Nutritional and Immunopotentiating Function of Methionine for Poultry
Article Information
Nour Ramadan1, Houssam Shaib1*, Mohamad Farran11Department of Agriculture, Faculty of Agricultural and Food Sciences, American University of Beirut, Beirut, Lebanon
*Corresponding Author: Houssam Shaib, Department of Agriculture, Faculty of Agricultural and Food Sciences, America University of Beirut, Riad El Solh 1107-2020, Beirut, Lebanon
Received: 04 April 2021; Accepted: 15 April 2021; Published: 13 July 2021
Citation: Nour Ramadan, Houssam Shaib, Mohamad Farran. Nutritional and Immunopotentiating Function of Methionine for Poultry. International Journal of Plant, Animal and Environmental Sciences 11 (2021): 363-390.
View / Download Pdf Share at FacebookAbstract
As an essential nutrient and a first limiting amino acid in several meal-based poultry diets, methionine (Met) is of rich nutritional value and physiological functions. Methionine’s multiple functions include but not limited to protein synthesis, feather development, and protection from oxidative stress, methylation of DNA reactions as well as several distinct molecules. Methionine is suggested to play an influential role in both humoral and cell-mediated immune responses. This is vital as the immune system prospers immensely from adequate nutrition, constituting of a balanced diet with supplementation of certain essential nutrients. Methionine’s immunopotentiating roles include detoxification, increasing humoral immune response, stimulation of the phagocytic activity of leukocyte, triggering serum lysozyme activity, and resistance for coccidia infection. These functions increase immune responses during stress cycles and disease outbreaks in poultry and other animals. Additional research is recommended to elucidate the role of the host genetic makeup involvement in the relationship between methionine and other disease factors.
Keywords
Methionine; Immunopotentiation; Nutrition; Poultry
Article Details
Abbreviations:
Met- Methionine; AA- Amino Acid; Arg- Arginine; His- Histidine; Iso- Isoleucine; Leu- Leucine; Lys- Lysine; Phe- Phenylalanine; Thr- Threonine; Try- Tryptophan; Val- Valine; Pro- Proline; Cys- Cysteine; Gly- Glycine; EAA- Essential AA; NEAA- Nonessential AA; CEAA- Conditionally EAA; FAA- Functional AAs; SAA- Sulfur amino acids; TSAA- Total sulfur amino acids; CAS- Chemical Abstracts Service; RN- Registry Number; FDA- US Food and Drug Administration; UNII- Unlicensed National Information Infrastructure; SAMe- S-adenosylmethionine; MAT I- Methionine adenosyl transferase I; MAT II- Methionine adenosyl transferase II; SAH- S-adenosylhomocysteine; HCys- Homocysteine; SucCoA- Succinyl-CoA; CAC- Citric Acid Cycle; TCA-Tricarboxylic acid cycle; MS- Methionine synthase; BHMT, E.C.2.1.1.5-Betaine-homocysteine methyltransferase; DLM- D, L-methionine; MHA-FA- L-methionine hydroxy analog-free acid; MHAC- DL- methionine hydroxyl analog calcium; AAFCO- American Feed Control Officials; CFR-Code of Federal Regulations; FDA- Food and Drug Administration; BCAA- Branched-chain AA; GMOs-Genetically modified organisms; E. coli- Escherichia coli; C. glutamicum- Corynebacterium glutamicum; ME- Metabolic energy; CP- Crude protein; N- Nitrogen; NRC- National Research Council’s; ME:CP- Metabolic energy to crude protein ratio; AF- Abdominal fat; FC- Feed conversion; FI - Feed intake; BWG- Body weight gain; FE- Feed efficiency; EW- Egg weight; EP- Egg production;
BW- Body weight; FCR- Feed conversion ratio; AFP- Abdominal fat pad; AME- Apparent metabolizable energy; DM- Dry matter; IP- Ideal protein; GI- Gastrointestinal tract; HI- Heat increment; ROS- Reactive oxygen species; AGP- a-1 acid glycoprotein; (IL)-l- Interleukin; LPS- Lipopolysaccharide; LMI- Leucocyte migration inhibition; PHA-P- Phaseolus vulgaris; IgG- Antibody immunoglobulin G; NDV-Newcastle disease virus; Ab- Antibody; SOD- Superoxide dismutase; GSH-Px- Glutathione peroxidase; MDA-Malondialdehyde; PHA- Phytohaemagglutinin; ConA- Concanamycin A; Th- T-lymphocyte helper cells; SRBC- Anti-sheep red blood cell antibody; CBH- Cutaneous basophilic hypersensitivity; CV- Coefficient of variation; HCT%- Hematocrit
1. Introduction
Amino acids (AA) are the building blocks of biological proteins. Over 500 AA exist in nature, though 20 of which can incorporate into proteins by arranging in myriad of ways (i.e metabolic proteins, structural proteins, enzymes, as well as precursors of multiple body constituents) serving variety of functions [1-3]. AA obtained from protein are utilized by avian species to fulfill a variety of function. Such that, proteins are underlying constituent of structural and protective tissues such as skin, feathers, ligaments, bone matrix, bone matrix, soft tissues, inclusive of organs as well as muscles [4].
The carbon skeleton of AA categorizes the dietary essentiality of AA [6]. According to the National Research Council’s (NRC), ten out of the twenty-two AA (arginine (Arg), histidine (His), isoleucine (Iso), leucine (Leu), lysine (Lys), Methionine (Met), phenylalanine (Phe), threonine (Thr), tryptophan (Try) and valine (Val) found in body proteins are classified as the essential AA (EAA) for the nutrient requirements of poultry [4]; that is, they are indispensable as their carbon skeleton cannot be synthesized de novo by the avian species. In contrast, AA that can be synthesized by the animal are known as nonessential AA (NEAA) [5-7]. It was assumed that animals do not require NEAA in their diets for maximal nutrition as they are capable of synthesizing adequate quantity of NEAA. Scientific research on animal as well as cell culture have nonetheless demonstrated evidence of NEAA carrying critical roles in multiple signaling pathways, while further demonstrating the importance of considering AA functions beyond their protein synthesis as young animals are unable to synthesize adequate amounts of NEAA particularly during early phase development to support their growth as the rates of utilization are relatively greater than rates of synthesis; to poultry this is true for Arg and proline (Pro). Such AAs are referred to as conditionally EAA (CEAA) [5, 8-10].
Functional AAs (FAA) is rather a notion developed to further define those AAs that regulate and engage in key metabolic pathways to improve health, survival, growth, development, and the reproduction of organisms. An observed deficiency in FAA whether it be EAA or NEAA hinders both the synthesis of protein as well as the species’ body homeostasis [11].
Methionine is an EAA as it cannot be biologically synthesized by the bird and due to its exceptional emphasis on poultry growth and production [12]. Met is also classified as a FAA. Animals ought to obtain EAA from the diet, however many feed ingredients lack some of the EAA. In a typical corn-soybean meal, birds’ requirement for fulfilling certain EAA may fail; this is the case for lysine (Lys), methionine (Met), threonine (Thr), and tryptophan (Trp). Met is considered the first limiting AA, with lysine and threonine being the second and third limiting AA respectively in a practical corn-soybean poultry fed diet [13, 14].
When formulating bird diet, sulfur amino acids (SAA) are crucial addition. Met and cysteine (Cys) are the two sulfur-containing AA (SAA); both are the principle providers of organic sulfur within the avian body and ought to be considered together as total sulfur amino acids (TSAA) nutrient requirements [15-19].
All AA must be present in cell into order for correct protein synthesis to take place. Therefore, it is crucial for protein and EAA to be supplied by the diet, where formulating dietary requirements for both ensures the appropriate approach for physiologically required AA to be implemented [7].
Met is ought to be available sufficiently in the diet to provide the building blocks of tissues, immune cells and to support the development of feathers [6, 20, 21]. Elevated levels of Met exist in eggs, sesame seeds, in addition to Brazilian nuts, fish meal, corn gluten meal, alfalfa meal, as well as sunflower seeds meal [20, 22-24]. Yet, Met is found to be limited in most natural sources of plant protein and it is therefore established as the first limiting AA for broilers and the second limiting AA for laying hens in a typical corn-soybean meal ration [24]. This article discusses the effect of immunopotentiation of methionine on broilers by going through methionine forms, metabolism, deficiency, and toxicity. It also shows the nutritional, fast-growing and slow-growing requirements in addition to the digestibility and the ideal protein ratio and choosing between cysteine and methionine as the Ideal sulfur amino acid in diets.
2. Methionine metabolism
Methionine possesses an empirical formula of C5H11NO2S, Chemical Abstracts Service (CAS) Registry Number (RN) 63-68-3; US Food and Drug Administration (FDA) Unlicensed National Information Infrastructure (UNII) with molecular structure demonstrated in Figure 1 [25, 26]. The sulfur-containing EAA has an asymmetric form, constructing both L- and D-isomers and the molecular structure of racemethionine is illustrated in Figure 2 below. The liver is the main site of Met metabolism where Met is the first limiting AA in commercial poultry [27]. The metabolism of Met is vital for numerous physiological processes (Figure 3). Expressly, Met is principally involved in the aid of methyl transfer reactions via its conversion to its active form as S-adenosylmethionine (SAMe) which is achieved by the catalyzation of methionine adenosyltransferase I (MAT I) and methionine adenosyltransferase II (MAT II) [10, 28]. The fact that vertebrates are unable to synthesize methyl groups which are essential constituents in their diets [29] proves Met methyl role a crucial one. Other than Met, potential dietary sources of methyl groups include choline, folic acid, and betaine [29]; however, they differentiate in their methylation availability and reactions. In the case of Met, it is necessary for protein synthesis. While as for instance with choline; it is primarily utilized in cell membranes as well as neurotransmitter [30, 31].
