Neurological Development of Infant after Surgery of Giant Parietal Encephalomeningocele- A Case Report
Article Information
Joanna Schreiber-Zamora1, Bozena Kociszewska-Najman1, Slawomir Barszcz2, Natalia Goluchowska1*, Piotr Rzepniewski1, Emilia Soltan2, Natalia Czaplinska1, Ewa Gluszczak-Idziakowska1, Michal Brzewski3
1Clinical Department of Neonatology, Pediatric Hospital of the Medical University of Warsaw, Warszawa, Poland
2Department of Neurosurgery, Pediatric Hospital of the Medical University of Warsaw, Warszawa, Poland
3Department of Pediatric Radiology, Pediatric Hospital of the Medical University of Warsaw, Warszawa, Poland
*Corresponding Author: Natalia Goluchowska, Clinical Department of Neonatology, Pediatric Hospital of the Medical University of Warsaw, Warszawa, Poland
Received: 17 August 2020; Accepted: 26 August 2020; Published: 18 September 2020
Citation:
Joanna Schreiber-Zamora, Bożena Kociszewska-Najman, Sławomir Barszcz, Natalia Gołuchowska, Piotr Rzepniewski, Emilia Sołtan, Natalia Czaplińska, Ewa Głuszczak-Idziakowska, Michał Brzewski. Neurological Development of Infant after Surgery of Giant Parietal Encephalomeningocele- A Case Report. Obstetrics and Gynecology Research 3 (2020): 190-198.
View / Download Pdf Share at FacebookAbstract
Encephalocele is a congenital neural tube defect associated with protrusion of the meninges and/or cerebral tissue through a defect in the cranium. Parietal encephalomeningoceles are rare and make 10% of these abnormalities. In this case we present neurological development of infant after a surgery of a giant parietal encephalomeningocele.
Keywords
Encephalocele, Neonate, Neurological Development, Meningoencephalocele, Congenital Defect
Encephalocele articles Encephalocele Research articles Encephalocele review articles Encephalocele PubMed articles Encephalocele PubMed Central articles Encephalocele 2023 articles Encephalocele 2024 articles Encephalocele Scopus articles Encephalocele impact factor journals Encephalocele Scopus journals Encephalocele PubMed journals Encephalocele medical journals Encephalocele free journals Encephalocele best journals Encephalocele top journals Encephalocele free medical journals Encephalocele famous journals Encephalocele Google Scholar indexed journals Neonate articles Neonate Research articles Neonate review articles Neonate PubMed articles Neonate PubMed Central articles Neonate 2023 articles Neonate 2024 articles Neonate Scopus articles Neonate impact factor journals Neonate Scopus journals Neonate PubMed journals Neonate medical journals Neonate free journals Neonate best journals Neonate top journals Neonate free medical journals Neonate famous journals Neonate Google Scholar indexed journals Neurological Development articles Neurological Development Research articles Neurological Development review articles Neurological Development PubMed articles Neurological Development PubMed Central articles Neurological Development 2023 articles Neurological Development 2024 articles Neurological Development Scopus articles Neurological Development impact factor journals Neurological Development Scopus journals Neurological Development PubMed journals Neurological Development medical journals Neurological Development free journals Neurological Development best journals Neurological Development top journals Neurological Development free medical journals Neurological Development famous journals Neurological Development Google Scholar indexed journals Meningoencephalocele articles Meningoencephalocele Research articles Meningoencephalocele review articles Meningoencephalocele PubMed articles Meningoencephalocele PubMed Central articles Meningoencephalocele 2023 articles Meningoencephalocele 2024 articles Meningoencephalocele Scopus articles Meningoencephalocele impact factor journals Meningoencephalocele Scopus journals Meningoencephalocele PubMed journals Meningoencephalocele medical journals Meningoencephalocele free journals Meningoencephalocele best journals Meningoencephalocele top journals Meningoencephalocele free medical journals Meningoencephalocele famous journals Meningoencephalocele Google Scholar indexed journals Congenital Defect articles Congenital Defect Research articles Congenital Defect review articles Congenital Defect PubMed articles Congenital Defect PubMed Central articles Congenital Defect 2023 articles Congenital Defect 2024 articles Congenital Defect Scopus articles Congenital Defect impact factor journals Congenital Defect Scopus journals Congenital Defect PubMed journals Congenital Defect medical journals Congenital Defect free journals Congenital Defect best journals Congenital Defect top journals Congenital Defect free medical journals Congenital Defect famous journals Congenital Defect Google Scholar indexed journals cerebral articles cerebral Research articles cerebral review articles cerebral PubMed articles cerebral PubMed Central articles cerebral 2023 articles cerebral 2024 articles cerebral Scopus articles cerebral impact factor journals cerebral Scopus journals cerebral PubMed journals cerebral medical journals cerebral free journals cerebral best journals cerebral top journals cerebral free medical journals cerebral famous