes.
2.7 Analysis
Soil digests were diluted 1:100 in 2% HNO3 and corn digests were diluted 1:10 in 2% HNO3 to a final volume of 10mL in transport tubes. Prior to dilution each sample had equal volumes of an internal standard of ruthernium-101 (Ru-101) added so that all final concentrations of Ru-101 would be 1μg/L in solution. External calibration standards of the elements of interest (As, Cd, Pb, and U) where made in 2% HNO3 with the same 1μg/L Ru-101 internal standard concentration. In addition, NIST SRM 1640a (trace metals in natural water) was diluted 1:10 in 2% HNO3 with 1μg/L of Ru-101.
Heavy metal and metalloid concentrations of standards and samples were determined by induc-tively couple plasma – mass spectrometry (ICP-MS). Raw counts per minute of analytes were collected and used to generate calibration curves of showing the concentrations of each analyte to its response. The linear fit equation was used to calculate each unknown samples diluted concentration of each select analyte. Utilizing dilution factors and masses of dried samples, the undiluted concentration could be computed for reporting.
Analyte specific limits of detection for the Thermo XSeries II ICP-MS were determined by running blank solutions. Any instrument response that fell below the following limits of detection after calculation with the linear fit equation were deemed not quantifiable under our instrument configurations: As=0.026ug/L, Cd=0.039ug/L, Pb=0.008ug/L and U=0.014ug/L. These results were reported as below detection limits (BDL).
3. Results and Discussion
3.1 Soil
Analysis by ICP-MS determined that the As, Cd, Pb, and U concentrations in all agri-cultural field topsoil samples did not exceed the Agency for Toxicological Substances and Disease Reg-istry (ATSDR) US natural soil averages: As 3-4mg/kg, Cd 0.06-1.1mg/kg, Pb 50-200mg/kg and U 3-5mg/kg. In addition, the topsoil results were far below the New Mexico Department of Environment soil screening levels (SSLs) for residential soils of 13, 70.5, 400, and 234 mg/kg for As, Cd, Pb, and U re-spectively. This indicates that no action is needed to remediate this soil for these select elements.
An emergent trend was that control site C1 topsoil had lower As, Cd, Pb, and U than all test site topsoil. A student T-test was used to test the statistical significance of this trend. All of the p-values of C1 to the test sites (T1,T2,T3) for each analyte were less than the alpha value of 0.05 and confirming C1 to have statically different average concentrations of As, Cd, Pb, and U. This could indicates some source of elevation in the test sites that use San Juan River water to irrigate, but it is likely the natural difference in the geological composition from site to site because the concentrations are not elevated above natural levels.
The low topsoil concentrations are consistent with the US EPA’s estimation that 500,000 kg of particulate and dissolved metals was released during the spill event rushing down the Animas River in the form of the yellow water plume before temporary storage in riverbanks and sediments [25]. The EPA concluded that all the GKMS metals were delivered to Lake Powell after the remobilization by flood events and snowmelt by the end of the 2016 snowmelt season because the 2017 snowmelt data was back to pre-spill ranges for the Animas River [26].
|
SITE |
As (ppm) |
Cd (ppm) |
Pb (ppm) |
U (ppm) |
|
C1 |
1.61 ± 0.34 |
0.07 ± 0.02 |
4.21 ± 0.26 |
0.16 ± 0.04 |
|
T1 |
3.49 ± 0.24 |
0.19 ± 0.02 |
19.07 ± 4.89 |
0.48 ± 0.04 |
|
T2 |
3.62 ± 0.22 |
0.33 ± 0.04 |
21.92 ± 1.58 |
0.57 ± 0.05 |
|
T3 |
4.54 ± 0.23 |
0.33 ± 0.02 |
28.39 ± 0.30 |
0.60 ± 0.05 |
Table 2: Agricultural topsoil concentrations and standard deviations reported in ppm for each site.
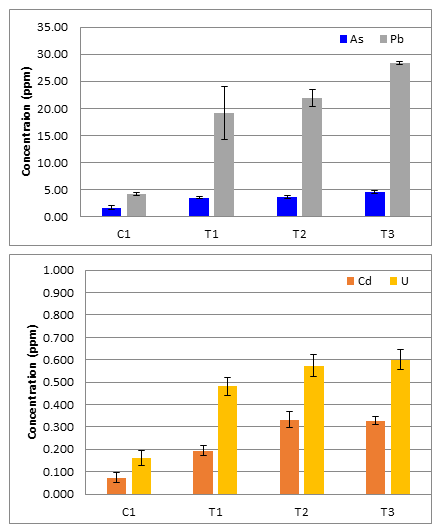
Figure 2: (A) Average topsoil As and Pb concentrations with standard deviations for error bars (N=5); (B) Average topsoil Cd and U concentrations with standard deviations for error bars (N=5).
