Physicochemical and Flow Characterization of a Mustard-Vinaigrette Salad Dressing
Article Information
Lozano-Gendreau M1, Vélez-Ruiz JF1,2*
1Department of Chemical Engineering and Food Engineering, University of the Americas Puebla, Sta. Catarina Mártir, Cholula, Puebla, Mexico
2FN Consultores, S.A. de C. V. Institute of Design and Technological Innovation, Boulevard del Niño Poblano 2901, Atlixcayotl Territorial Unit, Puebla, Mexico
*Corresponding Author: Vélez-Ruiz JF, Department of Chemical Engineering and Food Engineering, University of the Americas Puebla, Sta. Catarina Mártir, Cholula, Puebla, CP 72820, Mexico
Received: 30 August 2019; Accepted: 16 September 2019; Published: 27 September 2019
Citation: Lozano-Gendreau M, Vélez-Ruiz JF. Physicochemical and Flow Characterization of a Mustard-Vinaigrette Salad Dressing. Journal of Food Science and Nutrition Research 2 (2019): 253-269.
View / Download Pdf Share at FacebookAbstract
In the last decade, consumption and development of salad dressings has shown a continuous growing and their importance is increasing in Mexico and worldwide. These products are composed of oil, vinegar, spices, flavors and some hydrocolloids, among others. The formulation or specific composition is determinant on their characteristics and particularly, some physicochemical and flow properties. The objective of this study was to characterize and analyze a new salad dressing, and to observe how the incorporation of some components affects its properties. In order to study the physicochemical and flow behavior of a new mustard-vinaigrette salad dressing, different systems were elaborated. Two groups of samples were prepared; the first one included nine dressings or systems, with three different oil:vinegar rates and three mustard concentrations and stored four weeks. The second group consisted only of four systems with a constant concentration of mustard that were stored also through four weeks at two temperatures. Determinations of acidity, adhesivity, color, density, drop size, emulsion stability, flow properties, moisture, pH, retro-extrusion force, and water activity were carried out for most of the systems. Oil concentration had a significant effect on acidity, adhesivity, density, emulsion stability, flow properties, moisture, and water activity, in which the emulsifying capacity of the mustard was corroborated. The storage time showed a significant effect on adhesivity, emulsion stability, flow, luminosity and retro-extrusion properties. A modified Herschel and Bulkley model fitted better the flow behavior than other two equations. Analysis of variance allowed to know the significant effect of the studied variables on the yield stress, flow index and consistency coefficient obtained from the modified flow model.
Keywords
Mustard-vinaigrette salad dressing, Physicochemical properties, Flow behavior
Mustard-vinaigrette salad dressing articles, Physicochemical properties articles, Flow behavior articles
Mustard-vinaigrette salad dressing articles Mustard-vinaigrette salad dressing Research articles Mustard-vinaigrette salad dressing review articles Mustard-vinaigrette salad dressing PubMed articles Mustard-vinaigrette salad dressing PubMed Central articles Mustard-vinaigrette salad dressing 2023 articles Mustard-vinaigrette salad dressing 2024 articles Mustard-vinaigrette salad dressing Scopus articles Mustard-vinaigrette salad dressing impact factor journals Mustard-vinaigrette salad dressing Scopus journals Mustard-vinaigrette salad dressing PubMed journals Mustard-vinaigrette salad dressing medical journals Mustard-vinaigrette salad dressing free journals Mustard-vinaigrette salad dressing best journals Mustard-vinaigrette salad dressing top journals Mustard-vinaigrette salad dressing free medical journals Mustard-vinaigrette salad dressing famous journals Mustard-vinaigrette salad dressing Google Scholar indexed journals Physicochemical properties articles Physicochemical properties Research articles Physicochemical properties review articles Physicochemical properties PubMed articles Physicochemical properties PubMed Central articles Physicochemical properties 2023 articles Physicochemical properties 2024 articles Physicochemical properties Scopus articles Physicochemical properties impact factor journals Physicochemical properties Scopus journals Physicochemical properties PubMed journals Physicochemical properties medical journals Physicochemical properties free journals Physicochemical properties best journals Physicochemical properties top journals Physicochemical properties free medical journals Physicochemical properties famous journals Physicochemical properties Google Scholar indexed journals Flow behavior articles Flow behavior Research articles Flow behavior review articles Flow behavior PubMed articles Flow behavior PubMed Central articles Flow behavior 2023 articles Flow behavior 2024 articles Flow behavior Scopus articles Flow behavior impact factor journals Flow behavior Scopus journals Flow behavior PubMed journals Flow behavior medical journals Flow behavior free journals Flow behavior best journals Flow behavior top journals Flow behavior free medical journals Flow behavior famous journals Flow behavior Google Scholar indexed journals salad dressings articles salad dressings Research articles salad dressings review articles salad dressings PubMed articles salad dressings PubMed Central articles salad dressings 2023 articles salad dressings 2024 articles salad dressings Scopus articles salad dressings impact factor journals salad dressings Scopus journals salad dressings PubMed journals salad dressings medical journals salad dressings free journals salad dressings best journals salad dressings top journals salad dressings free medical journals salad dressings famous journals salad dressings Google Scholar indexed journals supermarkets articles supermarkets Research articles supermarkets review articles supermarkets PubMed articles supermarkets PubMed Central