journals cerebral Google Scholar indexed journals gestational articles gestational Research articles gestational review articles gestational PubMed articles gestational PubMed Central articles gestational 2023 articles gestational 2024 articles gestational Scopus articles gestational impact factor journals gestational Scopus journals gestational PubMed journals gestational medical journals gestational free journals gestational best journals gestational top journals gestational free medical journals gestational famous journals gestational Google Scholar indexed journals infant articles infant Research articles infant review articles infant PubMed articles infant PubMed Central articles infant 2023 articles infant 2024 articles infant Scopus articles infant impact factor journals infant Scopus journals infant PubMed journals infant medical journals infant free journals infant best journals infant top journals infant free medical journals infant famous journals infant Google Scholar indexed journals pregnancy articles pregnancy Research articles pregnancy review articles pregnancy PubMed articles pregnancy PubMed Central articles pregnancy 2023 articles pregnancy 2024 articles pregnancy Scopus articles pregnancy impact factor journals pregnancy Scopus journals pregnancy PubMed journals pregnancy medical journals pregnancy free journals pregnancy best journals pregnancy top journals pregnancy free medical journals pregnancy famous journals pregnancy Google Scholar indexed journals Neurological articles Neurological Research articles Neurological review articles Neurological PubMed articles Neurological PubMed Central articles Neurological 2023 articles Neurological 2024 articles Neurological Scopus articles Neurological impact factor journals Neurological Scopus journals Neurological PubMed journals Neurological medical journals Neurological free journals Neurological best journals Neurological top journals Neurological free medical journals Neurological famous journals Neurological Google Scholar indexed journals
Article Details
1. Introduction
Encephalocele is a congenital neural tube defect associated with protrusion of the meninges and/or cerebral tissue through a defect in the cranium. This malformation develops between 8 and 12 weeks of gestational age [1, 2]. Depending on the site of defect in the cranium, encephaloceles are classified as: occipital encephalomeningocele, encephalomeningocele of the cranial vault, fronto-ethmoidal encephalomeningocele, basal encephalomeningocele and cranioschisis [3]. According to the location relative to coronal suture encephaloceles are divided into posterior and anterior [4]. Other division defines hernations as frontal if located frontally to the coronal suture, parietal if between coronal and lamboidal sutures and occipital if located posterior to the lamboidal suture [5]. It is called a giant encephalomeninocele if the hernia sac is larger than the head size [6-8]. The prevalence of this abnormality varies based on the geographical location. The most common is occipital encephalocele, which makes 75-85% of cases and is diagnosed more often in North America and Western Europe. Frontal encephalocele is more common in south-western Asia, Africa, Malaysia and Russia. Parietal encephalocele makes 10% of cases [5, 7, 9, 10]. The target of this case report was to present neurological development after surgery of a giant parietal encephalomeningocele of an infant, born and treated in our clinic.
2. Case Description
A female infant from the first pregnancy was born at 38 weeks of pregnancy. The Apgar score was 7-10-10 points; weight 3300g (70 centile); body length 54cm (99 centile); head circumference 32cm (13 centile) at birth. Physical examination revealed large sack of the encephalomeningocele in the parietal region measuring 10x7x8 cm. Anterior fontanel measured 4x6 cm. Additionally complete cleft palate and cleft lip was found. Encephalocele was diagnosed prenatally at 32 weeks of pregnancy and confirmed in prenatal MRI. MRI showed defect of the cranium in parieto-occipital region (6-7mm in sagittal cross section) with hernia sac containing brain tissue that was spreading to occipital bone. Meningeal sac measured 76x67x41mm. Additionally asymmetry of the lateral ventricles and irregular fluid reservoir 18mm diameter, between posterior horns of lateral ventricles and additionally several minor subependymal focal changes of heterotrophic grey substance on the left side and dysgenesis of corpus callosum were found. Structures of posterior cranial fossa were without visible abnormalities and without signs of protrusion in the direction of spinal cord canal. No other pathologies of the infant were found. After birth cranial ultrasound scan showed asymmetrical, unwidened ventricles. Lateral ventricles were wide apart, split by midline cyst measuring 1,30/1,38 cm reaching to occipital region and communicating by narrow canal of 2mm width with meningoencephalocele space. Additionally, partial agenesis of corpus callosum was revealed. Cave of septum pellucidum wasn’t showed. External fluid spaces were unwidened. Two lobes of cerebellum were visible. The flow in superior sagittal sinus was maintained (Figure 4).