3.2 Corn
The average concentration for each analyte post ICP-MS analysis were produced after the blank matrix composed of diluted HNO3, HF, and H3BO4 was subtracted from all the corn segments to account for the metal and metalloids added during the digestion process (Table 3). Some of the segments were below detection level (BDL) for select analytes and the cobs of site T1 were not collected (NC). To compare each site side by side for each corn segment the bar charts were created for each segment (Figure 3). One trend was that roughly overall concentrations for each site decreased moving up from the roots to the shoots to the fruiting body. Currently there are very few limits for heavy metal and metalloids in plants. To focus on the im-pact of the part consumed by humans, the corn kernel was compared to the Food and Drug Admin-istration (FDA), Environmental Protection Agency (EPA), and European Commission (EC) for safe limits and daily intake levels of As, Cd, Pb, and U. Results concluded eating corn on the cob from each site was would not exceed any of these various agencies recommendations.
The Student TTEST for the control site, C1, kernels concentrations to each test site kernels concentrations with a two-tailed distribution and unequal variance produced some interesting develop-ments. The t-test confirmed the higher As concentration and lower Cd concentration for the control site was statically a different than the test sites with p-values less than 0.05. On the other hand the Pb and U concentrations of the control site were statistically similar with p-values greater than 0.05. Based on this information, there contradicting evidence none of the test sites are elevated above the control site for Cd, Pb, and U, but in fact the As is elevated at the control site. Still all concentrations are at very low levels in the corn kernels.
|
SITE |
As (ppb) |
Cd (ppb) |
Pb (ppb) |
U (ppb) |
|
C1-K |
1.51 ± 0.34 |
2.03 ± 0.04 |
10.03 ± 0.94 |
BDL |
|
T1-K |
BDL |
4.40 ± 0.05 |
6.02 ± 0.66 |
0.42 ± 0.02 |
|
T2-K |
BDL |
15.09 ± 0.08 |
3.25 ± 0.32 |
BDL |
|
T3-K |
BDL |
5.92 ± 0.21 |
1.59 ± 0.21 |
0.14 ± 0.03 |
|
C1-C |
15.55 ± 1.26 |
2.90 ± 0.10 |
34.76 ± 6.71 |
0.31 ± 0.18 |
|
T1-C |
NC |
NC |
NC |
NC |
|
T2-C |
4.13 ± 0.56 |
71.43 ± 7.23 |
23.62 ± 6.55 |
BDL |
|
T3-C |
14.74 ± 1.47 |
14.74 ± 1.47 |
2.83 ± 1.32 |
BDL |
|
C1-H |
BDL |
2.37 ± 0.18 |
3.14 ± 0.47 |
BDL |
|
T1-H |
BDL |
31.71 ± 0.32 |
23.48 ± 0.28 |
0.40 ± 0.04 |
|
T2-H |
BDL |
69.88 ± 0.56 |
7.67 ± 0.41 |
BDL |
|
T3-H |
8.78 ± 2.39 |
37.11 ± 0.36 |
57.04 ± 1.90 |
3.59 ± 0.20 |
|
C1-S |
121.2 ± 4.2 |
18.46 ± 0.18 |
131.7 ± 4.2 |
12.34 ± 0.37 |
|
T1-S |
20.90 ± 0.62 |
59.38 ± 0.62 |
121.2 ± 1.4 |
9.45 ± 0.37 |
|
T2-S |
30.50 ± 4.52 |
184.5 ± 1.4 |
252.5 ± 2.9 |
19.26 ± 0.69 |
|
T3-S |
67.31 ± 4.47 |
200.2 ± 1.5 |
186.8 ±5.3 |
16.01 ± 0.68 |
|
C1-R |
955.6 ± 7.1 |
63.16 ± 1.65 |
3707 ± 30 |
490.3 ± 6.0 |
|
T1-R |
685.3 ± 38.0 |
143.3 ± 3.0 |
6985 ± 78 |
487.1 ± 6.0 |
|
T2-R |
1432 ± 27 |
223.7 ± 10.3 |
8285 ± 107 |
789.6 ± 8.4 |
|
T3-R |
1831 ± 21 |
224.4 ± 4.0 |
10220 ± 192 |
759.4 ± 22.0 |
Table 3: Means and standard deviations for all sites separated by each corn segment in ng/g of dry biomass or ppb, (N=5 and BDL = below detection limit). Where the end letter K=kernels, C=cobs, H=husks, S=stems, and R=roots.