articles supermarkets 2023 articles supermarkets 2024 articles supermarkets Scopus articles supermarkets impact factor journals supermarkets Scopus journals supermarkets PubMed journals supermarkets medical journals supermarkets free journals supermarkets best journals supermarkets top journals supermarkets free medical journals supermarkets famous journals supermarkets Google Scholar indexed journals apple cider vinegar articles apple cider vinegar Research articles apple cider vinegar review articles apple cider vinegar PubMed articles apple cider vinegar PubMed Central articles apple cider vinegar 2023 articles apple cider vinegar 2024 articles apple cider vinegar Scopus articles apple cider vinegar impact factor journals apple cider vinegar Scopus journals apple cider vinegar PubMed journals apple cider vinegar medical journals apple cider vinegar free journals apple cider vinegar best journals apple cider vinegar top journals apple cider vinegar free medical journals apple cider vinegar famous journals apple cider vinegar Google Scholar indexed journals corn oil articles corn oil Research articles corn oil review articles corn oil PubMed articles corn oil PubMed Central articles corn oil 2023 articles corn oil 2024 articles corn oil Scopus articles corn oil impact factor journals corn oil Scopus journals corn oil PubMed journals corn oil medical journals corn oil free journals corn oil best journals corn oil top journals corn oil free medical journals corn oil famous journals corn oil Google Scholar indexed journals cheese articles cheese Research articles cheese review articles cheese PubMed articles cheese PubMed Central articles cheese 2023 articles cheese 2024 articles cheese Scopus articles cheese impact factor journals cheese Scopus journals cheese PubMed journals cheese medical journals cheese free journals cheese best journals cheese top journals cheese free medical journals cheese famous journals cheese Google Scholar indexed journals
Article Details
Introduction
In the last decade, the consumption and development of salad dressings has shown a continuous growing worldwide, and the importance of these food items has increased in Mexico. These products are oil in water emulsions, composed of oil, vinegar, spices, flavors and some hydrocolloids, among others; their composition being determinant on the physicochemical and flow properties. Mustard-vinaigrette is made from mustard flour and/or mustard seeds, water, vinegar and other ingredients, such as salt, sugar, and flavoring additives. As a salad dressing, it is an emulsion with a semisolid consistency and attractive flavor that is particularly appreciated by consumers, it is pungent, spicy-taste and commonly used as a condiment. It is well known, that sensory attributes of these emulsions are directly related to their rheological response [1-7].
Emulsions are thermodynamically unstable systems, due basically to a high interface area, which leads to a spontaneous phenomenon of aggregation of drops; thus, the presence of ingredients in less or more quantity will affect the flow and physicochemical properties of these dressings. Thus, many researches have been conducted, on one side, to reduce interfacial tension by adding emulsifiers; and on the other side to decrease drop mobility by increasing the viscosity of the system. The modification of ingredients is one of the most studied variables, analyzing how they affect the dressing properties.
Chantrapornchai et al. [8] studied the influence of the flocculation phenomenon on optical properties of some emulsions, in which droplets flocculation caused a decreasing in lightness and an augment of redness and yellowness values of the tested emulsions. Solano-Hurtado and Vélez-Ruiz [9] developed a work to study the rheology of dressings prepared with avocado oil, in which a pseudoplastic behavior, with flow index between 0.11 and 0.27 and a consistency coefficient that augmented with avocado oil, xanthan gum and egg yolk concentrations were observed. Alvarez et al. [10] carried out the rheological characterization of commercial sweet and salad sauces, finding that the Power Law model fitted very well the flow nature of the dressings, in which the mustard dressing (14% mustard, 55.2% moisture and pH 2.95) exhibited a flow index of 0.31-0.32 and a consistency coefficient that ranged from 9.9 to 15.0 Pasn. Physicochemical and rheological properties of seven commercial mustards were researched by Juszczak et al. [4] finding differences in physicochemical characteristics between the samples and a plastic, thixotropic and viscoelastic response of the commercial mustard. Dolz et al. [11] analyzed the influence of two gums on shear thinning and thixotropic behavior of food emulsions, in which the steady flow curves of all systems were well described by the Carreau model, whereas both up and down curves of thixotropy loops were well fitted by the Herschel-Bulkley relationship, thixotropy areas augmented with gum content.
It is very clear, that the formulation of the emulsion and its specific composition play an influencing role on the physicochemical and flow behavior of dressings, several works have been focused on the effects of formulation, by changes in composition and incorporation of new ingredients, and processing modifications, changing the process conditions or using new technologies [6, 7, 12-16]. Thus the objectives of this work were: to characterize the physicochemical and flow properties of a new mustard-vinaigrette salad dressing and to know the effect of mustard, oil and vinegar on the those properties, and also to determine the influence of storage time and temperature on the emulsion stability and physicochemical and flow behavior, through a selected period of four weeks.
2. Materials and Methods
2.1 Materials
Raw materials, such as apple cider vinegar (“Barrilito” brand), corn oil (“La Gloria” brand), mustard (McCormick brand), spices, and other ingredients, were acquired from local supermarkets.
