Multimodal Molecular Imaging Strategies using Functionalized Nano Probes
Article Information
Karunanithi Rajamanickam*
Faculty of Allied Health Sciences, Chettinad Academy of Research & Education (CARE), Chennai, India
*Corresponding author: Karunanithi Rajamanickam, Faculty of Allied Health Sciences, Assistant Professor (Sel. Gr) [physics], Chettinad Academy of Research & Education (CARE), Kelambakkam, Chennai 603 103, India
Received: 05 December 2019; Accepted: 17 December 2019; Published: 21December 2019
Citation:
Karunanithi Rajamanickam, Multimodal Molecular Imaging Strategies Using Functionalized Nano Probes. Journal of Nanotechnology Research 1 (2019): 119-135
View / Download Pdf Share at FacebookAbstract
Cancer prognosis was mainly done by imaging and biopsy with histological analysis of solid tumours. Identifying tumour margins and staging of the tumour are critical for choosing appropriate treatments so that the risk of reoccurrence could be minimized. Imaging modalities have their strengths and limitations. Hence multimodality imaging that takes advantage of strengths from two or more imaging techniques may serve improved diagnostic and therapeutic monitoring abilities. Radiolabelled small molecules have been used as contrast agents to detect tumour for prognosticating therapeutic interventions. However, these probes lack tissue specificity and stability for optimal usage. Quantum dots (QDs) are fluorescent probes for optical imaging, which are capable of tunable optical properties, ability to target tumours when their surface-functionalized with high stability. Recently, a wide application of biocompatible surface-functionalized QDs is evidenced as multimodal imaging probes. However, their pathway to clinical translation is not yet fully explored. In this review, different biocompatible functionalized nanoprobes, their in-vivo application in animal model followed by their future possible clinical applications, recent developments of optical fluorescence imaging probes and its integration with other imaging modalities such as X-ray computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), single-photon emission computed tomography (SPECT) and ultrasonography (US) are discussed.
Keywords
Multimodal molecular imaging, MRI, CT, SPECT, PET, Ultrasonography, Nanoprobes, Quantum dots
Multimodal molecular imaging articles Multimodal molecular imaging Research articles Multimodal molecular imaging review articles Multimodal molecular imaging PubMed articles Multimodal molecular imaging PubMed Central articles Multimodal molecular imaging 2023 articles Multimodal molecular imaging 2024 articles Multimodal molecular imaging Scopus articles Multimodal molecular imaging impact factor journals Multimodal molecular imaging Scopus journals Multimodal molecular imaging PubMed journals Multimodal molecular imaging medical journals Multimodal molecular imaging free journals Multimodal molecular imaging best journals Multimodal molecular imaging top journals Multimodal molecular imaging free medical journals Multimodal molecular imaging famous journals Multimodal molecular imaging Google Scholar indexed journals MRI articles MRI Research articles MRI review articles MRI PubMed articles MRI PubMed Central articles MRI 2023 articles MRI 2024 articles MRI Scopus articles MRI impact factor journals MRI Scopus journals MRI PubMed journals MRI medical journals MRI free journals MRI best journals MRI top journals MRI free medical journals MRI famous journals MRI Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals SPECT articles SPECT Research articles SPECT review articles SPECT PubMed articles SPECT PubMed Central articles SPECT 2023 articles SPECT 2024 articles SPECT Scopus articles SPECT impact factor journals SPECT Scopus journals SPECT PubMed journals SPECT medical journals SPECT free journals SPECT best journals SPECT top journals SPECT free medical journals SPECT famous journals SPECT Google Scholar indexed journals PET articles PET Research articles PET review articles PET PubMed articles PET PubMed Central articles PET 2023 articles PET 2024 articles PET Scopus articles PET impact factor journals PET Scopus journals PET PubMed journals PET medical journals PET free journals PET best journals PET top journals PET free medical journals PET famous journals PET Google Scholar indexed journals Ultrasonography articles Ultrasonography Research articles Ultrasonography review articles Ultrasonography PubMed articles Ultrasonography PubMed Central articles Ultrasonography 2023 articles Ultrasonography 2024 articles Ultrasonography Scopus articles Ultrasonography impact factor journals Ultrasonography Scopus journals Ultrasonography PubMed journals Ultrasonography medical journals Ultrasonography free journals Ultrasonography best journals Ultrasonography top journals Ultrasonography free medical journals Ultrasonography famous journals Ultrasonography Google Scholar indexed journals Nanoprobes articles Nanoprobes Research articles Nanoprobes review articles Nanoprobes PubMed articles Nanoprobes PubMed Central articles Nanoprobes 2023 articles Nanoprobes 2024 articles Nanoprobes Scopus articles Nanoprobes impact factor journals Nanoprobes Scopus journals Nanoprobes PubMed journals Nanoprobes medical journals Nanoprobes free journals Nanoprobes best journals Nanoprobes top journals Nanoprobes free medical journals Nanoprobes famous journals Nanoprobes Google Scholar indexed journals Quantum dots articles Quantum dots Research articles Quantum dots review articles Quantum dots PubMed articles Quantum dots PubMed Central articles Quantum dots 2023 articles Quantum dots 2024 articles Quantum dots Scopus articles Quantum dots impact factor journals Quantum dots Scopus journals Quantum dots PubMed journals Quantum dots medical journals Quantum dots free journals Quantum dots best journals Quantum dots top journals Quantum dots free medical journals Quantum dots famous journals Quantum dots Google Scholar indexed journals tomographic articles tomographic Research articles tomographic review articles tomographic PubMed articles tomographic PubMed Central articles tomographic 2023 articles tomographic 2024 articles tomographic Scopus articles tomographic impact factor journals tomographic Scopus journals tomographic PubMed journals tomographic medical journals tomographic free journals tomographic best journals tomographic top journals tomographic free medical journals tomographic famous journals tomographic Google Scholar indexed journals specimens articles specimens Research articles specimens review articles specimens PubMed articles specimens PubMed Central articles specimens 2023 articles specimens 2024 articles specimens Scopus articles specimens impact factor journals specimens Scopus journals specimens PubMed journals specimens medical journals specimens free journals specimens best journals specimens top journals specimens free medical journals specimens famous journals specimens Google Scholar indexed journals
Article Details
1. Introduction