On the second day of life surgical intervention of encephalocele was performed. Histopathological exam found skin, subcutaneous tissue and brain tissue which indicates meningoencephalocele. Infection markers were negative. Temporary, perioperative parenteral feeding through central line was introduced for 2 days followed by breastfeeding supplied by feeding with breast milk. On the 4th day after surgery control cranial ultrasound revealed widened right lateral ventricle, partially crossing midline and protruding to the left side. Left lateral ventricle was unwidened. The IV ventricle was slit. Between bodies of lateral ventricles in the midline fluid reservoir was found measuring 11x20mm. In the anterior fontanelle projection fluid spaces were unwidened. Small posterior cranial fossa was found. On the 14th day of life brain MRI showed several abnormalities in brain: wide interthalamic adhesion, defects in cortex such as polymicrogyria/ pachygyria bilaterally in temporo-parietal region, subependymal heterotrophy of grey substance, brain crease in the left occipital lobe and small posterior cranial fossa. Tonsils were on proper level. Quadruplet bodies were thickened and posterior to them in midline cystic fluid space was found. Occlusion of cerebral aqueduct was suspected. Ventricle IV was unwidened. Supratentorial ventricle system was widened (mostly right lateral ventricle) with communication between left lateral ventricle and pericerebral space. Postoperative sagittal sinus was partially narrowed or occluded but drainage was probably maintained due to visible veins (on the left side). Only genu and trunk of the corpus callosum were visible. On 19th day of life due to worsening hydrocephalus Ultra VS Neonate valve system was implanted. Implantation and postoperative period was uneventful. On 23rd day of life cranial ultrasound (4th after VS valve system was introduced) showed brain tissue without focal changes. Widened right lateral ventricle, deepness of the frontal horn and central part up to 16-18mm. Left ventricle wasn’t enlarged. The IV ventricle was slit. Fluid spaces in projection of anterior fontanelle were unwidened. Small posterior cranial fossa was found.
Neurological examination revealed symmetrical face with narrow facial skeleton. The patient fixated eyes, followed objects but weaker on the right side. Muscle tension was lower than normal. Deep reflexes were present. There was scant spontaneous movement scheme with anti-gravitational work of limbs. Traction and suspension tests were abnormal. The patient reacted to voice. The EEG didn’t show any abnormalities. Genetic examination found oxycephaly (result of primary defect) without signs of other dysmorphia. Ophthalmological examination revealed normal anterior segment of the eye. Fundus within physiology, mature retina. On the fundus near the optic disc atrophic limbus. In the macula smaller absorbing, temporal to the macula extensive supra-retinal hemorrhage without recent hemorrhages, right eye same as left apart from aforementioned pathologies. Ultrasound of the abdomen, heart and otoacoustic emission were normal. On 26th day of life the patient was discharged from the hospital in good general condition with weight of 3620g. Multidirectional stimulation of development was ordered as part of early support. On the follow-up normal weight gains were observed, which stayed on the 50th centile level. Control measurements of circumference of the head were on the 10th centile. Elongated shape of the head was still visible. The child was rehabilitated. On the 3rd month neurological examination revealed symmetric facial mimic, round, equal, reacting to light pupils, full range of movement of the eye and discrete esotropia. No nystagmus was found. Eyelid reflexes were present. Muscle tension was lower than normal, deep reflexes were present, symmetrical. Spontaneous movements of limbs were observed. Lower grasping reflex was present bilaterally. The child supported on the abdomen and the shoulder but shoulder protraction and bend retracted upper limbs were present. Moro reflex was symmetrical. In the cranial ultrasound asymmetry of lateral ventricles (right wider with rounded frontal horn) was still visible. No focal changes in brain were found. Pericerebral space under anterior fontanele were unwidened.