2.2 Emulsion preparation
Food emulsions, mustard-vinaigrette salad dressing, with a commercial composition were prepared in two sets. Nine systems or dressings with an oil:vinegar ratio of 2.5:1.0, 3.0:1.0 and 3.5:1:0, and three levels of mustard, 85.5, 92.0, and 98.5 g/L, were formulated for the first set, carrying out a more complete anlaysis. Whereas in the second set, the concentration of mustard was kept constant (98.0 g/L) and four ratios of oil:vinegar ratios, 2.6:1.0, 2.8:1.0, 3.0:1.0 and 3.2:1:0 were selected for the dressing preparation at two temperatures, being a complementary part of the study. All ingredients were gentle stirred and mixed at 60 rpm with a JR mixer of 1 HP (LM-12 model, N.L., Mexico)
2.3 Physicochemical determinations
The analytical procedures, with at least two determinations, included:
2.3.1 Acidity: Acidity titration of 5 g of sample with NaOH 0.1 N until pH of 8.3, following the procedure indicated by the correspondent Mexican norm [17, 18].
2.3.2 Color: Color measurement by using tristimulus Hunter parameters evaluation, with 10 g of sample in reflectance mode (Gardner Colorgard System 05, Reston, VA), and computing the net color change (DE) by the equation 1:
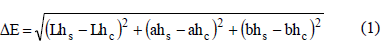
Where: Lhs, ahs and bhs are the Hunter parameters (luminosity, redness, and yellowness) in the dressing system and Lhc, ahc and bhc are the correspondent parameters of the control sample.
2.3.3 Density: Density was determined by utilizing Grease pycnometers for viscous materials (Fisherbrand, Ontario, Canada).
2.3.4 pH: pH by immersion of the electrode, with a Cole Parmer pH meter (Cole Parmer Instruments, Chicago, IL, USA), previously calibrated with buffers pH 4 and pH 7.
2.3.5 Total solids: Total solids supported on water evaporation of 3 g sample (17.007, [19]).
2.3.6 Water activity: Water activity was measured with a Decagon Aqua-labâ hygrometer (Decagon Devices Inc., Pullman, WA, USA) previously calibrated with distilled water.
2.3.7 Stability: Stability of the emulsion (SE) was measured by a centrifugal technique with 50 mL of sample, in which 4500 g were applied during 30 s [20], determining a percentage of stability (SE) as the rate between the volume of the sample without separation (RV) with respect to the initial volume (IV)

2.3.8 Droplet size: Droplet size determination was carried out following a methodology from DeCindio and Cacace [21], by analysis of an image corresponding to a given number of droplets from the emulsion sample in a microscope (Zeiss Axiover 26, Hollbergmoos, Germany) and utilizing a software KS300 (Imaging System 3.0, Hollbergmoos, Germany). Droplet sizes were obtained as the average of three measurements on the same sample.
2.3.9 Rancidity: Rancidity was completed by following of the 28.025 method [19] in which the oil separated from the emulsion in a centrifuge at 4500 g during 5 min, was titrated with 0.02N Na2S2O3, and expressed as peroxide value.
2.3.10 Textural attributes: Textural attributes, retro-extrusion and adhesivity forces, were quantified by following the technique cited by Yang and Coterrill [20], in a texture meter (Texture Analyzer TA.XT2, Texture Technologies, Scardale, NY) at room temperature (25 ± 1°C), inside of a container of 4.5 cm of diameter and 6 cm of height.
2.4 Rheological characterization
Flow measures were determined instrumentally with a Brookfield Viscometer (DV-III, Brookfield Engineering Laboratories Inc., Middleboro, MA, USA) using a programmable sequence with the small sample adapter, in which 10-11 mL of dressing was utilized, at selected temperature (20 and 40ºC). A set of velocities from an upward curve was programmed from 0 to 100 rpm and the corresponding torque values were registered. From pairs of the rotational velocity-torque values and applying those constants given by the manufacturer for the # 27 spindle, the shear rates and shear stresses were mathematically computed and graphically represented. From the flow curves, the flow parameters were evaluated by application of the Power Law and Herschel and Bulkley models (Equations 3 and 4) and quantifying the goodness of the fitting with the relative error (RE) and square root of mean error (SRME).


Where: s is the shear stress (Pa), K is the consistency coefficient (Pa sn), g is the shear rate, (s-1), n is the flow behavior index (dimensionless), and s0 is the yield stress (Pa).
All the properties determinations were measured twice at least, included the rheological characterization.
2.5 Statistical analyses
The statistical analyses (ANOVA) were done with the Minitab software, version16® (Minitab Inc., State College, PA, USA). A Tukey´s test (a=0.05) was used to establish significant differences.
4. Results and Discussion
This work covered two stages, a detailed first part with nine formulations stored through four weeks at ambient atmosphere; and a complementary and shorter second part with four different formulations at two temperatures, stored different days in which less dressing determinations were carried out.
3.1 First set of dressing samples
The samples from the first set, where the variables to study where the oil and water ratio, the mustard concentration and the storage time were identified with letters, as shown in Table 1. From the density consideration, in which the corn oil density is 902 kg/m3 and the vinegar density same as the water, it was possible to estimate an oil content in the samples between 68 and 76% and therefore 24 to 31% moisture content. These contents are comparable to the range reported by Ford et al. [22] for mustard dressings and sauces. And as part of our study, five commercial dressings (two mustard, one Cesar, one ranch and one Roquefort type) were analyzed, in order to have a comparison frame. In which their composition and properties were considered. Then, the first set of dressing samples gave the following results.
|
Sample |
Oil:Vinegar rate |
Mustard concentration (g/L) |
|
A |
2.5:1.0 |
85.5 |
|
B |
2.5:1.0 |
92.0 |
|
C |
2.5:1.0 |
98.5 |
|
D |
3.0:1.0 |
85.5 |
|
E |
3.0:1.0 |
92.0 |
|
F |
3.0:1.0 |
98.5 |
|
G |
3.5:1.0 |
85.5 |
|
H |
3.5:1.0 |
92.0 |
|
I |
3.5:1.0 |
98.5 |
Table 1: First Set of Dressing Samples.