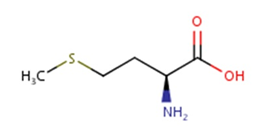
Figure. 1: Molecular structure of methionine, CAS RN: 63-68-3; UNII: AE28F7PNPL (ChemIDplus, 2017; FDA, 2017) [Diagram credit to ChemIDplus, 2017].
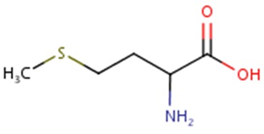
Figure. 2: Molecular structure of racemethionine, CAS RN: 59-51-8; UNII: 73JWT2K6T3 (ChemIDplus, 2017; FDA, 2017) [Diagram credit to ChemIDplus, 2017].
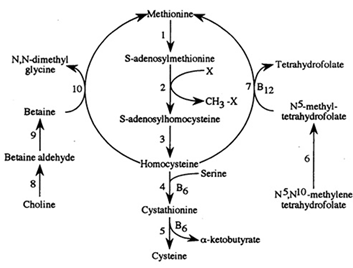
Figure. 3: Methionine metabolism. Enzymes depicted are as followed (1) methionine adenosyltrasferase, (2) various enzymes, (3) S-adenosylhomocysteine hydrolase (EC 2.1.1), (4) cystahionine ?-synthase, (5) cystathionine ?-lyase, (6) α-ketoacid dehydrogenase, (7) N5, N10- methylenetetrahydrofolate reductase, (8) methionine synthase, (9) choline dehydrogenase, (10) betaine aldehyde dehydrogenase, and (11) betaine-homocysteine methyltransferase (EC 2.1.1.5) (Illustration adopted from Emmert et al., 1996).
|
Name |
L-Methionine |
||
|
IUPAC |
L-2-amino-4-(methylthio)butyric acid, S-2-amino-4-(methylthio)butanoic acid, H-Met-OH |
||
|
Formula |
C5H11NO2S |
||
|
Molecular weight |
149.208g/mol |
||
|
Water solubility (20°C) |
53g/L |
||
|
Vapor Pressure |
5.23E-07mm Hg (25°C) |
||
|
Melting point |
283 dec °C |
||
|
pKa1 (-COOH) |
2.28 (25°C) |
||
|
pKa2 (-NH3) |
9.21 (25°C) |
||
|
Isolelectric point (pI) |
5.74 (25°C) |
||
|
Henry’s Law constant |
2.11E-11 atm-m3/mole (25°C) |
||
|
pH (1% aqueous solution) |
5.6-6.1 |
Table. 1: Chemical and Physical properties of Methionine1.
1Information and values credit to PubChem, 2017; ChemIDPlus, 2017.
Post its conversion in Met metabolism, same is next capable of behaving as a methyl-donor by releasing its terminal methyl group (and is the predominant methyl (−CH3) donor in Met metabolism) of which can occur in an assortment of methyltransferase reactions (E.C. 2.1.1) [31]. Such reactions permit the synthesis of choline, creatine, epinephrine, glutathionine, lipoic acid, DNA as well as several other necessary compounds [32, 33]. Once SAMe has donated its methyl group, it is irreversibly converted to S-adenosylhomocysteine (SAH). Via the removal of an adenosine molecule, adenosylhomocysteinase converts SAH to homocysteine (HCys). Then there are two fates of HCys; it is either transsulfurated to Cys or remethylated to Met in Met metabolism [27].
Governed by the activity of Cystathionine ?-Synthetase, HCys merges with serine constructing a molecule of cystathionine. This is broken down via cystathionine-?-lyase into a molecule of Cys and a molecule of ∝-ketobutyrate [23, 31]. In both of these reactions, a co-factor of vitamin B6 is obligatory in the form of pyridoxal phosphate [34]. The ∝-ketobutyrate generated through this pathway is later converted to propionyl-CoA via multiple steps using ∝-ketoacid dehydrogenase. Succinyl-CoA (SucCoA), the end product metabolized from propionyl-CoA then enters the Citric Acid Cycle (CAC) (also known as tricarboxylic acid (TCA) cycle or the Krebs cycle) to generate energy [27, 31]. Thereby, two molecules of the regenerated cysteine will compose a single molecule of cysteine, that is the structural component of keratin; the leading protein present in skin, hair, nails, and feathers [23].
Alternatively using vitamin B12 as a cofactor; HCys can be remethylated to Met from N5- methyl-tetrahydrofolate by the route of methionine synthase (MS) [27, 34]. In addition, post its development by the oxidation of choline; homocysteine can also be remethylated to Met from betaine via betaine-homocysteine methyltransferase (BHMT, E.C.2.1.1.5) [27, 31, 35].
3. Forms of methionine
Mueller, in 1923 was the first to isolate Methionine from casein and since then much research has been towards the importance of Met in nutrition and animal feed [36]. AA, apart from glycine (Gly) [5, 21] are available in two forms which are referred to as D- or L enantiomers. In nature, Met is predominantly present in the L-form. While, the D-enantiomer is biologically inactive. Poultry are capable of utilizing both D- and L- forms of Met [37]. Both forms can be metabolized by a DL-racemase, which is needed for supplementing the chemically synthesized DL-methionine racemate as feed additive in livestock farming [38]. Met usually exists as a white crystalline powder. As a limiting AA, Met is supplemented in the form of dry D,L-methionine (DLM), racemic 2-amino-4-methylthiobutyric acid, with approximately 99% purity [38] , or liquid as either D,L-methionine hydroxy analog-free acid (MHA-FA) with equivalency of 88% racemic 2-amino-4-methylthiobutyric acid or DL- methionine hydroxyl analog calcium (MHAC) with 97% racemic 2-amino-4- methylthibutyric acid and calcium salt activity post-conversion of the analog into the active biological form [38] according to the Association of American Feed Control Officials (AAFCO) Code of Federal Regulations (CFR), Title 21CFR 582.5477 [39]. As ?-keto acid analogs (the amine group replaced by a hydroxy group), MHA-FA and MHAC in avian species can be converted to the amino form via liver transamination applied by non-EAA (i.e. glutamic acid) [6]. DLM, MHA-FA, MHAC are all recognized as safe by the Food and Drug Administration (FDA) according to Title 21CFR 582.5475 and Title 21CFR 582.5477 [25, 39].
As mentioned earlier, it is not solely the level of methionine that is required but rather a balance of all EAA. Focusing only on methionine levels will generate AA imbalances and thus protein degeneration. Moreover, antagonistic relationships between certain AA have been present, an example of this exist between branched-chain AA (BCAA) leucine-isoleucine-valine, threonine and tryptophan and between arginine and lysine (37, 40].
Met is commercially synthesized via acrolein and methyl mercapton condensation [41, 42]. This compressed compound is then reacted with ammonia and hydrogen cyanide to generate a racemic mixture of the D and L isomers of Met which is effectively 100% pure [41]. The resulting compound is 1:1 ratio of D-Met and L- Met; where the racemic mixture DL-Met is a of 50% D-Met and 50% L-Met [38, 42].
Most of this manufactured Met is used for animal feed in livestock production amounting above 600,000 tons/year in 2013 according to the world market [38, 43]. This does not include organic farming due to the ban or high limitation on synthetic Met usage [20, 44]. Met has also been shown to be synthesized by bacterial fermentation [45-48] for up to 5 g/L with unemployment of genetically modified organisms (GMOs) [38]. The topmost concentration level of L-methionine via fermentation depicted at 35 g/L using a GMO of Escherichia coli (E. coli) [38]. Yet, to naturally synthesis Met, the process undergoes complex regulations including mutations, genetic modification, selection and optimization with only few strains of bacteria are capable of producing relevant amounts of Met, a major one being Corynebacterium glutamicum (C. glutamicum) [38, 49, 50].
As to whether which of L-, D-, or D-L- Methionine is more efficiently absorbed, statistical methodology and research trials to evaluate the precise bioefficacy for D-, L-, DL-Met have estimated to be comparable [51-53].