At the age of 5 months during neurological examination the child was active, interested in surrounding, it was smiling and articulated syllables. Facial mimic was symmetrical. Cranium was domed as previously. Esotropia was still visible, movement of the eye was full, nystagmus wasn’t found. Muscle tension was discreetly lower, deep reflexes were present, symmetrical. Traction test was normal. Spontaneous movement of limbs was symmetrical, the child grabbed toys but didn’t shuffle them. The patient was raising both lower limbs above the floor, didn’t grab knees. Lower grasping reflex was present, symmetrical. While supporting on the abdomen increased tension of spinal erectors and shoulder protraction were present. Abnormal position of upper limbs. During examination first phase Moro reflex was poorly visible. Another ophthalmological examination found no abnormalities. The ultrasound examination revealed that VP shunt works properly. At the age of 10 months the patient was vividly interested in surroundings, keeps eye contact, smiles, coos, articulates single syllables. Physical examination revealed general lower muscle tension also in facial region. Infant has sat independently since 9 months of life, has turn and crawled backwards. The patient played with both hands and shuffles toys from one hand to another. Deep and equivalent reflexes were symmetrical. EEG shows no signs of seizure symptoms. Head circumference was 43 cm (10-25 percentile). VP shunt worked properly. Cranial ultrasound showed asymmetry of lateral ventricles. In the region of corpus callosum agenesis fluid space is present. Otherwise no focal changes in brain were found. Pericerebral fluid spaces were unwidened. At the age of 12 months patient started to stand up by holding on the furnitures.
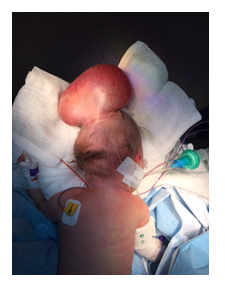
Figure 1: Pre-operative photo in prone position.
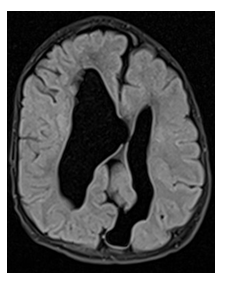
Figure 2: MR FLAIR in axial plane showing abnormal gyri in parietal and occipital regions, enlarged lateral ventricles of abnormal shape and brain fissure in the left occipital lobe.
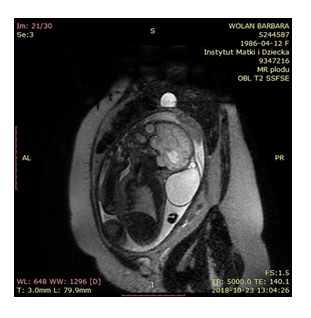
Figure 3: Ts trim tre dark fluid. SSFSE T2 ax sag cor Fiesta/2D ax sag cos GRE EPI ax DWI.
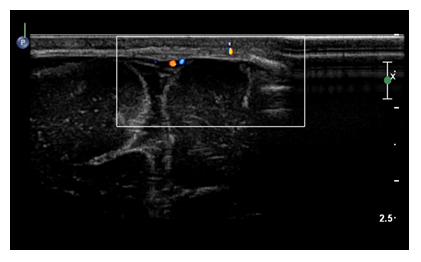
Figure 4: The postoperative US doppler study with the patent blood flow in the sagittal sinus close to the paramedian left sided skull defect and the dysplastic cystic lesion of the left cerebral hemisphere.
3. Discussion
The etiology of encephaloceles is not entirely defined. The probable cause include a genetic origin (the occurrence among siblings), ethnic and environmental factors, maternal nutritional deficiencies, viral infections, radiation, hipervitaminosis (high doses of vitamin A), using of salicylates in early pregnancy, valproic acid, cytostatic agents, hypoxia, hyperthermia, maternal chronic diseases and parental age. Encephaloceles were also observed in families with spina bifida history [5, 9, 11]. There are various data in the literature on the effect of folic acid deficiency during pregnancy on the incidence of encephaloceles. Rafaee et al. state, that contrasting to meningoceles, maternal folate does not have protective properties in preventing occurrence of encephaloceles [2]. Other data suggest, that encephaloceles may be associated with maternal fibrate deficiency [5, 12]. The congenital encephalocele is caused by lack of separation of the neuroectoderm and the ectodermic surface during neural tube formation, which blocks the interposition of the mesoderm between the two germinative layers. Another hypothesis proposes primary bone defect due to a failure of cartilage formation during skull ossification and secondary protrusion of intracranial structures through a defect in the cranium [5]. In almost all cases, encephalocele is diagnosed during the second trimester and 80% of cases in first trimester of pregnancy by ultrasonography. Cranial ultrasound exam reveales the bony skull defect, the size and morphology of encephalocele. However, the presence of neuronal migration disorders, or the developing white matter pathways, may be difficult to visualize by ultrasound.Fetal MRI allows for detailed morphological analysis of the fetus with encephalocele. The information from prenatal MRI of fetus are almost equivalent to the postnatal scan. Advanced MRI techniques may provide additional data useful in treatment after birth [13].