3.2 Physicochemical determinations
The humidity for the fresh dressing samples ranged between 27 and 34% (± 1-3%) that is up of the estimated value, it was influenced mainly by the oil content. Also, a water activity between 0.879 and 0.906 (± 0.02) was recorded. They may be observed with detail in Table 2.
Even though there were some small changes, these properties were constant, as expected. Moisture percentages are in a middle point of those reported by Chirife et al. [23] and other authors for mayonnaise dressings, in a wide range of 17 to 49% and Aw of 0.90-0.95 as a function of the dressing type. Additionally, the analyzed commercial samples had a moisture content higher to 48% and a range of Aw between 0. 94 to 0.97 as consequence of the high concentration of water.
The acetic acid content of the mustard systems ranged from 0.36 to 0.46% for fresh samples and a pH of 4.5 was measured in all the samples. The acidity and the pH showed low variation along the storage time. Density of the dressing samples varied between 940 and 973 kg/m³ (± 59), being smaller than the water density since the greater component was the oil. These characteristics were similar, to the five commercial dressings analyzed, with 0.18 to 0.33% of acetic acid and 959 to 983 kg/m³ of density, being 1085 kg/m³ for the light mustard dressing. The differences in density are due to the formulation of the prepared and commercial dressings. The color parameters ranged from 0.38 to 2.8 for redness (in the green side, ± 0.3), 1.15 to 10.2 for yellowness (in the yellow scale, ± 0.2) and 8.5 to 29.5 (± 0.7) for luminosity, these parameters changed within the week of storage. The specific average values may be observed in Table 3.
Since the Lh factor, in this work, was considered the most important color parameter, it was analyzed statistically (by an ANOVA). Despite the fact, that the samples with higher oil concentrations (G, H and I) had the lowest Lh values at the beginning of the storage, they exhibited the highest values after 4 weeks. This physical change is attributed to the fact that emulsions with higher disperse phase concentrations are more prone to develop the flocculation phenomenon. The flocculation rate depends mainly on the viscosity of the continuous phase, the temperature and the initial concentration of droplets per milliliter of emulsion. A higher flocculation rate will lead to drop coalescence [1]. This luminosity increasing or change can be further studied to establish a control mechanism for the food industry, where storage time of the dressings may be correlated with a Lh value. The net color change (Equation 1) on the other hand, computed values greater than 10, mainly after four weeks of storage and for the oily dressings (Table 4). The high values (seven from thirty six samples, almost 20%) indicated a significant color difference through storage, that can be attributed to the flocculation of the oil droplets in the disperse phase of the emulsion. In agreement with Chantrapornchai et al. [8] bigger oil droplets are crossed more efficiently by the light, increasing the luminosity of the readings. From the ANOVA results, it was obtained that the three studied variables (oil/vinegar ratio, mustard concentration and storage time) had a significant effect on luminosity.
|
System |
Humidity (%) |
Water Activity |
|||
|
0 |
2 |
4 (weeks) |
0 |
4 (weeks) |
|
|
A |
32.9 |
31.0 |
33.0 |
0.901 |
0.903 |
|
B |
31.9 |
30.8 |
32.6 |
0.904 |
0.904 |
|
C |
34.0 |
30.8 |
32.4 |
0.906 |
0.906 |
|
D |
31.0 |
29.2 |
29.0 |
0.889 |
0.888 |
|
E |
30.0 |
29.0 |
29.4 |
0.891 |
0.891 |
|
F |
30.6 |
30.0 |
31.0 |
0.893 |
0.892 |
|
G |
27.6 |
26.9 |
28.0 |
0.880 |
0.881 |
|
H |
29.5 |
27.7 |
27.2 |
0.879 |
0.880 |
|
I |
32.2 |
30.0 |
30.0 |
0.880 |
0.880 |
Table 2: Humidity and Water Activity for the First Set of Dressing Samples.