To date, there is a high demand for sensitive and accurate diagnostic and therapeutic approaches to alleviate numerous medical problems. There is a significant developments observed in imaging techniques in preclinical and clinical translational research in recent times [1]. In particular, fluorescence optical imaging is widely used in in-vitro investigations and gained clinical application in optical imaging-guided surgeries improving precise removal of tissue resection without affecting normal sections [2]. Cancer is a leading devastating cause of death accounting for an estimated 9.6 million deaths in 2018 worldwide. The most common cancers are cervical, lung, breast, colorectal, prostate, skin and stomach [3-7]. Diagnosing cancer depends on either examining the tumour by histopathalogical or non-invasive imaging methods or both in combination. In routine clinical investigations, disease diagnosis includes computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), single photon emission CT (SPECT) ultrasonography, and optical imaging. These tomographic techniques help to determine morphological changes only when the tumour reaches a significant size and are detectable with good resolution and cancerous tissue contrast with respect to healthy tissue background [8-10]. However, these imaging techniques demands high cost and requires relatively long imaging time [11]. Real-time imaging is important to completely excise the tumour without affecting normal tissue, which otherwise may lead to loss of function. Furthermore, it helps in the collection of biopsy specimens for staging cancer and serves clinician in surgical and therapeutic interventions. Fluorescent small molecules and radiolabeled albumins (e.g., technetium-99m) [12], are commonly used agents identify lymph nodes as they are highly sensitive, however, it lacks specificity for tumour and low signal-to-noise ratio and associated with harmful radiation effects. Fluorescence imaging is highly sensitive and its resolution is in the order of micro-meter at the tissue surface. However, fluorescence imaging in the clinical settings are limited in use due to requirement of more depth of penetration, good signal intensity, and stability [13]. Therefore, designing the probe that overcomes all these limitations is essential for extensive clinical application specifically during surgical procedure. Fluorescent molecular probes have been widely used to target biomolecules like expressed proteins, cells, and tissues fragments [14]. Fluorescent dye such as indocynanine green is used invivo in human for visualizing vertebral arteries prior surgical procedure [15-17]. Despite such wide applications, these probes are poor in their photostability and sensitivity. Prognosis at an early stage is a major challenge and is poor for solid tumours before it metastasis [18]. Therefore, early detection at molecular stage, which could be easier to treat is very important for asymptomatic and some aggressive cancers [19]. Quantum dots application has been growing exponentially not only in industry but also in medical domain [20]. QDs have great potential for various biomedical applications such as sensors, imaging and therapy agents. A single probe that integrates multiple imaging contrasting agents is ideal for multimodality imaging applications. It also should possess reduced toxicity for successful clinical translational research in later stages [21]. The present review summarizes the advantages and limitations of current diagnostic techniques and latest developments in this field using functionalized nano formulation for various medical applications.
2. Nanoprobes for Theranostic Application
Fluorescent dyes currently approved by the FDA for cancer imaging lack penetration depth more than 1cm [13]. QDs are versatile elements for optical cancer imaging as their optical properties are tunable and unique compared to aforementioned small molecule dyes. Quite a number of QDs formulations for theranostic application are currently available [22-26]. Due to their broad excitation and narrow emission spectrum, QDs can be used as multimodal contrast imaging agents which could be employed in positron emission tomography(PET), CT, infrared, fluorescence (optical imaging), MRI single photon emission computed tomography (SPECT) and ultrasonography (US) applications [27-29] (Figure – 1). Hence, the size, surface chemistry, spectral properties (fluorescence ranging from the UV-blue of the mid-IR), and stability (long photoluminescence lifetime) of QDs can be easily tuned to optimize in-vivo imaging. A large Stokes shift, long fluorescence property, narrow emission band and near-infra-red emission is the fundamental properties for an ideal QD which could make it suitable for deeper imaging with high resolution [30].
3. Surface Modification and Shell Capping of QDs
QDs are generally hydrophobic and therefore require suitable surface modification prior to biological applications. Also, most QDs include heavy metal ions which are toxic (e.g. Cd and Te). When these QDs encounters UV excitation source, these heavy metal ions escape from the crystal and may cause cytotoxicity. Hence several studies have been done on developing suitable QDs surface modification which improves stability, solubility in aqueous medium, biocompatibility, fluorescence intensity with reduced cytotoxicity [28, 31-35]. Hence, some fundamental properties are required to target solid cancerous tumour; viz., fluorescent core material that have broad excitable and emission range from ultra-violet to near-infra-red region (NIR) (e.g. Ag2Se); Semiconducting shell with improved quantum efficiency and enhanced photostability; Hydrophilic ligands for attaching biomolecules such as follate, anti-body etc., with the QDs (e.g. polyethylene glycol), and biomolecule conjugation for active targeting (e.g. folate, antibody etc.). For enhancing fluorescent property of QDs, a wide band gap semiconductor shell coating is practiced since 1990 [36, 37]. Coating with suitable material shell also enhances photostability and photoluminescence quantum yields [38]. To functionalize QDs with suitable biomolecules to enable it to target specific proteins expressed on the surface of the cancer cells, they can be surface functionalized depends on various aspects of tumour microenvironment such as heterogeneous upregulation of surface proteins [39], activity of enzymes [40] and pH [41]. Hence, for wide theranostic biomedical applications, QDs are defined as nanoparticles with fluorescent core, semiconductor shell, surface modified for dispersion in water and functionalized with suitable biomolecule to target expressed proteins by cancer cells. QDs are also said to leak especially through permeable tumour vessels and are retained due to reduced lymphatic drainage, which is known as the enhanced permeability and retention (EPR) effect [42]. However, tumour microenvironment factors and EPR effect alone may not be sufficient to target tumours. Hence recent studies have intended for incorporating active targeting moieties to improve tumour site accumulation and cancer cell-specific interactions with cancerous tissue compared to healthy tissue. QDs coated with polydentate phosphine aided in visual guidance throughout complete resection in the sentinel lymph node real-time mapping procedure in large animals [43].