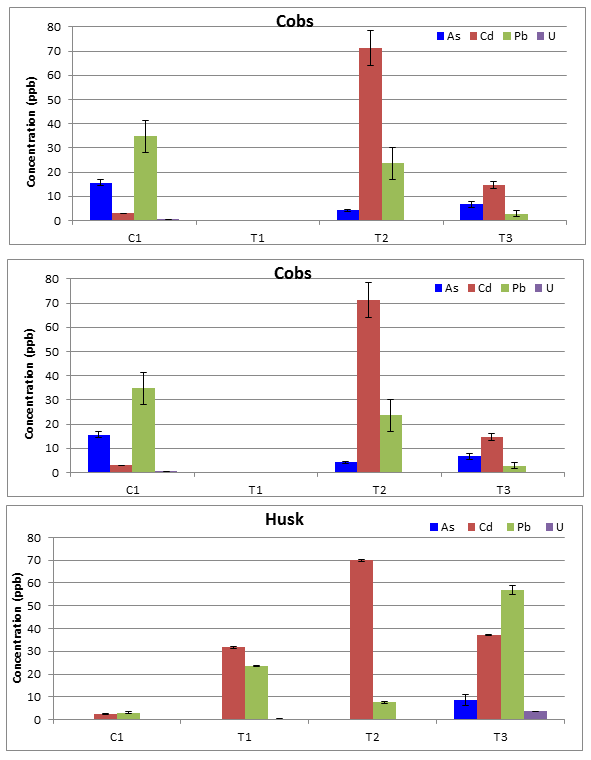
Figure 3: Average As, Cd, Pb, and U concentrations for corn segments with standard deviations for error bars, N=5, and BDL= below detection limits. (A) Kernels (B) Cobs (C) Husks (D) Stems/Leaves (E) Roots.
Bioaccumulation is defined as the buildup of contaminants in an organism from water, soil, air, and diet. To quantify the speed at which the buildup occurs, a bioaccumulation factor (BF) is used for biota. BF was calculated in this paper as the ratio of concentration of As, Cd, Pb, and U in the biomass part of the corn plant to the concentration in the corresponding soil in which it was grown: BF = Cpart/ Csoil. In general, when the BF<1 the plant can be an excluder, BF=1 the plant has no influence, and BF>1 the plant can be an accumulator. Another way to assess bioaccumulation in plants is to look at the translocation factor (TF) or the transfer of elemental concentrations through the plant moving from roots to above ground segments. TF is calculated by taking the ratio of the concentrations in the segments of the corn plant to the corn roots: TF = Cpart/Croots.
The key result from the BF was that the all sites kernels, cobs, and husks, and stems/leaves were poor accumulators or potential excluders of the As, Cd, Pb, and U with BF<1 from the soil. For the root segments As, Cd, and Pb were classified as excluded with BF<1 and U was with a BF>1 for all sites. This set uranium apart as the element with the best bioaccumulation in the corn plant compared to the other select analytes. For all segments of the corn plant the TF<1 for As, Cd, Pb, and U for all sites. The highest TF was the translocation from roots to stems/leaves, with much lower translocation to the fruit. An addition trend noted was that Cd had higher BFs and TFs relative to As, Cd, and Pb when looking at the plant as a whole. This indicated different biological processes allowing for the absorption and storage of Cd throughout the plant, not just the roots like uranium. Corn crops grown near an abandoned manganese mine in Guangxi Providence, China demonstrated similar Cd results to this study with an average BFCd around 0.66 for the whole plant [27]. Contrary to this study, corn grown in fields contaminated with dredging sludge confirmed Zn and Cd remediation would be possible with corn [28]. Both of the studies demonstrate that the unique elemental composition of the base soil has the greatest effect on the bioaccumulation and translocation of Zea mays.