|
System |
Parameter |
Week |
||||
|
0 |
1 |
2 |
3 |
4 |
||
|
A |
Lh |
23.19 |
19.50 |
24.97 |
29.54 |
26.71 |
|
ah |
0.61 |
0.74 |
0.87 |
1.18 |
1.01 |
|
|
bh |
9.78 |
9.71 |
9.05 |
10.20 |
8.75 |
|
|
B |
Lh |
27.87 |
12.74 |
18.66 |
22.41 |
28.49 |
|
ah |
0.92 |
2.52 |
1.21 |
0.89 |
1.13 |
|
|
bh |
8.63 |
7.98 |
9.82 |
10.09 |
8.97 |
|
|
C |
Lh |
19.90 |
14.91 |
17.12 |
19.54 |
23.67 |
|
ah |
0.47 |
1.61 |
1.33 |
0.98 |
0.67 |
|
|
bh |
9.25 |
8.93 |
9.37 |
9.69 |
9.02 |
|
|
D |
Lh |
25.21 |
14.58 |
20.98 |
22.89 |
27.94 |
|
ah |
0.66 |
1.69 |
0.71 |
0.87 |
1.07 |
|
|
bh |
8.47 |
8.75 |
9.41 |
9.54 |
8.42 |
|
|
E |
Lh |
21.59 |
9.92 |
20.56 |
22.21 |
25.83 |
|
ah |
0.38 |
2.69 |
0.63 |
0.94 |
0.91 |
|
|
bh |
9.24 |
6.41 |
9.65 |
9.60 |
8.53 |
|
|
F |
Lh |
16.13 |
12.13 |
16.90 |
29.30 |
23.79 |
|
ah |
1.14 |
2.48 |
1.39 |
1.06 |
0.78 |
|
|
bh |
1.15 |
2.08 |
1.33 |
0.91 |
1.43 |
|
|
G |
Lh |
16.25 |
8.53 |
16.67 |
23.70 |
31.36 |
|
ah |
1.15 |
2.08 |
1.33 |
0.91 |
1.43 |
|
|
bh |
9.11 |
8.20 |
9.22 |
9.96 |
8.52 |
|
|
H |
Lh |
13.56 |
10.73 |
20.47 |
20.28 |
25.76 |
|
ah |
1.72 |
2.75 |
0.78 |
0.94 |
0.86 |
|
|
bh |
8.23 |
6.77 |
9.69 |
9.84 |
9.36 |
|
|
I |
Lh |
13.96 |
14.13 |
16.88 |
21.09 |
25.93 |
|
ah |
1.92 |
1.77 |
1.44 |
0.88 |
0.76 |
|
|
b |
8.55 |
8.49 |
9.47 |
10.13 |
9.11 |
|
Table 3: Color Parameters for the First Set of Dressing Samples.
|
Net Change of Color (dimensionless) |
||||
|
System |
Week |
|||
|
0-1 |
0-2 |
0-3 |
0-4 |
|
|
A |
3.69 |
1.94 |
6.39 |
3.69 |
|
B |
15.23 |
9.30 |
5.66 |
0.73 |
|
C |
5.13 |
2.91 |
0.76 |
3.79 |
|
D |
10.68 |
4.34 |
2.57 |
2.76 |
|
E |
12.23 |
1.14 |
0.91 |
4.33 |
|
F |
4.51 |
0.85 |
13.21 |
7.67 |
|
G |
7.83 |
0.47 |
7.50 |
15.12 |
|
H |
3.34 |
7.12 |
6.96 |
12.28 |
|
I |
0.23 |
3.10 |
7.38 |
12.04 |
Table 4: Net Change of Color for the First Set of Dressing Samples.
The emulsion stability showed values between 53 and 82% (± 5%) through the whole period of analysis- Fresh samples exhibited a range of 73 to 81%, showing a small decrease during the storage; for the first week: 68-79%, for the second week: 63-82%, for the third week: 58-81% and for the fourth week: 53-82%. The least stable was the dressing B followed by A and C (stability <74%), whereas the sample I was the most stable (>78%); this property was significantly influenced by all the variables. These stability data are very good compared with those values reported by Perrechil et al. [13] for Italian salad dressing with 57-63% between two and ten days of storage, but it is lower than those stability values (81.5-100%) cited by Sanmartín et al. [7] for salad dressings made with caprine cheese whey powders and bovine whey protein concentrate This phenomenon occurred because the stability test measured the percentage of remaining emulsion after the samples were centrifuged and thus, samples with higher oil content tend to have a higher remanent volume. It is important to mention that as higher is a disperse phase concentration in the emulsion (corn oil), higher is the surface tension between both phases, leading to a more unstable emulsion [24]. This test also did reflect the positive effect of the mustard concentration on the stability of the dressing, its emulsifying effect has been corroborated by Fishbach and Kokini [25]. Then, a good stability was obtained in our formulations, the emulsion stability augmented with oil content and decreased with storage time.
The disperse phase droplet size is very important for emulsions stability [24-27]. In agreement with Coupland and Mc Clements [28], those emulsion properties that contributes the most to its perceptible characteristics are three, the disperse phase concentration, distribution and droplet size. Figures 1-3 show images used for the droplet size measurements. Even though the images look with some similarity, they exhibited a variety of characteristics: a more homogeneous size distribution may be observed in Figure 2, a bigger size of drops forming agglomerates may be observed in Figure 3, whereas a mix of small and medium oil droplets is present in Figure 1. Three pictures of each dressing were taken with the specialized software. In order to use the average diameter of the droplets as a comparison factor, it was necessary to determine previously if these values exhibited a normal distribution. This was done by the elaboration of the correspondent histograms of frequency [29], all the dressing samples showed the normal distribution (data not included). Thus, the droplet size ranged from 2.98 to 4.17 μm (± 1.3-2.2 μm, Table 5). These values are into the range reported by Coupland and Mc Clements [28] of 0.1 to 10 μm for food emulsions; Asano et al. [30] measured 5.48 to 29.97 μm for oil/water food emulsions; Moros et al. [31] cited 5.2 to 8.8 μm for spray-dried egg yolk-stabilized emulsions; De Cindio and Cacace observed [21] 8.1 to 30.4 μm for reduced-calorie food emulsions and Waite et al. [12] with 5.6 to 7.6 mm for fresh an treated ranch dressing, included the storage time at three temperatures (4, 26 and 37°C).