4. Methionine requirement
4.1. Nutritional requirement
Birds eat to satisfy their metabolic energy (ME) requirement [7]. As with all animals, poultry require energy (carbohydrates, and fats), protein, vitamins, minerals, and water [54, 7]. Formulation of diet must meet the EAA and this ought to be relative to the level of ME. Several factors can affect dietary AA requirement and their utilization efficiency [55]. These include age gender, genetics, reproductive state, stage of production, ambient temperature, housing system, immunological stressors, production aim as well as metabolic energy, the level of proteins, and the availabilities of vitamins and minerals in the diet (4, 6, 7]. Poultry do not have specific requirements for crude protein (CP) per se; rather only for AA levels. Yet, CP should be present to meet the requirements of EAA, and enough nitrogen (N) to synthesize the non-EAA. Amino acid requirements are usually presented as percentages of the diet or may percentage of protein requirement. National Research Council’s (NRC) Nutrient Requirements of Poultry is the fundamental reference for feed formulation. Dietary AA can be classified as both qualitative and quantitative [56]. Qualitative requirements rest under questions of “what” are the AA needed for maintenance, optimal performance, including growth, lactation and reproduction and optimal health including prevention of abnormalities, resistance, and the recovery to infectious diseases [5, 56]. Quantitative requirements follow questions of “how much” of an AA is needed for maintenance, optimal growth, and health [56]. The nutritional requirements of broilers are applied for starter, grower, and finisher phases as the requirements change with age; generally, with decrease in AA levels and an increase in ME. Overall CP contents of 23, 20, and 18% are applied for starter, grower, and finisher stages, respectively.
An unbalanced diet leads to poor poultry performance. In the case of deficient protein diet, i.e metabolic energy to crude protein (ME: CP) ratio; birds will overconsume energy to obtain sufficient protein [20]. Increasing the level of Met in broiler diets significantly reduces the abdominal fat (AF) content, this was reported by Summers and Leeson [57], whose work agreed with results by Mabray and Waldroup [58], demonstrating decreased abdominal fat (AF) weights as the levels of dietary Lys and Met increased. Deficient EAA diets can increase FI. According to Cherry & Siegel [59] pullets’ diets equal in energy and contained 15% crude protein with only difference in levels of Met and SAA observed increase in FI to compensate for a marginal SAA deficiency, and that the SAA requirement for maximum feed conversion (FC) efficiency was higher than the requirement for egg production (EP).
Including ME and AA content in feed, feed intake (FI) could be influenced by nutritional value and toxicity of the feed, palatability, particle size and environmental temperature [7, 60]. With high metabolic energy diets, poultry FI decreases, and AA intakes are thus restricted [6, 61].
During heat stress (high ambient temperature and humidity), FI decreases, evoking reduced growth and egg production, poor performance, leading to physiological and immunological stress, susceptibility to disease, reduced welfare status and high mortality rates. This results in detrimental effects of reduced welfare status of birds and economic losses to poultry production [62].
In period of heat waves, it is important to implement a combination of methods to aid in alleviating heat stress, ranging from housing, management, and feeding practices [62]. An additional method is nutritional manipulation. Administering high AA and high protein diets to birds during high ambient temperature demonstrated negative impact body weight gain (BWG), feed efficiency (FE), and carcass composition and yield [62, 63]. AA supplementation may partially prevent growth depression of heat stressed flocks, yet it is supplementation of low- apparent metabolizable energy (AME) and high AA diets at high temperature that significantly decreases the bird’s FI, BW, absolute carcass, breast, wing, front half, including back half weights [7, 55, 60].
Universally recognized as the first limiting AA in broilers, methionine is also the second limiting AA for laying hens fed on practical corn-soybean meal diets [7, 64]. NRC (1994) requirement for methionine are 0.50, 0.38 and 0.32% for starter (0-3 weeks of age), grower (3-6 weeks of age) and finisher (6-8 weeks of age) broiler phases respectively; with 90% dry matter (DM) basis of 0.90, 0.72, and 0.60% total TSAA requirement, respectively.
If the NRC requirement is not met for any EAA, the efficiency of poultry production is reduced immensely with great losses in broiler growth and egg size in laying hens [20, 64, 65]. Therefore, synthetic Met in form DLM, MHA-FA or MHAC are generally supplemented in poultry feed to meet their dietary requirements. It is crucial to adjust the concentration of all nutrients in diet in relation to the level of metabolic energy to provide a nutrient balanced diet. Thus, in the case of increased ME, Met requirement increases [66], and it is recommended to prepare poultry feed with AA needs calculated as percentage of ME. [67] observed that as wider the ratio of ME to protein tended to be, broiler consumed greater energy and deposited greater fat and less water in their carcasses. The present-day commercial bird is very distinct from commercial birds prior to 1991 [68, 69]. Some research suggests that AA requirement today differ greatly from those highlighted by the NRC (1994) as to reasons of genetic selection, management practice and feed-related alterations [68]. Other studies state that increasing levels of Met ought to be above the NRC (1994) recommendations [70, 71]. Further, studies by [72-74], report that methionine requirements for optimal immunity are higher than for optimal growth.
4.2. Fast-growing versus slow-growing broilers requirements
Dietary AA concentrations ought to match the needs for both maintenance and skeletal muscles [75]. The requirement of AA of fast-growing broiler breeds may be greater than slow-growing breeds. This is coherent with higher protein to fat ratio of fast- growing genotypes than slow-growing genotypes [20, 76]. Thus, higher AA to ME ratio is required for faster growing breeds [77]. As expected, the rapid growth rate of broiler today requires increased contents of nutrients and ME daily, however these demands for different nutrients are not in the same proportions as previously stated [77, 78].
Met requirement of fast-feathering versus slow-feathering genotypes has been indicated to be the same of Met level of 0.46% and 0.46% respectively for optimized nitrogen retention and 0.50% and 0.50% respectively for optimized version (FC) during the grower phase [79, 80]. As a sulfur AA and compared to Met, Cys requirement for fast and slow growing strain illustrated to be lower, with 0.44% and 0.39% respectively [79].
Methionine and total sulfur amino acid requirements for broilers with fast, medium, and slow growing genotypes have demonstrated to be analogous in the starter and grower phases, [7, 81, 82]. Increasing graded level of Met in basal diets has significantly increase BWG, but no interaction has been illustrated between the Met content and broiler genotype [20]. Yet an interaction was evident taking into consideration the measurement of breast yield. Breast yield of fast-growing broilers responded to increasing dietary content of Met, breast yield of medium-growing breeds responded solely to the intermediate content of Met, and breast yield of the slow-growing breeds responded solely to the diets with higher content of Met [20, 83].
5. Digestibility and ideal protein ratio
An ideal protein (IP) is one that consists of the explicit amounts of AA necessary for the animal without deficiencies or excesses [84]. No particular assortment of AA requirements is assigned to any animal following conditions of age, gender, body composition and nutrition combined [56]. The concept of IP holds a mixture of EAA that explicitly match protein accretion and maintenance demand i.e meeting the animal’s need for its specific growth stage or level of production, without under- or over-feeding of AA [22]. In order to determine the ideal amino acid ratio, it is mandatory to know the digestible amino acid requirement of each EAA for the chicken and its relationship to lysine [85]. IP ratios (AA-to-Lys ratios) are expressed as percentages of digestible Lys established on digestible AA requirements rather than TAA requirements [86]. This means that to consider Met requirement, it is crucial to focus on Met as well as a balanced profile of all the EAA [2, 86]. For this reason, the concept of IP is employed in diet formulation to aid in balancing and supplying with greater precision all the EAA in addition to the NEAA (such as glutamine, glutamate, proline, glycine, and arginine which are influential in regulating gene expression, cell signaling, anti-oxidative responses, fertility, neurotransmission, and immunity) for ideal performance and increased profitability [22, 56, 62].
Poultry possess very short digestive tract of which is particularly sensitive to pH alterations. Few natural crude proteins are gradually digested, thus the available AA that can be absorbed and deaminized prior to those that are gradually released are available for absorption. The liver however is not capable of storing AA, this means that if the AA are not absorbed, when necessary, they cannot be utilized for protein synthesis [87]. Several factors may influence protein digestibility. Yet AA content and chemical analysis may demonstrate a complete essential AA profile; factors as solubility, structure and type of proteins can affect digestibility [87].
Regular assays for requirements are not satisfactory. This is due to the factors affecting AA requirements: dietary ME or CP levels, age of birds, genetics, and gender. Thus, it is impossible to address all these factors in one trial, taking into consideration individual AA. This is the reason for a need of ideal ratios with Lys used as reference AA. The justifications for using Lys as a reference for ideal protein is 1) Lys is the second limiting AA in poultry diet, and in fact supplementing a limiting AA (i.e. methionine and lysine) to poultry diets increases the efficiency of protein utilization, and in turn N excretion will be reduced; 2) Lys is easier to analyze than Met or Cys, 3) Lys is almost exclusively used for body protein and thus not complicated by pathways related to maintenance and feathering 4) Ample of data for the digestible Lys requirement of poultry are available, and 5) Lys requirement for several dietary, environmental, and body compositional circumstances are readily available [7, 61, 88-90].
Yet, the NRC (1994) lists the TAA requirement as opposed to levels of digestible AA; and in ingredients of practical plant based diet, the content of AA is not equal to the available AA content for the presence of anti-nutritional factors [89, 91]. To determine the digestibility of feed ingredients, the below equation is used. This classical method for evaluating feed ingredients where measurements of digestibility allow for determining the amount of a certain nutrient absorbed in gastrointestinal tract (GI) from a given quantity of food. For instance, the % digestibility of protein is calculated as:
(DW diet eaten × %Pro diet – DW feces voided × %Pro feces) / DW diet eaten × %Pro diet
Whereby: DW goes for “Dry weight of”, Pro goes for “Protein in”.
The similar equation may be utilized to determine the percentage of digestibility of CP, fat, dry matter, energy, or any other nutrient [7].