|
Segment |
Site ID |
Bioaccumulation Factor, BF |
Translocation Factor, TF |
|||||||
|
BFAs |
BFCd |
BFPb |
BFU |
TFAs |
TFCd |
TFPb |
TFU |
|||
|
Kernels |
C1 |
0.001 |
0.028 |
0.002 |
0.000 |
0.002 |
0.032 |
0.003 |
0.000 |
|
|
T1 |
0.000 |
0.023 |
0.000 |
0.001 |
0.000 |
0.031 |
0.001 |
0.001 |
||
|
T2 |
0.000 |
0.045 |
0.000 |
0.000 |
0.000 |
0.067 |
0.000 |
0.000 |
||
|
T3 |
0.000 |
0.018 |
0.000 |
0.000 |
0.000 |
0.026 |
0.000 |
0.000 |
||
|
Cobs |
C1 |
0.010 |
0.040 |
0.008 |
0.002 |
0.016 |
0.046 |
0.009 |
0.001 |
|
|
T1 |
NC |
NC |
NC |
NC |
NC |
NC |
NC |
NC |
||
|
T2 |
0.001 |
0.215 |
0.001 |
0.000 |
0.003 |
0.319 |
0.003 |
0.000 |
||
|
T3 |
0.001 |
0.045 |
0.000 |
0.000 |
0.004 |
0.066 |
0.000 |
0.000 |
||
|
Husks |
C1 |
0.000 |
0.033 |
0.001 |
0.000 |
0.000 |
0.038 |
0.001 |
0.000 |
|
|
T1 |
0.000 |
0.164 |
0.001 |
0.001 |
0.000 |
0.221 |
0.003 |
0.001 |
||
|
T2 |
0.000 |
0.210 |
0.000 |
0.000 |
0.000 |
0.312 |
0.001 |
0.000 |
||
|
T3 |
0.002 |
0.113 |
0.002 |
0.006 |
0.005 |
0.165 |
0.006 |
0.005 |
||
|
Stems/ Leaves |
C1 |
0.075 |
0.254 |
0.031 |
0.076 |
0.127 |
0.292 |
0.036 |
0.025 |
|
|
T1 |
0.006 |
0.307 |
0.006 |
0.020 |
0.030 |
0.414 |
0.017 |
0.019 |
||
|
T2 |
0.008 |
0.555 |
0.012 |
0.034 |
0.021 |
0.825 |
0.030 |
0.024 |
||
|
T3 |
0.015 |
0.609 |
0.007 |
0.027 |
0.037 |
0.892 |
0.018 |
0.021 |
||
|
Roots |
C1 |
0.595 |
0.870 |
0.881 |
3.034 |
|||||
|
T1 |
0.196 |
0.741 |
0.366 |
1.013 |
||||||
|
T2 |
0.396 |
0.673 |
0.378 |
1.376 |
||||||
|
T3 |
0.403 |
0.683 |
0.360 |
1.264 |
||||||
Table 4: The bioaccumulation and translocation factors used to determine potential corn’s ability to be an accumulator or excluder plant.
4. Conclusion
In conclusion, the soil and corn grown in 2017 on all agricultural field sites in the investigation were safe under the current regulations. Based on the bioaccumulation and translocation, the corn plant is not an accumulator of As, Cd, Pb and U to levels that would aid in phytoremediation efforts. Information from this study could lead to more Cd studies to understand the transport in corn plants.
Further, elemental investigations with multiple factors in regions susceptible to environmental contami-nation from abandoned mine lands should be conducted to help understand the complex chemical in-teractions with the physiology of agricultural crops.
Acknowledgments
The authors would like to acknowledge the Ingram research lab and collaborators from University of Arizona and Dine’ College for their contributions to this work. They would also like to thank the community members of Shiprock and Upper Fruitland, New Mexico for their contributions.
Finally, the authors acknowledge the National Institute for Environmental Health Sciences (Grant Number R21ES026948) and Haury Foundation (Strength through the Diné (Navajo) clan system to respond to the Gold King Mine Spill) for funding support.
References
- USGS Professional Paper 1651. Integrated Investigations of Environmental Effects of His-torical Mining in the Animas River Watershed, San Juan County, Colorado (2008).
- Raines E. Colorado Gold: Part 2: The Discovery, Mining History, Geology, and Specimen Miner-alogy of Selected Occurrences in Central Colorado and the San Juans. Rocks Miner 72 (1997): 304-320.
- Buxton HT et al. A Science-Based, Watershed Strategy to Support Effective Remediation of Abandoned Mine Lands; Fourth International Conference on Acid Rock Drainage (ICARD); Vancouver, British Columbia, Canada (1997): 1869-1880.
- Blowes DW, Ptacek CJ, Jambor JL, et al. The Geochemistry of Acid Mine Drainage: Chapter 9 Section 5. In Treatise on Geochemistry; Holland HD. Turekian, K. K., Eds.; Pergamon: Oxford (2003): 149-204.