4. Multimodal Nano Probes
Currently there are several medical imaging diagnostic tools in use for routine clinical utility. Among them, fluorescence imaging (FLI), magnetic resonance (MR), and computed tomography (CT) imaging are the most commonly applied scanning techniques. However, each modality has its own limitation and cannot provide complete information [44]. For example FL imaging can provide high sensitive but poor resolution images [45, 46]. CT imaging can provide a high spatial resolution and anatomical information for the tissues but with poor soft tissue contrast and lack sensitivity [47]. MR on the other hand can provide soft tissue contrast but its sensitivity is poor [48]. The main challenge in developing multi-modality probes is that they must integrate multi imaging contrast agents. For instance, CT requires much larger amount contrast agents for efficient signal readout than what optical imaging require. This large amount of contrast agent may further increase the toxicity level and solubility issues. Hence, biocompatibility is critical to warrant the safety in their further in vivo bioimaging applications. Hence, development of multi modal imaging probes that combines the advantages of every imaging modality would definitely improve the accuracy and sensitivity in disease diagnosis. Studies have shown the utility of these multifunctional nanoprobes combining fluorescence, magnetic resonance and computed tomography that are promising and versatile agents for diagnosing tumours [49]. The use of these nano-probes in multimodal imaging is illustrated in figure – 2.
QDs can be synthesized for bimodal or multimodal applications suitable for infrared fluorescence, positron emission tomography (PET), SPECT, CT, MRI and ultrasonography imaging. Studies have proven QDs utilization in real-time imaging of cancer in-vitro and in-vivo [50-52]. Appropriate biomolecules are attached with QDs for targeting specific cancer markers which are found in the tumour microenvironment. For example, prostate-specific antigen [53-55], HER2 [56, 57], folic acid [58, 59] and CD44 [60]. The biomolecules that are attached to QDs are monoclonal antibody, or immunoglobulin , or peptide [61] [61, 62] depending on the target cell expressions. QDs encapsulated with paramagnetic liposomes are used to monitor tumour angiogenesis using MRI [63, 64]. Stroh M and colleagues showed permeability of the tumour microvasculature in its microenvironment using different sized QDs [62]. Functionalized QDs with paramagnetic dendritic wedges and (Asn-Gly-Arg) peptides were used to demonstrate angiogenesis in tumour microenvironment and in myocardial infarction using MR molecular imaging [65, 66]. Apart from MR applications, QDs also labelled for positron emission tomography (PET) imaging for monitoring tumour angiogenesis [67], vascular endothelial growth factor [68], by functionalizing QDs with suitable biomolecules (Figure – 3). The advantage of using functional QDs also extended to therapeutics [69] and enhanced specificity for targeting [70].
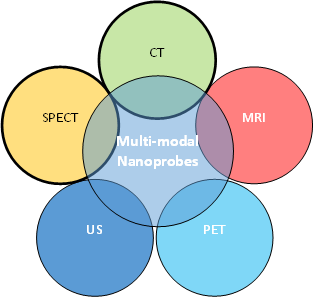
Figure 1: Nano imaging probe integrating with imaging contrast agents for multimodal molecular imaging to achieve improved sensitivity and specificity.
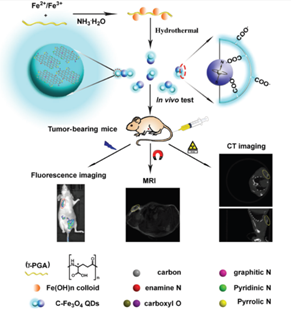
Figure 2: Applications of QD for multimodal imaging in-vivo in nude mice (reproduced with permission [49].
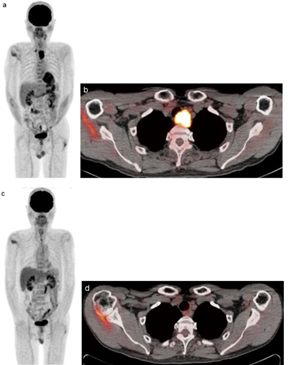
Figure 3: FDG-PET/CT scan of an advanced esophageal cancer, (a) Pretreatment PET scan shows two areas of strong FDG uptake (b) Pretreatment FDG-PET/CT scan shows a maximum standardized uptake value (SUVmax) in the primary tumor, (c) Three months post treatment PET scan showing faint FDG uptake and (d) FDG-PET/CT scan [71].
5. Functionalization for Cell Targeting, Imaging, Biocompatibility
QDs can be conjugated with peptides, proteins, antibodies, aptamers (oligonucleotides), polyethylene glycol (PEG) and small molecules [31]. These surface modified QDs are widely used for different modality imaging, targeting and tracking, both in-vitro and in-vivo [72]. It is important to select appropriate strategy for achieving solubility and stability of QDs in aqueous solutions under physiological condition for various biological applications. Hydrophobic interaction (with amphipilic molecules) and ligand exchange strategies are the two main surface modification procedures of QDs, based on the requirement the method of their synthesis vary. The surface modification protocol is chosen without impairing the photo-physical properties of QD. These conjugated molecules have binding sites to achieve cell targeting, increasing uptake of QDs in cells [73]. The amine and carboxyl groups present in these biomolecules couple with surface modified QDs by forming simple amide bonds [74]. For instance, DNA has several coupling sites such as amines, hydroxyl and phosphate in which it can bind non-specifically with QDs and other micro-environmental molecules present [75]. The complimentary binding of the DNA fragment in the DNA functionalized QDs are detectable by calorimetric detection assays [76]. Another important conjugating biomolecule is the poly ethylene glycol (PEG) which proved to enhance the cellular uptake and increase the retention time of these QDs in the body [77]. These PEG conjugated QDs are shown to have enhanced endothelial permeability retention (EPR) rate and are biocompatible [78]. However, these PEGylated QD needs amine, thiol or carboxyl functional groups to achieve covalent ligation. Apart from these, carbohydrates such as dextran have been used widely to provide solubility and biocompatibility to the QDs [79]. These dextrans when coated on QDs said to tolerate a wide range of pH without affecting their fluorescence and fluorescence resonance energy transfer (FRET) properties [80]. Hence, depending on the application of QDs, their surface is modified which ensures the functional aspect to the QDs. Table -1 summarises the various nano formulation suitable for different imaging modalities that can image in single mode or in combination. Multimodal molecular imaging pools two or more kinds of imaging techniques to for new fusion mode of imaging, which can pay way for obtaining further information in diagnosis and prognosis. Recently, multimodal molecular imaging has been extensively used to improve medical investigation and clinical practice. Multimodal molecular imaging has been successfully applied to diagnose various medical problems such as cardiovascular diseases [124], psychiatric abnormalities [125, 126], surgical resection of the tumour [127] etc. In summary, multimodal molecular imaging has a future potential development and will definitely bring a major breakthrough in the field of medical imaging and molecular science.