The use of an ideal AA ratio may aid in decreasing feed costs. Diets formulated on a digestible basis have illustrated to provide augmented performance when compared to diets formulated on a total amino acid basis [85, 92].
Note that proteins of those which supply solely the desired level of essential AA are preferred to those which provide a high excess of some essential with minimum level of another essential. This is due to the fact that large surplus of one type of AA can be antagonistic to another [87]. This antagonism relationship among AA particularly occurs between or among AAs belonging to the same group, such as Lys and Arg, or branched chain AA (Leu, Ile and Val) [40]. In such case increasing the level of one AA above its requirement necessitates increasing the level of the other AA [40]. It is important to highlight that this experimental thesis is aware and considers the antagonist relationship between AA as well as the previous research done on high excess of methionine demonstrating its negative turn out [65, 93-95]. This is why within this thesis experiment methionine is not supplemented in high excess but rather slightly above nutritional requirements for poultry.
It is also important to note that the utilization efficiencies of individual EAA are different [21, 96].
6. Choosing between cysteine and methionine as the ideal sulfur amino acid in diets
The reason for Met to be perceived as an ideal SAA than Cys is due mainly to the fact that Met is an EAA and Cys is not. In addition, TSAA requirement can be provided solely by the metabolism of Met. This is achieved via Met transsulfuration pathway where Met serves as a precursor of Cys. In contrast, Cys does not serve as the precursor of Met due to the irreversibility of Met transsulfuration pathway [27, 97-99]. Yet, studies on Met-sparing effect of Cys [97-99] where Met utilized in transsulfuration pathway was replaced with Cys demonstrated to be inadequate. This is because a raise dietary Cys and a reduction in dietary Met consequently raise dietary organic sulfur at the same concentration of TSAA, which means Met-sparing experiments may alter the quantity of organic sulfur in TSAA [100, 101].
7. Methionine deficiency
Malnutrition and infections are major obstacles to survival, growth, reproduction, and health [102, 104]. Dietary AA deficiency hinder concentrations of majority of AA found in plasma while damaging the lymphatic system [104, 105].
According to Elwinger and Tausen [106] reduced MET levels decreased feather cover and egg weight (EW), though egg production (EP) was not affected. Futher, they observed that FI increased as feather cover deteriorated, hence a reduction in feed efficient (FE) was apparent. Met deficiency in poultry is presented with reduced growth, performance, FI, BWG, FCR, breast meat yield, and increased abdominal fat pad (AFP) deposition [107, 108]. Met-deficient diet induces reduced growth and performance as feed lacking adequate level of Met to accommodate for maintenance, growth and production of poultry prompts for poor growth rate, FCR, BW, FCR, egg size and production for layers and breeders [20, 109]. As Met is a SAA and sulfur is a major constituent of feathers, Met-deficient diet is associated with poor feather development. Bird with met deficiency is likely to feed on feathers in an effort to satisfy a craving for Met and as such feather picking can lead to cannibalism circumstances in a flock or lead the bird to pick on its own feathers resulting in higher incidence and/or severity of bacterial infections [110, 111]. Further SAA execute antioxidant functions in the avian body preventing destruction of cells. Rubin et al [112] have reported that higher levels SAA may be beneficial to resilience to diseases. Swain and Johri, (2000) demonstrated Met incorporation of (0, 1.5, 2.0, and 4.5 g/kg diet) significantly augmented (P < 0.05) the cellular immune response. Avian met deficiency can well lead to a flawed lymphatic system, high morbidity, and mortality due to debilitated mechanisms of T and B lymphocytes [72].
Not incorporating Met to diet formulation leads to elevated levels of CP and unbalanced IP ratios, leaving excesses of AA behind unemployed for growth, production, etc. that ought to be metabolized and excreted. This therefore propagates incidence of kidney disorders and elevated heat increment (HI). Unable to cope with high HI, birds undergo HS particularly in warm regions establishing high mortality rates [62, 113]. On the other hand, the supplementation of Met aids in lowering the pH level of urine via excretion of sulfate anion and thus impeding the development of kidney stones, uroliths, or urologic syndromes [114].
8. Methionine toxicity
High levels of Met have been indicated to be toxic for avian species, this is in accordance with [93, 115-117] stated that the high excess of DLM or DL-HMB-FA Met supplementation reduced WG and FI of ducks significantly. Particularly growth depression was observed in broilers as well as turkeys when Met content in diet was supplemented above 1% [116-119]. It is worth noting that high levels of plasma homocysteine (HCys) are suggested to be an index of overage dietary Met considering HCys is a precursor for Met synthesis and a metabolite of Met degradation [27, 120].
9. Immunopotentiating function of methionine
As an essential and first-limiting amino acid, Methionine is suggested to play an influential role in both humoral and cell-mediated immune responses. Amino acids are required for clonal proliferation of lymphocytes; the delivering of new bone marrow monocytes and heterocytes and synthesis of effector molecules (immunoglobulins, lysozyme, nitric oxide, complement); formation of bursa of Fabricius’ germinative centers to perfect immunoglobulin affinity; and the development of communication molecules (such as cytokines and eicosanoids) [65, 112]. Multiple functions are dependent upon Methionine, however its notable roles include 1) protein synthesis; 2) precursor for glutathione; a tripeptide that curtails reactive oxygen species (ROS) safeguarding cells from oxidative stress; 3) methionine is required for the synthesis of spermine and spermidine which are polyamines that engage in nucleus and cell division; and 4) methionine is a vital methyl donor, methylating the reaction of DNA as well as several distinct molecules [5, 75, 112, 121].
Considerable scientific research studies have demonstrated methionine protagonistic interference in the immune system of poultry, leading to an enhancement in both humoral and cellular responses. One reason for such effect is employed by the intracellular glutathione and cysteine levels [112]. Immune cells proliferation is sensitive to intracellular disparities in glutathione and cysteine levels which are also involved in the metabolism of methionine [73]. Glutathione, carrying multiple important activities is known as the most abundant intracellular antioxidant compound and is crucial for the protection against the emergence of oxidative stress occurring posts inflammatory processes [112, 122]. For protein synthesis to occur in immune cells, sufficient dietary intake of both methionine and cysteine [sulfur-containing amino acids (SAA)] is important [123].
A study by Takahashi et al. [124] illustrated both Methionine and Cysteine (SAA) exerting beneficial aspect on immune and inflammatory responses to stress induced Escherichia coli lipopolysaccharide injection, and concanavalin A in male broiler chickens. Results indicated plasma a-1 acid glycoprotein (AGP) concentration and interleukin (IL)-l-like activity in chicks fed on the SAA- sufficient diet were higher following a single e injection of lipopolysaccharide (LPS) than those in chicks fed on the SAA- deficient diet [124].
Swain and Johri (2000) indicated that cellular immune response measured as leucocyte migration inhibition (LMI) increased significantly (P< 0·05) at supplemented concentrations of methionine in broiler diets at 21 d of age demonstrating enhanced immunity [74]. Such observation was in conformity with study by Tsiagbe et al. [72], that revealed enhanced mitogen stimulation by Phaseolus vulgaris (PHA-P) as responses to phytohaemagglutinin and significant increase in total antibody immunoglobulin G (IgG) in chicks fed on corn-soybean diets supplemented with methionine [72]. Increased methionine is reported to be critical for the synthesis of the IgG necessary for Th cells function [72].
Experiment carried out to study Methionine deficient diet in challenged chicks with infectious bursal disease demonstrated significant decrease (P<0.05) in monocyte ratio and blood triglyceride; in addition to protein efficiency ratio, body weight gain, and feed conversion ratio [125].
Moreover, dietary supplementation with Methionine or Cysteine was beneficial for the lymphatic system under various catabolic conditions. In chickens challenged Newcastle disease virus (NDV) increasing dietary levels of Methionine (from 0.4 to 0.6, 1.2 and 1.8% respectively) in diets noticeably augmented T-lymphocyte proliferation in response to mitogen stimulation as well as IgG plasma levels [126]. While, an increased level of dietary Cysteine (from 0.185 to 0.37%) has shown similar effects as Methionine. However, high supplemental levels of Methionine and Cysteine (1.8 and 0.37%, respectively) were inimical to the chickens’ performance and immune responses. This can be explained by the excess production of highly toxic elements such as homocysteine and sulfuric acid [65, 95] and thus a higher Cysteine content supplemented in poultry diet is considered to be toxic [65, 127]. While, lower sulfur-containing amino acid levels have resulted in a severe lymphocyte depletion in the Peyer’s patches and in the lamina propria [74, 112].
Hence, it is crucial to remark that neither the excess nor the deficiency of methionine in diets influence the generation of primary antibodies in chickens [65, 74, 128, 129]. Moreover, according to Rubin et al., (2007) vaccines administered on 1-day of age can impair the bird’s performance up to 21 days of age. Thus, it is recommended to carefully administer vaccines, considering the risk of mortality caused by disease as compared to mortality caused by vaccines [130].
Majority of experimental studies conducted have focused on the effect of methionine deficiency on selected immune mechanisms in chickens [74, 112, 125, 131], and only a few researches investigated the influence of methionine on lymphocytes in peripheral blood and lymphoid organs in broilers [93, 115, 132].