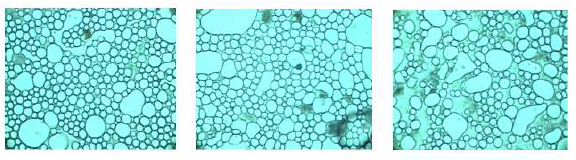
Figure 1: Photographs of dressing B (2.5:1.0 volume oil:vinegar and 92.0 g/L of mustard).
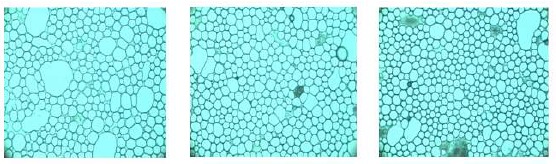
Figure 2: Photographs of dressing E (3.0:1.0 volume oil:vinegar and 92.0 g/L of mustard).
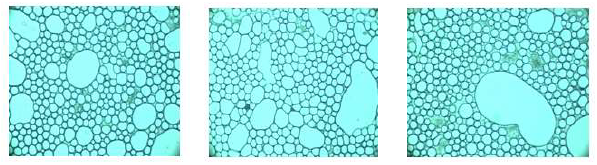
Figure 3: Photographs of dressing G (3.5:1.0 volume oil:vinegar and 85.5g/L of mustard).
|
Fresh dressings (week 0) |
|||
|
System |
Droplets number |
Average (μm) |
Stand. Dev. |
|
A |
445 |
3.39 |
1.70 |
|
B |
812 |
3.71 |
1.93 |
|
C |
1517 |
2.98 |
1.12 |
|
D |
1020 |
3.63 |
1.36 |
|
E |
1176 |
3.70 |
1.32 |
|
F |
1006 |
3.73 |
1.65 |
|
G |
973 |
3.73 |
2.23 |
|
H |
1005 |
4.17 |
1.72 |
|
I |
1503 |
3.37 |
1.33 |
Table 5: Average Droplet Size for the First Set of Dressing Samples.
Finally, the peroxide content exhibited initial values in a range of 0.83 to 1.56 meq/kg that increased to 1.73-2.56 meq/kg during 4 weeks (64-108% of increasing) and to 5.26-8.79 meq/kg in 8 weeks. Commercially a dressing should not exceed 20 meq/kg, this tracking value is important to establish the shelf life of the dressings; therefore, our dressing samples are acceptable even at the eighth week. Min et al. [32] reported similar peroxide values of 1.85 to 5.67 meq/kg for the fresh prepared salad dressings, with higher oxidation (3.70-30.91 meq/kg) after five days of storage. It is clear that some dressing properties are comparable, analogue or different, but properties, as it is known, are a function of the formulation and other process variables.
3.3 Textural attributes
In order to characterize ths physical condition of the prepared dressings, two textural attributes were measured. The retro-extrusion varied between 1.97 and 2.82 N in fresh samples (Table 6), it increased with all the factors. The main increase in this property was attributed to the coalescence and resistance of the droplets to mechanical action. This force reached values between 2.36 and 3.26 N after storage. While the adhesivity was different between dressing samples (-0.42 to -1.56 N.s), it decreased with the storage time and ranged from -1.09 to -2.90 N.s after four weeks of storage, expressing a loss of structure stability. Lower retro-extrusion and adhesivity values were recorded in A, B, C samples, intermediate values were observed in D, E, F samples, while higher values were obtained for G, H, I dressings. Thus, these textural parameters were related to the oil content, as well as other properties. In general terms, the recorded forces of the prepared dressings were lower than those obtained for commercial ones (1.68 to 5.02 N, and -0.41 to -2.99 N.s, respectively).
|
Week: |
0 |
1 |
2 |
3 |
4 |
|
System |
Force (N) |
Force (N) |
Force (N) |
Force (N) |
Force (N) |
|
A |
1.97 |
2.26 |
2.23 |
2.62 |
2.57 |
|
B |
2.20 |
2.58 |
2.16 |
2.46 |
2.36 |
|
C |
2.47 |
2.70 |
2.58 |
2.92 |
2.85 |
|
D |
2.37 |
2.84 |
2.73 |
3.03 |
2.99 |
|
E |
2.51 |
3.21 |
2.72 |
3.12 |
3.06 |
|
F |
2.44 |
3.03 |
2.78 |
2.94 |
2.98 |
|
G |
2.70 |
2.94 |
2.77 |
2.94 |
2.80 |
|
H |
2.76 |
2.75 |
2.68 |
2.92 |
3.08 |
|
I |
2.82 |
3.14 |
2.88 |
3.19 |
3.26 |
Table 6: Retro-extrusion Force for the First Set of Dressing Samples.
3.4 Flow characterization
Based on the rheograms (Figure 4), the flow nature of the dressing samples was determined to be of plastic-pseudoplastic type, with a measured yield stress and a flow index behavior lower than one. This food emulsion’s behavior has widely been recognized and reported before by Paredes et al., [33, 34], Ma and Barbosa-Cánovas [2], Campanella et al. [34], Solano Hurtado and Vélez Ruiz [9], Dolz et al. [11], Perrechil et al. [13] and Pero et al. [6] or as a viscoelastic material by Sanmartin et al. [7], with higher elastic component than the viscosity [36, 37].