In our case, prenatal MR was performed, which was a sufficient to perform the surgery after birth. Control examination was ordered on the 14th day after surgery. In a significant number of encepaloceles (20, 5-60%), microcephaly, absence of the corpus callosum, optic nerve abnormalities, hypothalamic/pituitary dysfun-ction, fusion of thalami, schizencephaly, holopro-sencephaly, arachnoid cyst, craniosynostosis, hypertelorism, Dandy-Walker malformation, Walker-Warburg syndrome, Chiari malformation, Klippel-Feil malformation, myelomeningocele, and hydrosy-ringomyelia, hemifacial microsomia, cleft lip/palate and defects of kidneys, heart and limbs, may also occur [4, 11, 13]. Gao et al. state, that parietal encephalocele sac may contain abnormal blood vessels and neuroglial elements, and be associated with abnormalities like corpus callosum agenesis, hydrocephalus, intracranial cysts and gray matter heterotopia [14]. Atretic parietal encephaloceles are most often associated with midline anomalies, porencephalies, interhemispheric cysts, corpus callosum agenesis, ventriculomegaly, cystic malformation, heterotopias of posterior fossa and intracranial venous anomalies [15]. In the presented case, MR scan revealed multiple cerebral abnormalities: wide interthalamic adhesion, defects in cortex such as polimicrogyria/ pachygyria bilaterally in temporo-parietal region, subependymal heterothrophy of grey substance, thickened quadruplet bodies, brain crease in left occipital lobe, partial agenesis of the corpus callous and small posterior cranial fossa. Additionally, complete cleft palate was found. The encephalocele sac is generally covered with skin. Large meningoencephaloceles usually require urgent surgical intervention due to the risk of injuring sac and cerebrospinal fluid (CSF) leakage, which may lead to meningitis and prevention of future neurological deficits. Surgery of large meningoencephaloceles can sometimes be extremely difficult due to presence of ischemic or necrotic tissue in the sac, or intracranial vessels, which may come out from the sac to supply normal brain parenchyma. Brain tissue excision can also lead to cerebral infarction [6, 11, 16]. Before surgical intervention it is essential to ascertain preoperatively if the herniated structure contains critical venous drainage. According to embryological theory it is impossible for intracranial dural sinuses to herniate into the sac of encephalocele, because dysraphism of neural tube occurs earlier than the maturation of venous system and development of dural sinuses would adapt for anomalous circumstances: superior sagittal sinus will be divided and disconnected sagittal sinuses will run separately avoiding the neck of encephalocele. Regardless of its size, encephalocele may be safely resected at the neck without subsequent to venostasis [17]. Congenital encephalocele contains abnormal tissue, which is routinely removed without additional neurological sequelae [18]. Ramdurg et al. state, that in all of their patients with the occipital, parietal and nasal encephaloceles there was dysplastic brain tissue which was removed safely [16]. In presented case, the encephalocele sac also contained abnormal brain tissue, which was removed. In case of secondary protrusion of the properly developed parts of brain into broad hernial ring they need to be relocated back to cranial cavity before plasticity of meninges if possible. Gandhoke et al. described the case of child with secondary occipital encephalocele containing normal tissue of cerebellum which was put back during operation to cranial cavity. Postoperatively neurological development of patient was normal [18]. Microcephaly may be another problem, where herniated brain tissue cannot be pushed back inside the small existing cranial cavity during the surgery. Expansile cranioplasty has been in practice to expand the cranial cavity. Owing to the fact, that dura mater is osteogenic and it helps in significant bone growth, there is no need to restore bone defect [9, 10, 19]. The biggest problems with giant encephalocele may be: loss of blood and cerebrospinal fluid during surgery, electrolyte disturbances, hypothermia, bradycardia and cardiac arrest [7, 20, 21]. Other postoperative complications include: cerebrospinal fluid leakage, infection, meningitis and skin necrosis [11, 16, 19]. In patients with encephalomeningocele, the complication may be hydrocephalus, which may not be visible before surgery and develop after the procedure. An associated Arnold Chiari malformation, aqueductal stenosis or reduction of fluid reserve after removal of large sac may be possible etiological factors [9, 19, 22].