|
Imaging Modality |
Contrast Probe |
Reference |
|
Computed Tomography (CT) |
Conjugation of nanoparticle with X-ray-absorbing atoms and liposomes/ emulsion/ lipoproteins/ polymers |
de Vries A et al [81], Elrod DB et al [82], Attia M et al. [83], Sung June Kim et al [84] |
|
Polymer-coated bismuth sulfate (Bi2 S3) |
Rabin et al [85] |
|
|
Gold labelled 2-deoxy-d-glucose |
Hyafil et al [86] |
|
|
Gold labelled 2-deoxy-d-glucose |
Li J et al [87] |
|
|
Gold nanoparticles with a prostate-specific membrane antigen |
Kim D et al [88] |
|
|
Gold nanoparticles with liposomal iodine |
Kayyali MN et al [89] |
|
|
Iodine-contained diatrizoic acid (DTA) conjugated to glycol chitosan (GC - DTA) |
Choi D et al [90] |
|
|
Nano composite of folic acid (FA), and iron platinum-dimercaptosuccinnic acid/PEGylated graphene oxide |
Yue L et al [91] |
|
|
Magnetic Resonance Imaging (MRI) |
Diffusion weighted imaging (DWI), Apparent diffusion coefficient (ADC), Hydrogen proton magnetic resonance spectroscopy (MRS), Magnetization transfer ratio (MTR), other active nuclei such as hydrogen, phosphorous, sodium, carbon, and fluorine |
[92-98] |
|
Superparamagnetic agents for altering proton relaxation time T1, T2, or T2* |
[99] |
|
|
Non-specific, tumour-specific, antibody conjugated, specific proteinases or pH sensitive, T-cell/stem-cell labelled contrast agents |
[100-103] |
|
|
Gold core with slilica coated trans-1,2-bis(4-pyridyl)-ethylene (MRI –Photoacoaustic Raman Imaging - MPR) |
Moritz F Kircher et al [104] |
|
|
Nano composite of folic acid (FA), and iron platinum-dimercaptosuccinnic acid/PEGylated graphene oxide |
Yue L et al [91] |
|
|
Positron Emission Tomography (PET) |
2-fluoro-2-deoxy-glucose ([18F]-FDG) |
|
|
Single Photon Emission Computed Tomography (SPECT) |
Technetium 99 m, Copper 64, Gallium 68, Iodine 124 |
[108-112] |
|
Iodine 123, Indium (In-111), Gallium 67 |
||
|
Ultrasonography (US) |
GC-DTA Encapsulated with perfluoropentane (Bimodal probe for CT and US) |
Choi D et al [90] |
|
Antibody attached micro bubbles |
||
|
Peptide attached micro bubbles |
||
|
Optical Imaging Fluorescence, Bioluminescence, Fluorescent Molecular Tomography (FMT), etc. |
Lanthanide-based probes, Arginyl peptides to cross-linked iron oxide amine (amino-CLIO), Fluorescent dye-doped silica (DySiO2), green fluorescent protein (GFP) |
[119-123] |
Table 1: Functionalized contrast probes for different Molecular Imaging Modality.
6. Summary
Molecular imaging using QDs is found to be effective for cancer imaging both in-vitro and in-vivo conditions. Though this technique is simple, low-cost, and highly sensitive in comparison to other imaging techniques it bench side to bed side transformation is still underway due to many reasons such as fluorescence particles inside the body, limitations in deep tissue penetration, etc. However, for studies involving detecting cancer biomarkers non-invasively which are expressed on cancer cell membrane at the early stage of cancer, application of QDs is promising. NIR-emitting QDs are used widely for imaging solid tumour, tumour vasculatures and sentinel lymph nodes. Surface modification is necessary to achieve high stability, biocompatibility, clearance, specificity in tumour targeting, biodistribution, suitability of multimodal imaging, etc. In summary, the surface of the QDs needs to be engineered specific to each application and acts as a single unit that compliments the limitation of the imaging modality and suitable for unique bioimaging applications. Future research should be focused on not only multimodal but also functionalized imaging probes for effective targeting tumour for diagnosis and therapy. Non-invasive cancer imaging, drug delivery, real-time guidance for the surgery, continuous monitoring for drug therapy, imaging metastasis, detecting circulating tumour cells, imaging angiogenic vasculatures, sentinel lymph node is the basic requirement for effective cancer management. All these requirements could be satisfied in future by using suitable multimodal QDs. It is anticipated to achieve translational research activity in the near future using appropriate imaging probes for managing cancer and other dreadful disease.
7. Conclusion
The multimodal QDs devoloped in future should satisfy all the requirements suitable for cancer theranostic applications. It is anticipated to achieve translational research activity in the near future using appropriate imaging probes for managing cancer and other dreadful disease.
Acknowledgments
The author would like to thank the Director- Research, Chettinad Academy of Research and Education for his moral support and encouragements.
References
- Alberti C. From molecular imaging in preclinical/clinical oncology to theranostic applications in targeted tumor therapy. European review for medical and pharmacological sciences 16 (2012): 1925-1933.
- Bu L, Shen B, Cheng Z. Fluorescent imaging of cancerous tissues for targeted surgery. Adv Drug Deliv Rev 76 (2014): 21-38.
- Bosch F X, Manos M M, Muñoz N, Sherman M, Jansen A M, Peto J, et al. Prevalence of Human Papillomavirus in Cervical Cancer: a Worldwide Perspective. JNCI: Journal of the National Cancer Institute 87 (1995): 796-802.
- Haas G P, Delongchamps N, Brawley O W, Wang C Y, de la Roza G. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol 15 (2008): 3866-3871.
- Dela Cruz C S, Tanoue L T, Matthay R. A Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 32 (2011): 605-644.
- Peto J, Collins N, Barfoot R, Seal S, Warren W, Rahman N, et al. Prevalence of BRCA1 and BRCA2 Gene Mutations in Patients With Early-Onset Breast Cancer. JNCI: Journal of the National Cancer Institute 91 (1999): 943-949.
- Bray F, Ferlay J, Soerjomataram I, Siegel R L, Torre L A, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 68 (2018): 394-424.
- Kubota S I, Takahashi K, Nishida J, Morishita Y, Ehata S, Tainaka K, et al. Whole-Body Profiling of Cancer Metastasis with Single-Cell Resolution. Cell reports 20 (2017): 236-250.