As usually, the rheograms were modeled with different approaches, one without and other two with yield stress consideration. Thus, after comparing the fitting of models by RE (relative error) and SMRE (square mean root error) as goodness tests, it was concluded that the modified Herschel-Bulkley model (t0.25=to0.25 + Kg0.25) that also may be considered as modified Casson equation, had the smallest magnitudes for RE and SMRE, being then the better fitting. Table 7 shows the obtained magnitudes for flow properties by the non-Newtonian nature corresponding to this modified equation (R2 > 0.986), in which an average relative error of 8.6 was obtained (5.6% average for fresh samples, 10.2% average for first week, 7.3% average for second week, 9.3% average for third week and 10.6% average for fourth week of storage), in comparison to 8.9% for Power Law model and 17.4% for Herschel and Bulkley relationship.
The flow behavior index was higher in samples A and B (0.39-0.51), whereas the rest of the dressings exhibited a range of 0.29 to 0.39, but most of them had a n of 0.30-0.35 (eighteen of thirty determinations). Whereas the consistency coefficient ranged from 2.34 to 9.73 Pa sn, with not a generalized trend. And all the dressings showed the presence of a yield stress (0.92-2.04 Pa). Alvarez et al. [10], that fitted a variety of dressings reported a consistency coefficient of 9.9-14.9 Pa sn as a function of temperature and a constant flow behavior of 0.31-0.32. The Herschel and Bulkley model has been applied to a lot of dressing [2, 4, 6, 9, 11, 14] and other type of foods, with diversity of ingredients, such is the case of the prepared mustard dressings. The ANOVA results indicated that the yield stress was significantly affected by the three studied factors; while the consistency coefficient was just affected by two of them, the oil:vinegar ratio and the storage time, and the flow index was not affected by the storage time but it was influenced by the other two factors.
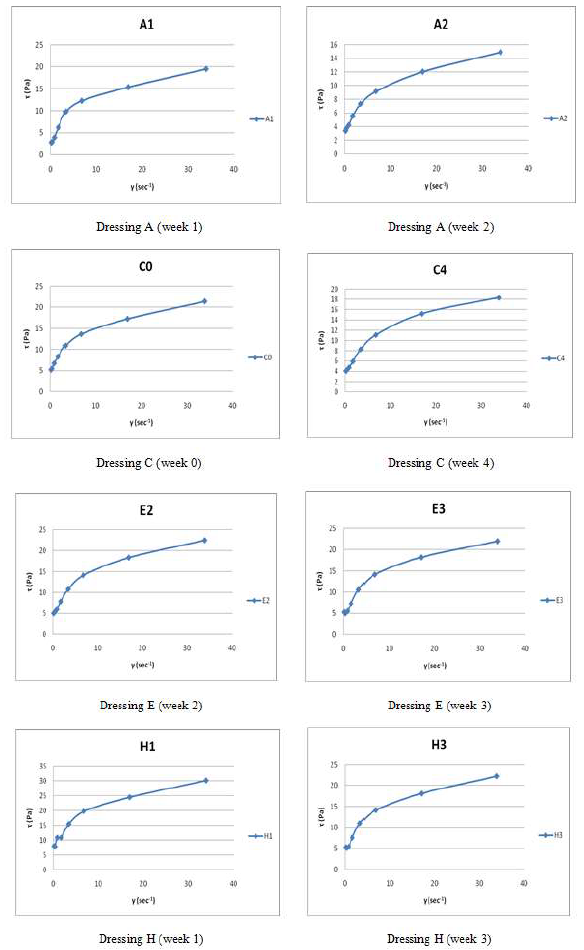
Figure 4: Rheograms for Some Dressing Systems of the First Set at Different Storage Weeks.