However, there is no clear correlation between size of the encephaloceles and the occurrence of hydroce-phalus. Postoperative course after surgical removal of the encephalocele should be concentrate on observation of symptoms of increased intracranial pressure, monitoring the width of the ventricular system by measuring head circumference and size of the fontanelles. Likewise, imaging tests are helpful. If hydrocephalus or intracranial pressure increase, the amount of cerebrospinal fluid should be reduced. In some cases with preoperative ventriculomegaly, postoperative stabilization of the ventricular size and intracranial pressure can occur without the need for a ventriculoperitoneal shunt. However, implantation of the VP shunt together with resection of the encephalocele in the same procedure is not recommended [2]. In case of our patient, the causes of the hydrocephalus were cerebral aqueduct obstruction and reduction of the CSF space as a result of removal of a large hernia sac, which compensated for pressure increases. On the 19th day of life due to worsening hydrocephalus the VP shunt was implanted and improvement was observed. Outcome of encephalocele treatment is determinate by the site, size, contents of the hernial sac, patency of CSF pathway, presence of hydrocephalus and associated congenital anomalies, microcephaly and infections [6, 19, 22]. Newborns with parietal encephalocele have a worse prognosis than occipital encephalocele, which is associated with more cerebral malformations occurring [23]. Additionally, patients with small encephaloceles with small or none amount of nerve tissue in the hernia sac may have normal neurological function. Larger hernias may be associated with neurological disorders, cranial nerve abnormalities, suction disorders, spasticity, blindness, developmental delay and microcephaly [12, 18]. Refaee et al. assessed the neurological development of children born with an occipital or a frontal encephaloceles and ascertain, that all patients with ventriculomegaly had a neurologic delay with variable presentations. As a comparison among patients without ventriculomegaly, some showed no signs of delayed neurological development, and in the remaining cases only minimal delay was observed [2]. According to Ozdemir and coauthors, size of encephalocele, amount of nerve tissue in the hernial sac and presence of hydrocephalus may impact on social development, major and minor motility and pronunciation in children, and therefore, to prognosis [7]. Da Silva et al. assessed neurological development of children after surgery of the anterior or posterior encephalocele and stated, that the presence of hydrocephalus in all cases was associated with abnormal neurological development.
However location of encephalocele wasn’t statistically connected with prevalence of hydrocephalus and neurological disorders [4]. Patients may not initially present substantial abnormalities in neurological examination (paralysis) but neurological disorders, different severities of retardation, difficulties in learning or changes in image examination of CNS (ventriculomegaly) may show in later period of children life [1, 4, 22]. The literature describes also psychological development of children after encephalocele surgery. Dutta et al. assessed children after anterior encephalocele surgeries and stated that patients without additional intracranial defects had good outcomes of development [24]. Lo et al. assessed development of patients after surgeries of occipital, frontal and parietal encepahlocele and revealed that presence of encephalocele shall not affect developmental retardation. However concomitant microcephaly, presence of brain tissue in sac, intracranial defects, hydrocephalus and seizures were associated with retardation [25]. Muralidharan observed severe retardation in patients with encephalocele and comorbid anomalies (Walker- Warburg syndrome, ventriculomegaly) [15].
4. Conclusion
Treatment of encephalocele requires individual approach. MRI in fetal period allows to plan the neurosurgical treatment after birth. Patients with encephalocele need to be regularly checked for complications such as hydrocephalus, neurological defects, developmental disorders which may occur later in life. Good outcomes of preoperative and postoperative treatment is affected by cooperation of: obstetricians, neurosurgeons, anesthesiologists, neonatologists, neurologists, radiologists, pediatricians, physiotherapists, ophthalmologists and geneticists. Multidirectional stimulation of development as part of early support is advised.
References
- Menekse G, Celik H, Bayar MA. Giant Parietal Encephalocele with Massive Brain Herniation and Suboccipital Encephalocele in a Neonate: An Unusual Form of Double Encephalocele. World Neurosurg 98 (2017): 867
- Refaee EAE, Refaat MI, Reda M. Incidence of Secondary Hydrocephalus after Excision of Huge Encephaloceles in Neonates: Case Study. J Neurol Surg A Cent Eur Neurosurg 79 (2018): 15-18.