According to [93] methionine deficient diet in broilers caused ultrastructural pathological changes in the thymus, decreased T-lymphocyte populations, reduced serum concentrations of interleukine-2 and T-lymphocyte proliferation via increase in the percentage of apoptotic cells. In another study, Wu et al., 2013 cited relative decrease in weights of the thymus and bursa of Fabricius as well as decrease in proliferative of thymocytes and bursal cells with lower levels methionine in diet than recommended by NRC (1994).
Thus, methionine deficiency can lead to lymphoid organs dysplasia [115, 133, 134], and decrease the relative weight of thymus, spleen and bursa of Fabricius [132, 135].
With regards to immune cell response, low level of methionine cause receding tubercularization reaction which proposes decline in Th1 lymphocytes proliferation within inflammation sites [130]. Such results were compatible with [74, 136] displaying levels of methionine below 0.50% spawn feeble immune response as distinguished to higher concentrations [74, 130, 136].
Methionine deficiency can significantly inhibit the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) and the prevention of hydroxyl radicals while augmenting malondialdehyde (MDA) levels [137]. Further, methionine deficiency causes oxidative stress and lipid peroxidation, forcing the aggregation of free radicals, and hence destroying biofilm structure of lymphocytes. It also greatly hinders the reactivity of phytohaemagglutinin (PHA), the stimulus reaction of spleen lymphocytes to concanamycin A (ConA) as well as the mitogenesis of thymic cells [137, 138].
Histological studies acknowledge congestion in cortex and medulla of thymic lobule with loosely arranged and significantly reduced quantity of lymphocytes in the medulla in methionine deficient formulated diet [93]. Decline in lymphocytes were also observed in lymphoid follicles with thinner cortices and wider medullae in the bursa of Fabricius [135]. The histological structure of spleen was disarranged, and its lymphocytes were significantly decreased in the white and red pulp [135]. Vacuolated mitochondria of lymphocytes and greater apoptotic lymphocytes were detected in the spleens of broiler on methionine deficient ration [93, 132, 137, 138].
Commercial broiler chickens do not require above 0.50% and 0.38% Methionine in starter and grower diets, respectively for optimum growth and feed efficiency, while it has been observed that higher incorporation rates of Methionine are necessary to prompt immune responses [65, 73, 74, 139].
Elevated Methionine content, above the required dose for optimal growth, augments the immune response through both direct effects (protein synthesis and breakdown) and indirect effects involving Methionine derivatives. Few studies show the effects of methionine on nonspecific immune function as stimulating phagocytic activity of the leukocyte [140, 141], peripheral blood lymphocyte activity, as well as serum lysozyme activity [98, 142]. As with methionine derivatives, methionine is a substrate for the synthesis of choline and therefore resulted compounds phosphatidylcholine and acetylcholine play essential role in leucocyte metabolism and nerve function [127, 143]. Furthermore, Methionine has demonstrated important physiological function in regard to detoxification [143] and resistance for coccidium infection [136].
Antibody titers (IgG) in broilers supplemented with 1.2 and 0.9% methionine diet were significantly higher than those of low levels of methionine [144]. Such experiment illustrated significant increase of total leukocyte, percentage of lymphocytes and heterophils as well as significant weight changes of bursa and spleen. Further, increase body weights and higher feed intake were obtained at high methionine and low energy diet [144].
Turkeys that received methionine supplemented diet with at 5.98 g/kg; only slightly (by 8.7%) above the NCR (1994) recommendations have shown increase percentage in IgM+ B-cell subpopulation in the spleen [145].
Also, optimizing leukocyte migration inhibition assay required greater content of methionine than the growth promoting level in broiler chicks [146]. Similarly, higher methionine was required for antibody response and thymus derived T-lymphocyte helper cells (Th) function in full-feathered broiler, in addition to a higher leucocyte migration inhibition value and enhanced antibody titer of New Castle Disease virus in full-feathered broilers [147].
The results of recent experiments on poultry are insufficient to define the optimal dietary levels of Methionine for sparking immunoexertory activity. However, a study by Rama Rao et al. (2003) have illustrate an elevated anti-sheep red blood cell (SRBC) antibody titers and greater cutaneous basophilic hypersensitivity (CBH) response with higher supplemented methionine diet and that the optimal methionine concentration for antibody production was the highest at 0.55% [136]. The SRBC administration pathway, genetic traits and gender may have influenced antibody production. However, in this study, the female birds of four different genetic strains were used, and SRBC was intravenously injected [136]. Similar results were observed in different study where male Ross broilers were used and SRBC was injected in the muscle [65, 112].
Findings illustrated that methionine can increase the relative weight of bursa of Fabricius and spleen in chicken [138, 144, 148] and while it can also increase the weight of thymus and bursa of Fabricius of layers in the brood rearing stage, it shows no significant effect on the spleen during this stage [131]. So, it is concluded that the development of the primary lymphoid organs: thymus and bursa of Fabricius possibly be easily influenced than the secondary lymphoid organs such as the spleen by methionine [134, 138, 149, 150].
Appropriate methionine concentration can significantly increase the degree of antibody and the sheep red blood cells antibody titer as previously explained and cited by the following authors [128, 129, 144, 147]. Methionine is capable of encouraging T-lymphocyte proliferation of the peripheral blood, thymus, and spleen, while methionine deficiency diminishes the transformation of T-lymphocyte proliferation [151]. It has also been evident that with higher levels of methionine in ration, serum antibody rises in the broilers with coccidium infection [138, 151], and as several research have pointed out the leukocyte migration and antibody titer also have increased in chickens infected by Newcastle disease virus (NDV) [74, 152].
On the other hand, Wu et al. (2012) reported that methionine deficiency can significantly decrease serum IgG, IgA and IgM content which in turn suggests that the humoral immunity is jeopardized [93]. [153] illustrated methionine’s ability to significantly affect the content of serum IgM and IgA in a meat rabbit.
Deficiency in methionine influences the relative percentage of T-lymphocyte subsets which includes CD3+, CD3+ CD8+ and CD3+ CD4+ of broilers [93].
Recent study by Ramadan et al [154] demonstrated that 20% excess methionine above modern broiler requirement significantly increased average body weight (BW) at 10 days of age; with no significant differences in BW and feed conversion ratio (FCR) at 17 and 35 days of age [154]. In Mycoplasma gallisepticum-challenged birds, the 20% excess methionine treatment significantly increased IgG titers (3170) in comparison to adequate methionine level (1843) along with coefficient of variation (CV) of 14.84 and 66.38%, respectively [154]. Results of Ramadan et al. (2018) also illustrated excess methionine had significantly increased bursal indices in Mycoplasma gallisepticum -challenged broilers at 35 days of age and augmented hematological parameters with a significant increase of hematocrit (HCT%) compared to tilmicosin-based antimicrobial that significantly decreased HCT%. Further, excess methionine significantly decreased the percentage of birds with severe tracheatis caused by Mycoplasma gallisepticum-infection from 40 to 10% at 17 to 35 days of age [154].
In addition, the level of the methionine is found to be higher in the immune response than in normal circumstance [130, 138] deducing that there exists a close relationship between the methionine and immune response or disease resistance [112, 130, 138].
10. Conclusion
- Such substantiated extensive research review on methionine proves its multiple roles in poultry physiology and vitally highlights its immunopotentiating function.
- Understanding the underlying mechanisms and metabolism of methionine and the process by which it alleges an impact on the lymphatic system is essential in decoding complex interactions between nutrition and disease.
- The treatment or resistance against an infection necessitates an augmented response congregated by the immune system. From nutritional point of view, amino acids are required to stimulate an immune response including, but not limited to, lymphocytes proliferation, germinal centers enactment in the bursa of Fabricius, immunoglobulins affinity, synthesis of effector B-lymphocytes, nitric oxide, lysozyme, complement in addition to cytokines and eicosanoids. Several points were emphasized on methionine’s role as an immunopotentiator; however, additional research is also recommended to elucidate the precise genetic makeup involvement in the relationship between methionine and other disease factors.
- It is important to note that albeit the indispensable function of cell-mediated immunity, the intervention of methionine, as a sulfur donor, on diseases may heavily rely on humoral immunity due to its role in the disulfide-bridge linkage formation in immunoglobulins.
Conflicts of Interest
The authors declare that they have no competing interests or personal relationships that could have influenced the work reported in this paper.
References
- Wandita TG, Joshi N, Nam IS, et al. Dietary supplementation of purified amino acid derived from animal blood on immune response and growth performance of broiler chicken. Tropical Animal Science Journal 41 (2018): 108-113.
- Applegate T, Angel R. Protein, and amino acid requirement for poultry. Purdue University Extension Publication AS-581-W. Purdue University, West Lafayette, IN (2008).
- Emmert JL, Garrow TA, Baker DH. Hepatic betaine-homocysteine methyltransferase activity in the chicken is influenced by dietary intake of sulfur amino acids, choline, and betaine. Journal of Nutrition 126 (1996): 2050-2058.
- National Research Council (NRC). Nutrient Requirements of Poultry. 9th rev. ed. National Academy Press, Washington, D.C (1994).
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids 37 (2009): 1-17.
- Leeson S, Summers J. Commercial Poultry Nutrition. 3 edition. University Books, Guelph, Ontario, Canada. Print (2009).