- James M L, Gambhir S S. A molecular imaging primer: modalities, imaging agents, and applications. Physiological reviews 92 (2012): 897-965.
- Fass L. Imaging and cancer: a review. Molecular oncology 2 (2008): 115-152.
- Muzic R F, Jr DiFilippo F P. Positron emission tomography-magnetic resonance imaging: technical review. Semin Roentgenol 49 (2014): 242-254.
- Schwochau K. Technetium: Chemistry and radiopharmaceutical applications; Wiley-VCH: Germany, (2000).
- Leblond F, Davis S C, Valdes P A, Pogue B W. Pre-clinical whole-body fluorescence imaging: Review of instruments, methods and applications. Journal of photochemistry and photobiology. B, Biology 98 (2010) 77-94.
- Santra S, Xu J, Wang K, Tan, W. Luminescent nanoparticle probes for bioimaging. Journal of nanoscience and nanotechnology 4 (2004): 590-599.
- de Oliveira J G, Beck J, Seifert V, Teixeira M J, Raabe A. Assessment of flow in perforating arteries during intracranial aneurysm surgery using intraoperative near-infrared indocyanine green videoangiography. Neurosurgery 61 (2007): 63-72.
- Chen S F, Kato Y, Oda J, Kumar A, WatabeT, Imizu S, et al. The application of intraoperative near-infrared indocyanine green videoangiography and analysis of fluorescence intensity in cerebrovascular surgery. Surg Neurol Int 2 (2011): 42-42.
- Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery 52 (2003): 132-139.
- Siegel R L, Miller K D, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 67 (2017): 7-30.
- Stephens F O. Induction chemotherapy: to downgrade aggressive cancers to improve curability by surgery and/or radiotherapy. European Journal of Surgical Oncology 27 (2001): 672-688.
- Matea C T, Mocan T, Tabaran F, Pop T, Mosteanu O, Puia C, et al. Quantum dots in imaging, drug delivery and sensor applications. Int J Nanomedicine 12 2017): 5421-5431.
- Louie A. Multimodality Imaging Probes: Design and Challenges. Chemical Reviews 110 (2010): 3146-3195.
- Frangioni J V, Kim SW, Ohnishi S, Kim S, Bawendi M G. Sentinel lymph node mapping with type-II quantum dots. Methods Mol Biol 374 (2007): 147-159.
- Kobayashi H, Hama Y, Koyama Y, Barrett T, Regino C A, Urano Y, et al. Simultaneous multicolor imaging of five different lymphatic basins using quantum dots. Nano letters 7 (2007): 1711-1716.
- Liu J, Erogbogbo F, Yong KT, Ye L, Liu J, Hu R, et al. Assessing Clinical Prospects of Silicon Quantum Dots: Studies in Mice and Monkeys. ACS Nano 7 (2013): 7303-7310.
- Yu C, Xuan T, Chen Y, Zhao Z, Liu X, Lian G, et al. Gadolinium-doped carbon dots with high quantum yield as an effective fluorescence and magnetic resonance bimodal imaging probe. Journal of Alloys and Compounds 688 (2016): 611-619.
- Tan A, Yildirimer L, Rajadas J, Peña H D L, Pastorin G, Seifalian A. Quantum dots and carbon nanotubes in oncology: a review on emerging theranostic applications in nanomedicine. Nanomedicine 6 (2011): 1101-1114.
- Michalet X, Pinaud F F, Bentolila L A, Tsay J M, Doose S, Li J J, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science (New York, N.Y.) 2005, 307, 538-544.
- Medintz I L, Uyeda H T, Goldman E R, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nature materials 4 (2005): 435-446.
- Probst C E, Zrazhevskiy P, Bagalkot V, Gao X. Quantum dots as a platform for nanoparticle drug delivery vehicle design. Adv Drug Deliv Rev 65 (2013): 703-718.
- McHugh K J, Jing L, Behrens A M, Jayawardena S, Tang W, Gao M, et al. Biocompatible Semiconductor Quantum Dots as Cancer Imaging Agents. Advanced Materials 30 (2018): 1706356.
- Xing Y, Rao J. Quantum dot bioconjugates for in vitro diagnostics & in vivo imaging. Cancer biomarkers : section A of Disease markers 4 (2008): 307-319.
- Smith A M, Dave S, Nie S, True L, Gao X. Multicolor quantum dots for molecular diagnostics of cancer. Expert review of molecular diagnostics 6 (2006): 231-244.
- Gao X, Yang L, Petros J A, Marshall F F, Simon JW, Nie S. In vivo molecular and cellular imaging with quantum dots. Current opinion in biotechnology 16 (2005): 63-72.
- Gao J, Chen X, Cheng Z. Near-infrared quantum dots as optical probes for tumor imaging. Curr Top Med Chem 10 (2010): 1147-1157.
- Rogach A L, Ogris M. Near-infrared-emitting semiconductor quantum dots for tumor imaging and targeting. Current opinion in molecular therapeutics 12 (2010): 331-339.
- Dabbousi B O, Rodriguez-Viejo J, Mikulec F V, Heine J R, Mattoussi H, Ober R, et al. (CdSe)ZnS Core−Shell Quantum Dots: Synthesis and Characterization of a Size Series of Highly Luminescent Nanocrystallites. The Journal of Physical Chemistry B 101 (1997): 9463-9475.
- Chan W C, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science (New York, N.Y.) 281 (1998): 2016-2018.
- Hu J, Liu A, Jin H, Ma D, Yin D, Ling P, et al. A Versatile Strategy for Shish-Kebab-like Multi-heterostructured Chalcogenides and Enhanced Photocatalytic Hydrogen Evolution. Journal of the American Chemical Society 137 (2015): 11004-11010.
- Howarth M, Takao K, Hayashi Y, Ting A Y. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proceedings of the National Academy of Sciences of the United States of America 102 (2005): 7583-7588.
- Knudsen B R, Jepsen M L, Ho Y P. Quantum dot-based nanosensors for diagnosis via enzyme activity measurement. Expert review of molecular diagnostics 13 (2013): 367-375.
- Orte A, Alvarez-Pez J M, Ruedas-Rama M J. Fluorescence lifetime imaging microscopy for the detection of intracellular pH with quantum dot nanosensors. ACS Nano 7 (2013): 6387-6395.