- Suwanwela C, Suwanwela N. A morphological classification of sincipital encephalo-meningoceles. J Neurosurg 36 (1972): 201-211.
- Da Silva SL, Jeelani Y, Dang H, et al. Risk factors for hydrocephalus and neurological deficit in children born with an encephalocele. J Neurosurg Pediatr 15 (2015): 392-398.
- Rosildo JF, Dos Santos MF, de Santa Barbara Rde C. Huge interparietal posterior fontanel meningohydroencephalocele. Autops Case Rep 5 (2015): 43-48.
- Kumar V, Kulwant SB, Saurabh S, et al. Giant Occipital Meningoencephalocele in a Neonate: A Therapeutic Challenge. J Pediatr Neurosci 12 (2017): 46-48.
- Özdemir N, Ozdemir SA, Özer EA. Management of the giant occipital encephaloceles in the neonates. Early Human Development 103 (2016): 229-234.
- Agarwal A, Chandak AV, Kakani A, et al. A Giant Occipital Encephalocele. APSP J Case Rep 1 (2010): 16.
- Sharma S, Ojha BK, Chandra A, et al. Parietal and occipital encephalocele in same child: A rarest variety of double encephalocele. Eur J Paediatr Neurol 20 (2016): 493-496.
- Verma SK, Satyarthee GD, Singh PK, et al. Torcular occipital encephalocele in infant: Report of two cases and review of literature. J Pediatr Neurosci 8 (2013): 207-209.
- Yucetas SC, Uçler N. A retrospective Analysis of Neonatal Encephalocele Predisponsing Factors and Ouctcomes. Pediatr Neurosurg 52 (2017): 73-76.
- Jimenez DF, Barone CM. Encephaloceles, Meningoceles and Dermal Sinuses. In: Albright AL, Pollack IF, Adelson PD (eds.). Principles and Practice of Pediatric Neurosurgery. (3rdedn), Thieme Medical Publishers, Inc. New York, USA (2014): 205-223
- Kasprian GJ, Paldino MJ, Mehollin-Ray AR, et al. Prenatal imaging of occipital encephaloceles. Fetal Diagn Ther 37 (2015): 241-248.
- Gao Z, Massimi L, Rogerio S, et al. Vertex cephaloceles: a review. Childs Nerv Syst 30 (2014): 65-72.
- Muralidharan CG, Aggarwal R, Singh D. Atretic parietal encephalocoele - An unusual diagnosis. Med J Armed Forces India 69 (2013): 83-85.
- Ramdurg SR, Sukanya M, Maitra J. Pediatric encephaloceles: A series of 20 cases over a period of 3 years. J Pediatr Neurosci 10 (2015): 317-320.
- Ohba H, Yamaguchi S, Sadatomo T, et al. Surgical resection of large encephalocele: a report of two cases and consideration of resectability based on developmental morphology. Childs Nerv Syst 33 (2017): 541-545.
- Gandhoke GS, Goldschmidt E, Kellogg R, et al. Encephalocele development from a congenital meningocele: case report. J Neurosurg Pediatr 20 (2017): 419-422.
- KIymaz N, YIlmaz N, Demir ?, et al. Prognostic Factors in Patients with Occipital Encephalocele. Pediatr Neurosurg 46 (2010): 6-11.
- Mahapatra AK. Giant Encephalocele: A Study of 14 Patients. Pediatr Neurosurg 47 (2011): 406-411.
- Chaturvedi J, Goyal N, Arora RK, et al. Giant Occipitocervical Encephalocele. J Neurosci Rural Pract 9 (2018): 414-416.
- Nayak A, Sharma S, Vadher RK, et al. Congenital interparietal encephalocele: a case report. J Clin Diagn Res 9 (2015): PD09-PD10.
- Yokota A, Kajiwara H, Kohchi M, et al. Parietal cephalocele: clinical importance of its atretic form and associated malformations. J Neurosurg 69 (1988): 545-551.
- Dutta HK, Khangkeo CW, Baruah K, et al. Growth and Psychological Development in Postoperative Patients With Anterior Encephaloceles. Pediatr Neurol 71 (2017): 29-34.
- Lo BW, Kulkarni AV, Rutka JT, et al Clinical predictors of developmental outcome in patients with cephaloceles. J Neurosurg Pediatr 2 (2008): 254-257.


 Impact Factor: * 3.2
Impact Factor: * 3.2 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 