- Leeson S, Summers J. Nutrition of the Chicken. 4th edition. University Books, Guelph, Ontario, Canada. Print (2001).
- Shen Y. Functional Role and Application of Tryptophan and Methionine in Animals. North Carolina State University. Animal Science & Poultry Science (2013): 292 pages.
- Kim S, Wu G. Dietary arginine supplementation enhances the growth of milk-fed young pigs. Journal of Nutrition 134 (2004): 625-630.
- Mato JM, Alvarez L, Ortiz P, et al. S-adenosylmethionine synthesis: molecular mechanisms and clinical implications. Pharmacology & Therapeutics 73 (1997): 265-280.
- Wu X, Ruan Z, Gao Y, et al. Dietary supplementation with L-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids 39 (2010): 831-839.
- Osti N, Pandey S. Methionine and Lysine Supplementation in Low Quality Feed Ingredient based diets on the performance of broiler chicken. Fourth National Conference on Science and Technology, Royal Nepal Academy of Science and Technology (RONAST), Kathmandu, Nepal from March (2004).
- Gill J, Malin M, Sutherland J, et al. Thymic generation, and regeneration. Immunological Reviews 195 (2003): 28-50.
- Fernandez SR, Aoyagi S, Han Y, et al. Limiting order of amino acids in corn and soybean meal for growth of the chick. Poultry Science 73 (1994): 1887-1896.
- Castro FLS, Kim HY, Hong YG, et al. The effect of total sulfur amino acid levels on growth performance, egg quality, and bone metabolism in laying hens subjected to high environmental temperature. Poultry Science 98 (2019): 4982-4993.
- Aldrich G. DL-methionine: several vital functions in Petfood Industry. Watt Publishing Co., Rockford, IL (2007).
- Cole DJA, Haresign W. Recent Developments in Poultry Nutrition. (ed.) Butterworths, Essex, UK (2004): Pp 131-144.
- Novak C, Yakout H, Scheideler S. The combined effects of dietary lysine and total sulfur amino acid level on egg production parameters and egg components in Dekalb Delta laying hens. Poultry Science 83 (2004): 977-984.
- Knowles TA, Southern LL. The lysine requirement and ratio of total sulfur amino acids to lysine for chicks fed adequate or inadequate lysine. Poultry Science 77 (1998): 564-569.
- Fanatico A. Organic poultry production: providing adequate methionine. ATTRA-National Sustainable Agriculture Information Service, IP363 (2010).
- Baker D. Advances in protein–amino acid nutrition of poultry. Amino Acids 37 (2009): 29-41.
- Burley HK, Anderson KE, Patterson PH, et al. Formulation challenges of organic poultry diets with readily available ingredients and limited synthetic methionine, The Journal of Applied Poultry Research 25 (2016): 443-454.
- Burley H. Enrichment of methionine from naturally concentrated feedstuffs for use in organic poultry diets. PhD Diss. The Pennsylvania State University, University Park, PA (2012).
- Blair R. Nutrition and Feeding of Organic Poultry. CABI, Wallingford, UK (2008).
- Substances generally recognized as safe Electronic Code of Federal Regulations: Part 582 - Food and Drug Administration, Department of Health and Human Services (2017).
- U.S. National Library of Medicine, National Institutes of Health, Health & Human Services, Bethesda, MD (2017).
- Finkelstein J. Methionine metabolism in mammals. Journal of Nutritional Biochemistry 1 (1990): 228-237.
- Horikawa S, Tsukada K. Molecular cloning and nucleotide sequence of cDNA encoding the human liver S-adenosylmethionine synthetase. Biochemistry International 25 (1991): 81-90.
- Kidd MT, Ferket PR, Garlich JD. Nutritional and osmoregulatory functions of betaine. World's Poultry Science Journal 53 (1997): 125-139.
- De Lima MB, Da Silva EP, Pereira R, et al. Estimate of choline nutritional requirements for chicks from 1 to 21 days of age. Journal of Animal Physiology and Animal Nutrition 102 (2018): 780-788.
- Metzler-Zebeli BU, Eklund M, Mosenthin R. Impact of osmoregulatory and methyl donor functions of betaine on intestinal health and performance in poultry. 1Institute of Animal Nutrition, University of Hohenheim, Emil-Wolff-Str. 10, 70599.Stuttgart, Germany; Present address: Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton T6G 2P5, Canada (2009).
- Kanehisa Laboratories. Kyoto Encyclopedia of Genes and Genomes (KEGG). Cysteine and methionine metabolism - Reference pathway (2017).
- Rack AL, Lilly KGS, Beanan KR, et al. The effect of genotype, choice feeding, and season on organically reared broilers fed diets devoid of synthetic methionine, The Journal of Applied Poultry Research 18 (2009): 54-65.
- Brosnan JT, Brosnan ME. The sulfur-containing amino acids: an overview. Journal of Nutrition 136 (2006): 1636S-1640S.
- Škovierová H, Vidomanova E, Mahmood S, et al. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Biomedical Center Martin, Department of Molecular Medicine (2016).
- Mueller J. A new sulfur-containing amino acid isolated from the hydrolytic products of protein. Journal of Biological Chemistry (1923): 157-169.
- Jacob J. Synthetic Methionine and Organic Poultry Diets. University of Kentucky (2013).
- Willke T. Methionine production—a critical review. Applied Microbiology and Biotechnology 98 (2014): 9893-9914.
- National organic program: amendment to the national list of allowed and prohibited substances (livestock). Federal Register 76 (2001): 13501-13504.
- Farran M, Thomas O. Valine deficiency. 1. The effect of feeding a valine-deficient diet during the starter period on performance and feather structure of male broiler chicks. Poultry Science 71 (1992): 1879-1884.
- Goldfarb AS, Goldgraben GR, Herrick EC, et al. Organic Chemicals Manufacturing Hazards. Ann Arbor Science Publishers Inc., Ann Arbor, MI (1981).
- Lussling GT, Gerd KPM, Ferdin S, et al. Patent to Deutsche Gold- und Silber-Scheideanstalt formerly Roessler (Degussa): Process for the recovery of methionine and potassium bicarbonate. International Journal of Pharmaceutical Investigation 2 (1981): 176-182.
- Methionine: supply/demand overview (2014). FeedInfo news service. www.feedinfo.com.
- National Organic Program (NOP). Legal Rule 205.603: Synthetic substances allowed for use in organic livestock production. In: Agricultural Marketing Service, Electronic Code of Federal Regulations Subpart D-Administrative. Part 205-(2014).
- Xing S, Huang C, Wu R, et al. Breed Differences in The Expression Levels of Gga-Mir-222a In Laying Hens Influenced H2S Production by Regulating Methionine Synthase Genes in Gut Bacteria. Research Square, (2020): 1-24.
- Kim SY, Shim MJ, Shin Y, et al. Patent to CJ CheilJedang: Microorganism producing L-methionine precursor and method of producing L-methionine and organic acid from L-methionine precursor. WO (2008): 0134329 (A9).
- May O, Nguyen PT, Arnold FH. Inverting enantioselectivity by directed evolution of hydantoinase for improved production of L-methionine. Nature Biotechnology 18 (2000): 317-320.
- Syldatk C, May O, Altenbuchner J, et al. Microbial hydantoinases – industrial enzymes from the origin of life? Applied Microbiology and Biotechnology 51(1999):293-309.
- Bolten CJ, Schröder H, Dickschat J, et al. Towards methionine overproduction in Corynebacterium glutamicum - methanethiol and dimethyldisulfide as reduced sulfur sources. Journal of Microbiology and Biotechnology 20 (2010): 1196-1203.
- Lee HS, Hwang BJ. Methionine biosynthesis and its regulation in Corynebacterium glutamicum: Parallel pathways of transsulfuration and direct sulfhydrylation. Applied Microbiology and Biotechnology 62 (2003): 459-467.
- Sauer N, Emrich K, Piepho HP, et al. Meta-analysis of the relative efficiency of methionine-hydroxy-analogue-free-acid compared with DL-methionine in broilers using nonlinear mixed models. Poultry Science 87 (2008):2023-2031.
- Dilger RN, Baker DH. DL-Methionine is as efficacious as L-methionine, but modest L-cystine excesses are anorexigenic in sulfur amino acid-deficient purified and practical-type diets fed to chicks. Poultry Science 86 (2007): 2367-2374.
- Littell RC, Henry PR, Lewis AJ, et al. Estimation of relative bioavailability of nutrients using SAS procedures. Journal of Animal Science 75 (1997): 2672-2683.
- Ahiwe EU, Omede AA, Abdallah MB, et al. Managing dietary energy intake by broiler chickens to reduce production costs and improve product quality. Animal Husbandry and Nutrition (2018): 115-145.
- Zhang B, Zhang X, Schilling MW, et al. Effects of Broiler Genetic Strain and Dietary Amino Acid Reduction on (Part I): Growth Performance and Internal Organ Development. Poultry Science 99 (2020): 3266-3279.
- Wu G. Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. Journal of Animal Science and Biotechnology 5 (2014): 34.
- Summers JD, Leeson S. Broiler carcass composition as affected by amino acid supplementation. Cano Journal of Animal Science 65 (1985): 717-723.