- Johnson DB, Hallberg KB. Acid Mine Drainage Remediation Options: A Review. Sci. Total Environ 338 (2005): 3-14.
- Mast MA, et al. Natural Sources of Metals to Surface Waters in the Upper Animas River Watershed, Colorado. Soc Mining Metallurgy and Exploration Inc; Littleton (2000): 513-522.
- Chief K, Artiola JF, Wilkinson ST, et al. Understanding the Gold King Mine Spill 11 (2016).
- Mines E. The Mineralogy, Geology, and Mining History of the Telluride District, San Miguel County, Colorado: A Historical Overview of the District and the Smuggler Union and Associated Mines and Veins of Marshall Basin. vdocuments. site 75 (2000): 318-342.
- Roberts K. A Legacy That No One Can Afford to Inherit: The Gold King Disaster and the Threat of Abandoned Hardrock Legacy Mines. J. Natl. Assoc. Adm. Law Judic 36 (2016): 361-363.
- Rodriguez-Freire L, Avasarala S, Ali AM S, et al. Post Gold King Mine Spill Investigation of Metal Stability in Water and Sediments of the Animas River Watershed. Environ. Sci. Technol 50 (2016): 11539-11548.
- Health MS, Friday, topics reporter; Aug. 5; Saturday, 2016 10:00 PM Updated; Aug. 6; Am 11 (2016): 13.
- Hensch M. Navajo Nation sues EPA over mine spill (2018).
- Superfund: EPA’s Gold King cleanup workers find lead contamination (2018).
- US EPA. Bonita Peak Mining District Site Profile (2019).
- Frossard E, Bucher M, Mächler F, et al. Potential for Increasing the Content and Bioavailability of Fe, Zn and Ca in Plants for Human Nutrition. J. Sci. Food Agric 80 (2000): 861-879.
- Avci H, Deveci T. Assessment of Trace Element Concentrations in Soil and Plants from Cropland Irrigated with Wastewater. Ecotoxicol. Environ. Saf 98 (2013): 283-291.
- Lidon FC, Azinheira HG, Barreiro MG. Aluminum Toxicity in Maize: Biomass Pro-duction and Nutrient Uptake and Translocation. J. Plant Nutr 23 (2000): 151-160.
- Ma JF, Sasaki M, Matsumoto H. Al-Induced Inhibition of Root Elongation in Corn, Zea Mays L. Is Overcome by Si Addition. Plant Soil 188 (1997): 171-176.
- Ali N A, Bernal MP, Ater M. Tolerance and Bioaccumulation of Copper in Phragmites Australis and Zea Mays. Plant and Soil 239 (2002): 103-111.
- Weissenhorn I, Leyval C, Belgy G, et al. Arbuscular Mycorrhizal Contribution to Heavy Metal Uptake by Maize (Zea Mays L.) in Pot Culture with Contaminated Soil. Mycorrhiza 5 (1995): 245-251.
- Yu Y, Zhang S, Huang H, et al. Arsenic Accumulation and Speciation in Maize as Affected by Inoculation with Arbuscular Mycorrhizal Fungus Glomus Mosseae. J. Agric. Food Chem 57 (2009): 3695-3701.
- Brugge D, Goble R. The History of Uranium Mining and the Navajo People. Am. J. Public Health 92 (2002): 1410-1419.
- McLemore V. Uranium Industry in New Mexico-History, Production, and Present Status. New Mexico Geology (1983): 45-51.
- Radiation Exposure Compensation Act. (2019).
- One Year After the Gold King Mine Incident: A Retrospective of the EPA’s Efforts to Re-store and Protect Impacted Communities ( 2016).
- Kathleen Sullivan, Cyterski M. Post Gold King Mine Release Water Quality in the Animas and San Juan Rivers during Spring Snowmelt Updated with 2017 Water Sampling (2018).
- Liu K, Fan L, Li Y, et al. Concentrations and Health Risks of Heavy Metals in Soils and Crops around the Pingle Manganese (Mn) Mine Area in Guangxi Province, China. Environ. Sci. Pollut. Res. Int 25 (2018): 30180-30190.
- Arbaoui S, Evlard A, Mhamdi MEW, et al. Potential of Kenaf (Hibiscus Cannabinus L.) and Corn (Zea Mays L.) for Phytoremediation of Dredging Sludge Contaminated by Trace Metals. Biodegradation 24 (2013): 563-567.


 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 