- Nakamura Y, Mochida A, Choyke P L, Kobayashi H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjugate chemistry 27 (2016): 2225-2238.
- Kim S, Lim Y T, Soltesz E G, De Grand A M, Lee J, Nakayama A, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nature biotechnology 22 (2004): 93-97.
- Louie A. Multimodality imaging probes: design and challenges. Chem Rev 110 (2010): 3146-3195.
- Santra S, Yang H, Holloway P H, Stanley J T, Mericle R A. Synthesis of Water-Dispersible Fluorescent, Radio-Opaque, and Paramagnetic CdS:Mn/ZnS Quantum Dots: A Multifunctional Probe for Bioimaging. Journal of the American Chemical Society 127 (2005): 1656-1657.
- Cormode D P, Skajaa T, van Schooneveld M M, Koole R, Jarzyna P, Lobatto M E, et al. Nanocrystal core high-density lipoproteins: a multimodality contrast agent platform. Nano letters 8 (2008): 3715-3723.
- Wu Y, Sun Y, Zhu X, Liu Q, Cao T, Peng J, et al. Lanthanide-based nanocrystals as dual-modal probes for SPECT and X-ray CT imaging. Biomaterials 35 (2014): 4699-4705.
- Tang Y, Zhang C, Wang J, Lin X, Zhang L, Yang Y, e al. MRI/SPECT/Fluorescent Tri-Modal Probe for Evaluating the Homing and Therapeutic Efficacy of Transplanted Mesenchymal Stem Cells in a Rat Ischemic Stroke Model. Advanced functional materials 25 (2015): 1024-1034.
- Liu X, Jiang H, Ye J, Zhao C, Gao S, Wu C, et al. Nitrogen-Doped Carbon Quantum Dot Stabilized Magnetic Iron Oxide Nanoprobe for Fluorescence, Magnetic Resonance, and Computed Tomography Triple-Modal In Vivo Bioimaging. Advanced functional materials 26 (2016): 8694-8706.
- Ghazani A A, Lee J A, Klostranec J, Xiang Q, Dacosta R S, Wilson B C, et al. High throughput quantification of protein expression of cancer antigens in tissue microarray using quantum dot nanocrystals. Nano letters 6 (2006): 2881-2886.
- Smith B R, Cheng Z, De A, Koh A L, Sinclair R, Gambhir S S. Real-time intravital imaging of RGD-quantum dot binding to luminal endothelium in mouse tumor neovasculature. Nano letters 8 (2008): 2599-2606.
- Yu X, Chen L, Li K, Li Y, Xiao S, Luo X, et al. Immunofluorescence detection with quantum dot bioconjugates for hepatoma in vivo. Journal of biomedical optics 12 (2007): 014008.
- Gokarna A, Jin L H, Hwang J S, Cho Y H, Lim Y T, Chung B H, et al. Quantum dot-based protein micro- and nanoarrays for detection of prostate cancer biomarkers. Proteomics 8 (2008): 1809-1818.
- Kerman K, Endo T, Tsukamoto M, Chikae M, Takamura Y, Tamiya, E. Quantum dot-based immunosensor for the detection of prostate-specific antigen using fluorescence microscopy. Talanta 71 (2007): 1494-1499.
- Shi C, Zhu Y, Xie Z, Qian W, Hsieh C L, Nie S, et al. Visualizing human prostate cancer cells in mouse skeleton using bioconjugated near-infrared fluorescent quantum dots. Urology 74 (2009): 446-451.
- Tada H, Higuchi H, Wanatabe T M, Ohuchi N. In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer research 67 (2007): 1138-1144.
- Chen C, Peng J, Xia H, Wu Q, Zeng L, Xu H, et al. Quantum-dot-based immunofluorescent imaging of HER2 and ER provides new insights into breast cancer heterogeneity. Nanotechnology 21 (2010): 095101.
- Yong K T, Roy I, Hu R, Ding H, Cai H, Zhu J, et al. Synthesis of ternary CuInS(2) /ZnS quantum dot bioconjugates and their applications for targeted cancer bioimaging. Integrative biology: quantitative biosciences from nano to macro 2 (2010): 121-129.
- Manzoor K, Johny S, Thomas D, Setua S, Menon D, Nair S. Bio-conjugated luminescent quantum dots of doped ZnS: a cyto-friendly system for targeted cancer imaging. Nanotechnology 20 (2009): 065102.
- Snyder E L, Bailey D, Shipitsin M, Polyak K, Loda M. Identification of CD44v6 (+) /CD24- breast carcinoma cells in primary human tumors by quantum dot-conjugated antibodies. Lab Invest 89 (2009): 857-866.
- Orndorff R L, Rosenthal S J. Neurotoxin quantum dot conjugates detect endogenous targets expressed in live cancer cells. Nano letters 9 (2009): 2589-2599.
- Stroh M, Zimmer J P, Duda D G, Levchenko T S, Cohen K S, Brown E B, et al. Quantum dots spectrally distinguish multiple species within the tumor milieu in vivo. Nature medicine 11 (2005): 678-682.
- Mulder W J, Castermans K, van Beijnum J R, Oude Egbrink M G, Chin P T, Fayad Z A, et al. Molecular imaging of tumor angiogenesis using alphavbeta3-integrin targeted multimodal quantum dots. Angiogenesis 12 (2009): 17-24.
- Mulder W J M, Strijkers G J, Nicolay K, Griffioen A W. Quantum dots for multimodal molecular imaging of angiogenesis. Angiogenesis 13 (2010): 131-134.
- Oostendorp M, Douma K, Hackeng T M, Dirksen A, Post M J, van Zandvoort M A, et al. Quantitative molecular magnetic resonance imaging of tumor angiogenesis using cNGR-labeled paramagnetic quantum dots. Cancer research 68 (2008): 7676-7683.
- Oostendorp M, Douma K, Wagenaar A, Slenter J M, Hackeng T M, van Zandvoort M A, et al. Molecular magnetic resonance imaging of myocardial angiogenesis after acute myocardial infarction. Circulation 121 (2010): 775-783.
- Cai W, Chen K, Li Z B, Gambhir S S, Chen X. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 48 (2007): 1862-1870.
- Chen K, Li Z B, Wang H, Cai W, Chen X. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. European journal of nuclear medicine and molecular imaging 35 (2008): 2235-2244.
- Lukyanov A N, Torchilin V P. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev 56 (2004): 1273-1289.