- Mabray CJ, Waldroup PW. The influence of dietary energy and amino acid levels on abdominal fat pad development of the broiler chicken. Poultry Science 60 (1981): 151-159.
- Cherry JA, Siegel PB. Compensatory increase in feed consumption in response to marginal levels of the sulfur containing amino acids. Archiv fur Ge ooghelkunde 45 (1981): 269-273.
- Zhai W, Peebles ED, Mejia L, et al. Effects of dietary amino acid density and metabolizable energy level on the growth and meat yield of summer- reared broilers. Journal of Applied Poultry Research 23 (2014): 501-515.
- Emmert J, Baker D. Use of the ideal protein concept for precision formulation of amino acid levels in broiler diets. Journal of Applied Poultry Research 6 (1997): 462-470.
- Daghir N. Poultry Production in hot Climates. 2nd CABI. Print (2008).
- Abu Dieyeh ZH. Effect of high temperature per se on growth performance of broilers. International Journal of Poultry Science 5 (206): 1006-1011.
- Wen C, Wu P, Chen Y, et al. Methionine improves the performance and breast muscle growth of broilers with lower hatching weight by altering the expression of genes associated with the insulin-like growth factor-I signaling pathway. British Poultry Science 111 (2014): 201-206.
- Jankowski J, Kubinska M, Zdunczyk Z. Nutritional, and immunomodulatory function of methionine in poultry diets – a review. Annals of Animal Science 14 (2014): 17-31.
- Ewing WR. The Ray Ewing Co., Pasadena, CA. Poultry Nutrition. 5th Ed (1963).
- Donaldson WE, Combs GF, Romoser GL. Studies on energy levels in poultry rations. 1. The effect of calorie-protein ratio of the ration on growth, nutrient utilization, and body composition of chicks. Poultry Science 35 (1956): 1100-1105.
- Applegate T, Angel R. Protein, and amino acid requirement for poultry. Extension Project funded by the USDA NRCS CIG program (2014).
- Havenstein GB, Ferklet PR, Qureshi MA. Growth, livability, and feed conversion of 1991 vs 1957 broilers when fed “typical” 1957 and 1991 broiler diets. Poultry Science 73 (1994): 1785-1794.
- Wallis I. Dietary supplements of methionine increase breast meat yield and decrease abdominal fat in growing broiler chickens. Australian Journal of Experimental Agriculture 39 (1999): 131-141.
- Schutte JB, Pack M. Effects of dietary sulphur-containing amino acids on performance and breast meat deposition of broiler chicks during the growing and finishing phases. British Poultry Science 36 (1995): 747-762.
- Tsiagbe VK, Cook ME, Harper AE, et al. Enhanced immune responses in broiler chicks fed methionine supplemented diets. Poultry Science 66 (1987a): 1147-1154.
- Shini S, Li X, Bryden WL. Methionine requirement and cell-mediated immune in chicks. Asia Pacific Journal of Clinical Nutrition 14 (2005): S123.
- Swain BK, Johri TS. Effect of supplemental methionine, choline and their combinations on the performance and immune response of broilers. British Poultry Science 41 (2000): 83-88.
- Kidd MT, Mcdaniel CD, Branton SL, et al. Increasing amino acid density improves live performance and carcass yields of commercial broilers. Journal of Applied Poultry Research 13 (2004): 593-604.
- Morris TR, Gous RM, Abebe S. Effects of dietary protein concentration on the response of growing chicks to methionine. British Poultry Science 33 (1992): 795-803.
- Gous RM. Nutritional limitations on growth and development in poultry. Livestock Science 130 (2010): 25-32.
- Morris TR, Njuru DM. Protein requirement of fast- and slow-growing chicks. British Poultry Science 31 (1990): 803-809.
- Kalinowski A, Moran Et JR, Wyatt C. Methionine and cystine requirements of slow- and fast-feathering male broilers from zero to three weeks of age. Poultry Science 82 (2003): 1423-1427.
- Ajang OA, Prijono S, Smith WK. Effect of dietary protein content on growth and body composition of fast and slow feathering broiler chickens. Br. Poultry Science 34 (1993): 73-91.
- Fanatico AC, Owens CM, Emmert JL. Organic poultry production in the United States: Broilers. Journal of Applied Poultry Research 18 (2009): 355-366.
- Fanatico AC, Owens CM, Emmert JL. Methionine requirements of alternative slow-growing genotypes. Poultry Science 86 (2006): (Suppl. 1).
- Fanatico AC, Pillari PB, Emmert JL, et al. Meat quality of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poultry Science 86 (2007): 2245-2255.
- Maharjan P, Mullenix G, Hilton K, et al. Effects of dietary amino acid levels and ambient temperature on mixed muscle protein turnover in Pectoralis major during finisher feeding period in two broiler lines. Journal of Animal Physiology and Animal Nutrition 104 (2020): 1351-1364.
- Moore DT, Baker K, Firman JD. Digestible Sulfur Amino Acid Requirements for Male Turkeys to Five Weeks of Age. Journal of Applied Poultry Research 10 (2001): 363-370.
- Vieira SL, Angel CR. Optimizing broiler performance using different amino acid density diets: What are the limits? The Journal of Applied Poultry Research 21(2012): 149-155.
- Patrick H, Schaible P. Poultry: Feeds and nutrition. Second Edition. AVI publishing company, INC. Westport, Connecticut (1980).
- Brown J, Firman JD, Sun SS, et al. Digestible Lysine Requirements for Maintenance in the Starting Turkey. International Journal of Poultry Science 5 (2006): 740-743.
- Schutte J, De Jong J. Ideal amino acid profile for poultry. In: Brufau J. (ed.), Tacon A. (ed.). Feed manufacturing in the Mediterranean region: Recent advances in research and technology. Zaragoza: CIHEAM, (1999): p. 259-263.
- Fisher C. Amino acid requirements of broiler breeders. Poultry Science 77 (1998): 124-133.
- Baker DH, Batal AB, Parr TM, et al. Ideal ratio (relative to lysine) of tryptophan, threonine, isoleucine, and valine, for chicks during the second- and third-week’s post hatch. Poultry Science 81 (2002): 485-494.
- Fernandez SR, Zhang Y, Parsons CM. Dietary formulation with cottonseed meal on a total amino acid versus a digestible amino acid basis. Poultry Science 74 (1995): 1168-1179.
- Wu B, Cui H, Peng X, Fang J, Cui W. Effect of Methionine Deficiency on the Thymus and the Subsets and Proliferation of Peripheral Blood T-Cell, and Serum IL-2 Contents in Broilers. Journal of Integrative Agriculture 11 (2012): 1009-1019.
- Zhang LB, Wo YM. Effects of Liquid DL2Methionine Hydroxy Analogue on Growth Performance and Immune Responses in Broiler Chickens. Acta Veterinaria and Zootechnica Sinica 39 (2008): 1204-1211.
- Wu G, Meininger C. Regulation of nitric oxide synthesis by dietary factors. Annual Reviews of Nutrition 22 (2002): 61-86.
- Batterham ES. Ileal digestibilities of amino acids in feedstuffs, in: Amino Acids in Farm Animal Nutrition. D’Mello, J.P.F. (Eds) CAB International (1994) pp. 113-131. (Wallingford, Oxon, U.K.).
- Baker DH, Fernandez SR, Webel DM, et al. Sulfur amino acid requirement and cystine replacement value of broiler chicks during the period three to six weeks posthatching. Poultry Science 75 (1996): 737-742.
- Finkelstein JD, Martin JJ, Harris BJ. Methionine metabolism in mammals. The methionine-sparing effect of cystine. The Journal of Biological Chemistry 263 (1988): 11750-11754.
- Wheeler K, Latchaw J. Sulfur amino acid requirements and interactions in broilers during two growth periods. Poultry Science 60 (1981): 228-236.
- Adegboyega Fatufe A. Dose response relationships between intake and efficiency of utilisation of individual amino acids in chicken. Cuvillier Verlag Publishing (2004): 164 pages.
- Chung TK, Baker DH. Apparent and true amino acid digestibility of a crystalline amino acid mixture and of casein: comparison of values obtained with ileal-cannulated pigs and cecectomized cockerels. Journal of Animal Science 70 (1992): 3781-3790.
- Calder PC, Yaqoob P. Amino acids, and immune function. In Metabolic & Therapeutic Aspects of Amino Acids in Clinical Nutrition, 2nd Ed (2004) pp. 305-320.
- Field CJ, Johnson IR, Schley PD. Nutrients, and their role in host resistance to infection. Journal of Leukocyte Biology 71 (2002): 16-32.
- Dasgupta M, Sharkey JR, Wu G. Inadequate intakes of indispensable amino acids among homebound older adults. Journal of Nutrition for the Elderly 24 (2005): 85- 99.
- Woodward B. Protein, calories, and immune defenses. Nutrition Reviews 56 (1998): S84-S92.
- Elwinger K, Tausen R. Low-methionine diets are a potential health risk in organic egg production. European Symposium on Poultry Nutrition, Edinburgh, Scotland (2009).
- Corzo A, Kidd MT, Dozier WA, et al. Protein expression of pectoralis major muscle in chickens in response to dietary methionine status. British Poultry Science 95 (2006): 703-708.
- Leclercq B. The influence of dietary protein content on the performance of genetically lean or fat growing chickens. British Poultry Science 24 (1983): 581-587.