- Kluza E, van der Schaft D W, Hautvast P A, Mulder W J, Mayo K H, Griffioen A W, et al. Synergistic targeting of alphavbeta3 integrin and galectin-1 with heteromultivalent paramagnetic liposomes for combined MR imaging and treatment of angiogenesis. Nano letters 10 (2010): 52-58.
- Kitajima K, Nakajo M, Kaida H, Minamimoto R, Hirata K, Tsurusaki M, et al. Present and future roles of FDG-PET/CT imaging in the management of gastrointestinal cancer: an update. Nagoya J Med Sci 79 (2017): 527-543.
- Bilan R, Fleury F, Nabiev I, Sukhanova A. Quantum Dot Surface Chemistry and Functionalization for Cell Targeting and Imaging. Bioconjugate chemistry 26 (2015): 609-624.
- Rosenthal S J, Chang J C, Kovtun O, McBride J R, Tomlinson I D. Biocompatible quantum dots for biological applications. Chem Biol 18 (2011): 10-24.
- Sperling R A, Parak W J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philosophical transactions. Series A, Mathematical, physical, and engineering sciences 368 (2010): 1333-1383.
- Murcia M J, Minner D E, Mustata G M, Ritchie K, Naumann C A. Design of quantum dot-conjugated lipids for long-term, high-speed tracking experiments on cell surfaces. J Am Chem Soc 130 (2008): 15054-15062.
- Zhang Y, Wang T H. Quantum dot enabled molecular sensing and diagnostics. Theranostics 2 (2012): 631-654.
- Oh E, Delehanty J B, Sapsford K E, Susumu K, Goswami R, Blanco-Canosa J B, et al. Cellular uptake and fate of PEGylated gold nanoparticles is dependent on both cell-penetration peptides and particle size. ACS Nano 5 (2011): 6434-6448.
- Susumu K, Mei B C, Mattoussi H. Multifunctional ligands based on dihydrolipoic acid and polyethylene glycol to promote biocompatibility of quantum dots. Nature protocols 4 (2009): 424-436.
- Thanh N T K, Green L A W. Functionalisation of nanoparticles for biomedical applications. Nano Today 5 (2010): 213-230.
- Robert W, David G, S Alison B, Ian A, P Violaine S e. Highly Stable Dextran-Coated Quantum Dots for Biomolecular Detection and Cellular Imaging (2010).
- de Vries A, Custers E, Lub J, van den Bosch S, Nicolay K, Grull H. Block-copolymer-stabilized iodinated emulsions for use as CT contrast agents. Biomaterials 31 (2010): 6537-6544.
- Elrod D B, Partha R, Danila D, Casscells S W, Conyers J L. An iodinated liposomal computed tomographic contrast agent prepared from a diiodophosphatidylcholine lipid. Nanomedicine 5 (2009): 42-45.
- Attia M F, Anton N, Chiper M, Akasov R, Anton H, Messaddeq N, et al. Biodistribution of X-ray iodinated contrast agent in nano-emulsions is controlled by the chemical nature of the oily core. ACS Nano 8 (2014): 10537-10550.
- Kim S J, Xu W, Ahmad M W, Baeck J S, Chang Y, Bae J E, et al. Synthesis of nanoparticle CT contrast agents:in vitroandin vivostudies. Science and Technology of Advanced Materials 16 (2015): 055003.
- Rabin O, Manuel Perez J, Grimm J, Wojtkiewicz G, Weissleder R. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nature materials 5 (2006): 118-122.
- Hyafil F, Cornily J C, Feig J E, Gordon R, Vucic E, Amirbekian V, et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nature medicine 13 (2007): 636-641.
- Li J, Chaudhary A, Chmura S J, Pelizzari C, Rajh T, Wietholt C, et al. A novel functional CT contrast agent for molecular imaging of cancer. Physics in medicine and biology 55 (2010): 4389-4397.
- Kim D, Jeong Y Y, Jon S. A drug-loaded aptamer-gold nanoparticle bioconjugate for combined CT imaging and therapy of prostate cancer. ACS Nano 4 (2010): 3689-3696.
- Kayyali M N, Brake L, Ramsey A J, Wright A C, O'Malley B W, Li D D. A Novel Nano-approach for Targeted Inner Ear Imaging. Journal of nanomedicine & nanotechnology 8 (2017).
- Choi D, Jeon S, You D G, Um W, Kim J Y, Yoon H Y, et al. Iodinated Echogenic Glycol Chitosan Nanoparticles for X-ray CT/US Dual Imaging of Tumor. Nanotheranostics 2 (2018): 117-127.
- Yue L, Wang J, Dai Z, Hu Z, Chen X, Qi Y, et al. pH-Responsive, Self-Sacrificial Nanotheranostic Agent for Potential In Vivo and In Vitro Dual Modal MRI/CT Imaging, Real-Time, and In Situ Monitoring of Cancer Therapy. 28 (2017): 400-409.
- Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clinical pharmacology and therapeutics 69 (2001): 89-95.
- Therasse P, Arbuck S G, Eisenhauer E A, Wanders J, Kaplan R S, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 92 (2000): 205-216.
- Gore J C, Manning H C, Quarles C C, Waddell K W, Yankeelov T E. Magnetic resonance in the era of molecular imaging of cancer. Magnetic resonance imaging 29 (2011): 587-600.
- Mahon M M, Williams A D, Soutter W P, Cox I J, McIndoe G A, Coutts G A, et al. 1H magnetic resonance spectroscopy of invasive cervical cancer: an in vivo study with ex vivo corroboration. NMR in biomedicine 17 (2004): 1-9.
- Majos C, Julia-Sape M, Alonso J, Serrallonga M, Aguilera C, Acebes J J, et al. Brain tumor classification by proton MR spectroscopy: comparison of diagnostic accuracy at short and long TE. AJNR. American journal of neuroradiology 25 (2004): 1696-1704.
- Babsky A M, Hekmatyar S K, Zhang H, Solomon J L, Bansal N. Application of 23Na MRI to monitor chemotherapeutic response in RIF-1 tumors. Neoplasia 7 (2005): 658-666.
- Hu H, Katyayan K K, Czeskis B A, Perkins E J, Kulanthaivel P. Comparison between Radioanalysis and <sup>19</sup>F Nuclear Magnetic Resonance Spectroscopy in the Determination of Mass Balance, Metabolism, and Distribution of Pefloxacin. Drug Metabolism and Disposition 45 (2017): 399.