- Sekiz SS, Scott ML, Nesheim MC. The effect of methionine deficiency on body weight, food and energy utilization in the chick. Poultry Science 54 (1975): 1184-1188.
- Ashton C. Keeping Geese: Breeds and Management. Crowood Publishing (2015).
- Dahiya JP, Hoehler D, Van Kessel AG, et al. Effect of different dietary methionine sources on intestinal microbial populations in broiler chickens. Poultry Science 86 (2007): 2358-2366.
- Rubin LL, Cabal CW, Ribeiro ALM, et al. Effects of methionine and arginine dietary levels on the immunity of broiler chickens submitted to immunological stimuli. British Journal of Poultry Science 9 (2007): 241-247.
- Khalil AA, Thomas OP, Combs GF. Influence of body composition, methionine deficiency or toxicity and ambient temperature on feed intake in the chick. Journal of Nutrition 96 (1968): 337-341.
- Wideman RFJR, Roush WB, Satnick JL, et al. Methionine hydroxy analog (free acid) reduces avian kidney damage and urolithiasis induced by excess dietary calcium. Journal of Nutrition 119 (1989): 818-828.
- Zhang LB, Guo YM. Effects of liquid DL-2-hydroxy-4-methylthio butanoic acid on growth performance and immune responses in broiler chickens. Poultry Science 87 (2008): 1370-1376.
- Scherer CS, Baker DH. Excess dietary methionine markedly increases the vitamin B-6 requirement of young chicks. Journal of Nutrition 130 (2000): 3055-3058.
- Acar N, Patterson PH, Barbato GF. Appetite suppressant activity of supplemental dietary amino acids and subsequent compensatory growth of broilers. Poultry Science 80 (2001): 1215-1222.
- Carew LB, Evarts KG, Alster FA. Effects of methionine deficiencies on plasma levels of thyroid hormones, insulin-like growth factors-I and -II, liver and body weights, and feed intake in growing chickens. Poultry Science 82 (2003): 1932-1938.
- Harter J, Baker D. Factors affecting methionine toxicity and its alleviation in the chick. Journal of Nutrition 108 (1978):1061-1070.
- Frontiera MS, Stabler SP, Fred J, et al. Regulation of methionine metabolism: effects of nitrous oxide and excess dietary methionine. The Journal of Nutritional Biochemistry 5 (1994): 28-38.
- Ugarte N, Ladouge R, Radjei S, et al. Proteome Alteration in Oxidative Stress-Sensitive Methionine Sulfoxide Reductase-Silenced hek293 Cells. Free Radical Biology and Medicine 65 (2013): 1023-36.
- Le Floch N, Melchior D, Obled C. Modification of protein and amino acid metabolism during inflammation and immune system activation. Livestock Production Science 87 (2004): 37-45.
- Grimble R. The effects of sulfur amino acid intake on immune function in humans. Journal of Nutrition 136 (2006): 1660-1661.
- Takahashi K, Kanashi S, Akiba Y. Influences of dietary methionine and cysteine on metabolic responses to immunological stress by Escherichia coli lipopolysaccharide injection, and mitogenic response in broiler chickens. British Journal Nutrition 78 (1997): 815-821.
- Hashemi SM, Loh TC, Foo HL, et al. Effects of putrescine supplementation on growth performance, blood lipids and immune response in broiler chickens fed methionine deficient diet. Animal Feed Science and Technology 194 (2014): 151-156.
- Tsiagbe VK, Cook ME, Harper AE, et al. Efficacy of cysteine in replacing methionine in the immune responses of broiler chicks. Poultry Science 66 (1987b): 1138-1146.
- Lip Yin YL, Li D, Kim SW, et al. Amino acids and immune function. British Journal of Nutrition 98 (2007): 237-252.
- Takahashi K, Konashi S, Akiba Y, et al. Effects of Dietary Threonine Level on Antibody Production in Growing Broilers. Animal Science and Technology 65 (1994): 956-960.
- Takahashi K, Konashi S, Akiba Y, et al. Effects of Marginal Excess or Deficiency of Dietary Methionine on Antibody Production in Growing Broilers. Animal Science and Technology 64 (1993): 13-19.
- Rubin LL, Ribeiro AML, Canal CW, et al. Influence of Sulfur Amino Acid Levels in Diets of Broiler Chickens Submitted to Immune Stress. Revista Brasileira de Ciência Avícola 9 (2007b): 305-311.
- Deng K, Wong CW, Nolan JV. Carry-over effects of early-life supplementary methionine on lymphoid organs and immune responses in egg-lying strain chickens. Animal Feed Science and Technology 134 (2007): 66-76
- Wu B, Cui H, Peng X, et al. Pathology of bursae of Fabricius in methionine-deficient broiler chickens. Nutrients 5 (2013): 877-886.
- Zhang YC, Li FC. Effect of Dietary Methionine Supplement Levels on Growth Performance, Immunity Performance and Blood Metabolites of 2–3-Month-Old Rabbits. Southwest China Journal of Agricultural Sciences 1 (2008): 472-475.
- Konashi S, Takahashi K, Akiba Y. Effects of Dietary Essential Amino Acid Deficiencies on Immunological Variables in Broiler Chickens. British Journal of Nutrition 83 (2000): 449-456.
- Wu B, Cui H, Peng X, et al. Pathology of Spleen in Chickens Fed on a Diet Deficient in Methionine. Health 4 (2012b): 32-38.
- Rama Rao SV, Praharaj NK, Panda AK, et al. Interaction between Genotype and Dietary Concentrations of Methionine for Immune Function in Commercial Broilers. British Poultry Science 44 (2003): 104-112.
- Wu B, Cui H, Peng X, et al. Pathology of Spleen in Chickens Fed on a Diet Deficient in Methionine. Health 4 (2012a): 32-38.
- Ruan T, Li L, Peng X, et al. Effects of Methionine on the Immune Function in Animals. Scientific Research publishing. Health 9 (2017): 857-869.
- Bunchasak C. Role of dietary methionine in poultry production. Journal of Poultry Science 46 (2009): 169-179.
- Elmada CZ, Huang W, Jin M, et al. The Effect of Dietary Methionine on Growth, Antioxidant Capacity, Innate Immune Response and Disease Resistance of Juvenile Yellow Catfish (Pelteobagrus fulvidraco). Aqua- culture Nutrition 22 (2016): 1163-1173.
- Shuai, K, Zhou, X. Effects of Methionine on Young Carp Digestion Function, and Immune Function. Research on Animal Nutrition and Feed, the Fifth National Academic Symposium on Feed Nutrition (2006).
- Chen N, Ma J, Zhou H, et al. Assessment of Dietary Methionine Requirement in Largemouth Bass, Micropterus Salmoides. Journal Fisheries China 34 (2010): 1244-1253.
- Kim WK, Froelich CA, Patterson PH, et al. The Potential to Reduce Poultry Nitrogen Emissions with Dietary Methionine or Methionine Analogues Supplementation. World Poultry Science Journal 62 (2006): 338-353.
- Mirzaaghatabar F, Saki P, Zamani AA, et al. Effect of Different Levels of Diet Methionine and Metabolisable Energy on Broiler Performance and Immune System. Food and Agricultural Immunology 22 (2011): 93-103.
- Kubi?ska M, Tyka?owski B, Jankowski J, et al. Immunological and biochemical indicators in turkeys fed diets with a different Methionine content. Polish Journal of Veterinary Sciences 17 (2014): 687-695.
- Kidd MT. Nutritional modulation of immune function in broilers. Poultry Science 83 (2004): 650-657.
- Bhanja S, Mandal A. Optimizing dietary energy and amino acid levels in the diets of naked neck broilers for growth, nutrient utilisation and immune response in a hot climate. Journal of the Science of Food and Agriculture 87 (2007): 945–950.
- Al-Mayah A. Immune Response of Broiler Chicks to DL-Methionine Supplementation at Different Ages. International Journal of Poultry Science 5 (2006): 169-172.
- Sahu T, Naik SK, Tiwari ST. Effect of Taurine and Methionine on Lymphoid Organs of Vencobb Broilers. Journal of Animal Research 9 (2014): 857-869.
- Deng K, Wong CW, Nolan JV. Long-Term Effects of Early-Life Dietary L-Carnitine on Lymphoid Organs and Immune Responses in Leghorn-Type Chickens. Journal of Animal Physiology and Animal Nutrition 90 (2006): 81-86.
- Jin LM, Wang M, Meng XY, et al. Effect of Con- tent of Dietary Methionine on the Value of Blood Sugar of Broiler Chikens with E. tenella. Acta Ecologiae Animalis Domastici 26 (2005): 61-64.
- Bouyeh M. Effect of Excess Lysine and Methionine on Immune System and Performance of Broilers. Annals of Biological Research 3 (2012): 3218-3224.
- Zheng H, Li B, Fan W, et al Effect of Two Kinds of Methionine Additives on the Production Performance and Development of Immune Organs in Broiler Chickens. Feed China (2008): 25-27.
- Ramadan NM, Farran MT, Shaib HA. DL-Methionine: An immunopotentiator in Mycoplasma gallisepticum challenged broilers treated with Pulmotil AC®. MS Thesis, American University of Beirut (2018).

 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 