- Ta H T, Li Z, Hagemeyer C E, Cowin G, Zhang S, Palasubramaniam J, et al. Molecular imaging of activated platelets via antibody-targeted ultra-small iron oxide nanoparticles displaying unique dual MRI contrast. Biomaterials 134 (2017): 31-42.
- Bellin M F, Vasile M, Morel-Precetti S. Currently used non-specific extracellular MR contrast media. European radiology 13 (2003): 2688-2698.
- Mohs A M, Lu Z R. Gadolinium (III) - based blood-pool contrast agents for magnetic resonance imaging: status and clinical potential. Expert opinion on drug delivery 4 (2007): 149-164.
- Artemov D. Molecular magnetic resonance imaging with targeted contrast agents. Journal of cellular biochemistry 90 (2003): 518-524.
- Lepage M, Dow W C, Melchior M, You Y, Fingleton B, Quarles C C, et al. Noninvasive detection of matrix metalloproteinase activity in vivo using a novel magnetic resonance imaging contrast agent with a solubility switch. Molecular imaging 6 (2007): 393-403.
- Kircher M F, de la Zerd A, Jokerst J V, Zavaleta C L, Kempen P J, Mittra E, et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nature medicine 18 (2012): 829-834.
- Bao C, Wei J, Zhao X, Lin L, Chen D, Liu K, et al. Prognostic value of fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography in primary hepatic mucosa-associated lymphoid tissue lymphoma: A case report and review of the literature. Medicine 97 (2018): e9877.
- Rudroff T, Kindred J H, Koo P J, Karki R, Hebert J R. Asymmetric glucose uptake in leg muscles of patients with Multiple Sclerosis during walking detected by [18F] -FDG PET/CT. NeuroRehabilitation 35 (2014): 813-823.
- Fledelius J, Winther-Larsen A, Khalil A A, Hjorthaug K, Frokiaer J, Meldgaard P. Assessment of very early response evaluation with (18) F-FDG-PET/CT predicts survival in erlotinib treated NSCLC patients-A comparison of methods. American journal of nuclear medicine and molecular imaging 8 (2018): 50-61.
- Elvas F, Vangestel C, Rapic S, Verhaeghe J, Gray B, Pak K, et al. Characterization of [(99m)Tc]Duramycin as a SPECT Imaging Agent for Early Assessment of Tumor Apoptosis. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging 17 (2015): 838-847.
- Clough A V, Audi S H, Haworth S T, Roerig D L. Differential lung uptake of 99mTc-hexamethylpropyleneamine oxime and 99mTc-duramycin in the chronic hyperoxia rat model. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 53 (2012): 1984-1991.
- Shokeen M, Anderson C J. Molecular imaging of cancer with copper-64 radiopharmaceuticals and positron emission tomography (PET). Accounts of chemical research 42 (2009): 832-841.
- Wadas T J, Wong E H, Weisman G R, Anderson C J. Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem Rev 110 (2010): 2858-2902.
- Holland J P, Williamson M J, Lewis J S. Unconventional nuclides for radiopharmaceuticals. Molecular imaging 9 (2010): 1-20.
- McKeith I, O'Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. The Lancet. Neurology 6 (2007): 305-313.
- Wadas T J, Wong E H, Weisman G R, Anderson C J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chemical Reviews 110 (2010): 2858-2902.
- Otani K, Yamahara K. Development of antibody-carrying microbubbles based on clinically available ultrasound contrast agent for targeted molecular imaging: a preliminary chemical study. Molecular imaging and biology: MIB: the official publication of the Academy of Molecular Imaging 13 (2011): 250-256.
- Kiessling F, Fokong S, Bzyl J, Lederle W, Palmowski M, Lammers T. Recent advances in molecular, multimodal and theranostic ultrasound imaging. Adv Drug Deliv Rev 72 (2014): 15-27.
- Zhang H, Ingham E S, Gagnon M K J, Mahakian L M, Liu J, Foiret J L, et al. In vitro characterization and in vivo ultrasound molecular imaging of nucleolin-targeted microbubbles. Biomaterials 118 (2017): 63-73.
- Dayton P A, Ferrara K W. Targeted imaging using ultrasound. Journal of Magnetic Resonance Imaging 16 (2002): 362-377.
- Thibon A, Pierre V C. Principles of responsive lanthanide-based luminescent probes for cellular imaging. Analytical and bioanalytical chemistry 394 (2009): 107-120.
- Hyde D, de Kleine R, MacLaurin S A, Miller E, Brooks D H, Krucker T, et al. Hybrid FMT–CT imaging of amyloid-β plaques in a murine Alzheimer's disease model. NeuroImage 44 (2009): 1304-1311.
- Josephson L, Kircher M F, Mahmood U, Tang Y, Weissleder R. Near-Infrared Fluorescent Nanoparticles as Combined MR/Optical Imaging Probes. Bioconjugate chemistry 13 (2002): 554-560.
- Cheon J, Lee J H. Synergistically Integrated Nanoparticles as Multimodal Probes for Nanobiotechnology. Accounts of chemical research 41 (2008): 1630-1640.
- Yang M, Baranov E, Jiang P, Sun F X, Li X M, Li, et al. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proceedings of the National Academy of Sciences of the United States of America 97 (2000): 1206-1211.
- Bruckman M A, Jiang K, Simpson E J, Randolph L N, Luyt L G, Yu X, et al. Dual-Modal Magnetic Resonance and Fluorescence Imaging of Atherosclerotic Plaques in Vivo Using VCAM-1 Targeted Tobacco Mosaic Virus. Nano letters 14 (2014): 1551-1558.
- O'Halloran R, Kopell B H, Sprooten E, Goodman W K, Frangou S. Multimodal Neuroimaging-Informed Clinical Applications in Neuropsychiatric Disorders. Frontiers in psychiatry 7 (2016): 63.
- Voss H U, Heier L A, Schiff N D. Multimodal imaging of recovery of functional networks associated with reversal of paradoxical herniation after cranioplasty. Clinical imaging 35 (2011): 253-258.
- van Dam G M, Themelis G, Crane L M, Harlaar N J, Pleijhuis R G, Kelder W, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nature medicine 17 (2011): 1315-1319.

 Impact Factor: * 1.2
Impact Factor: * 1.2 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 