|
Sample: |
A |
B |
C |
||||||
|
Storage |
τo |
K |
n |
τo |
K |
n |
τo |
K |
n |
|
(week) |
(Pa) |
(Pa.sn) |
(Pa) |
(Pa.sn) |
(Pa) |
(Pa.sn) |
|||
|
0 |
0.93 |
2.34 |
0.49 |
0.98 |
2.67 |
0.46 |
1.60 |
5.94 |
0.34 |
|
1 |
1.00 |
3.91 |
0.51 |
1.05 |
4.28 |
0.48 |
1.95 |
8.68 |
0.29 |
|
2 |
1.26 |
3.69 |
0.37 |
1.00 |
3.11 |
0.45 |
1.37 |
4.80 |
0.37 |
|
3 |
1.11 |
3.34 |
0.42 |
0.92 |
2.84 |
0.47 |
1.29 |
4.60 |
0.39 |
|
4 |
1.10 |
3.58 |
0.40 |
0.97 |
3.14 |
0.45 |
1.30 |
4.50 |
0.37 |
|
Sample: |
D |
E |
F |
||||||
|
Storage |
τo |
K |
n |
τo |
K |
n |
τo |
K |
n |
|
(week) |
(Pa) |
(Pa.sn) |
(Pa) |
(Pa.sn) |
(Pa) |
(Pa.sn) |
|||
|
0 |
1.33 |
4.66 |
0.38 |
1.62 |
6.35 |
0.35 |
1.48 |
5.09 |
0.36 |
|
1 |
1.74 |
7.63 |
0.33 |
2.04 |
9.57 |
0.30 |
1.72 |
7.18 |
0.32 |
|
2 |
1.39 |
5.43 |
0.38 |
1.48 |
5.81 |
0.37 |
1.39 |
5.19 |
0.38 |
|
3 |
1.37 |
5.21 |
0.37 |
1.45 |
5.66 |
0.36 |
1.37 |
5.04 |
0.36 |
|
4 |
1.39 |
5.24 |
0.36 |
1.53 |
6.03 |
0.34 |
1.47 |
5.43 |
0.34 |
|
Sample: |
G |
H |
I |
||||||
|
Storage |
τo |
K |
n |
τo |
K |
n |
τo |
K |
n |
|
(week) |
(Pa) |
(Pa.sn) |
(Pa) |
(Pa.sn) |
(Pa) |
(Pa.sn) |
|||
|
0 |
1.72 |
6.94 |
0.33 |
1.65 |
7.05 |
0.35 |
1.81 |
7.52 |
0.32 |
|
1 |
1.84 |
8.72 |
0.33 |
1.99 |
9.08 |
0.31 |
1.97 |
9.73 |
0.32 |
|
2 |
1.69 |
6.72 |
0.33 |
1.57 |
6.20 |
0.35 |
1.71 |
7.79 |
0.34 |
|
3 |
1.55 |
5.98 |
0.34 |
1.46 |
5.71 |
0.36 |
1.65 |
7.09 |
0.35 |
|
4 |
1.50 |
5.66 |
0.35 |
1.40 |
5.68 |
0.37 |
1.58 |
6.90 |
0.35 |
Table 7: Flow Properties from the Modified Herschel and Bulkley Model for Dressing Samples from the First Set.
3.5 Second set of dressing samples
In the second set of samples, dressings with high mustard content (98 g/L) and four modifications in the oil:vinegar rate were prepared (Table 8), with the idea of researching the effect of small compositional changes and storage temperature. Properly, four different dressing formulations were prepared, while two controls were selected taking into account the good performance of the previous samples (physicochemical, textural and flow properties).
3.6 Storage at ambient temperature
Some few changes in some physicochemical properties were observed in this set of dressings at 20-25°C. Color parameters exhibited similarity, with 17.4-27.4 for luminosity, 0.73-1.68 for redness, and 7.8-9.1 for yellowness through four weeks of storage. Luminosity showed only significant effect (95%) from storage time. The net change of color was lower, from 0.47 to 7.74 as a function of the storage time, but without showing a relation to that time. The density of the samples ranged from 934 (± 47.0, sample L and M) to 976 kg/m3 (± 10.7, samples J and K); the humidity varied from 32.7 (± 2.45, samples P and Q) to 34.8% (± 0.78, samples J and K), with the highest content for the smallest oil:vinegar ratio. Acetic acid content (pH 4.5 for fresh samples) was recorded as 0.36 (± 0.04, samples P and Q) to 0.49% (± 0.05, samples J and K). And the droplet sizes were 3.3-4.0 μm, being similar to the first set of samples, as it was expected because the same preparation process was followed. The stability was also equivalent to 52-82%, with the lowest values (52-62) at the fourth storage week; two factors, oil concentration and time, influenced the stability significantly. With respect to the flow properties, measured only at days 0. 3 and 9 (Table 9), a good fitting was obtained with the modified Herschel and Bulkley model, consistent with the first set of samples; but in this case the Power Law also did a good fitting, in both models the average relative error was 17%, indicating that the modeling was not as good as the obtained for the first set. As expected with modified HB, fit all samples exhibited the same rheological behavior, of plastic-pseudoplastic type.
3.7 Storage at 40°C
The storage at this temperature contributed to modify the dressing properties, being more notable in some of them that in others. Color parameters did not show observable modification, 17.4-24.9 for luminosity, 0.73-1.45 for redness and 7.94-9.10. With net color change in the range of 1.23 to 9.13, through 12 days of storage; even though it was higher than at 20-25°C resulted lower than for the first set. The emulsion stability was very similar (51-82%) to the first set, that was decreasing as time advanced, through fifteen days of storage. The droplet sizes increased a little after fifteen days at which this parameter was determined: 4.1-4.4 μm. Probably the most important effect was on the rheological response. The rheograms were fitted with the same three equations, Power law and Herschel and Bulkley (normal: HB and modified MHB), finding in this set of dressing at 40°C that the best fit corresponded to the traditional Herschel and Bulkley model with an average relative error of 17.1%, with an increasing error as a function of the storage time: error 12.8% at day 0, error 12.5 at day 3, error 19.3% at day 9 and error 23.9 at day 15, and observing a very small difference with the Power Law (PL) fitting (average relative error of 17.2%). The flow parameters for HB are presented in Table 10, in which the argument to prefer the HB fitting over PL is the presence of yield stress, that is a physical and important parameter for fluids characterization, process and equipment design.
|
Sample |
Oil:Vinegar rate |
Mustard content (g/L) |
Storage T (°C) |
|
J |
2.6:1 |
98.5 |
20-25 |
|
K |
2.6:1 |
98.5 |
40 |
|
L |
2.8:1 |
98.5 |
20-25 |
|
M |
2.8:1 |
98.5 |
40 |
|
N |
3.0:1 |
98.5 |
20-25 |
|
O |
2.3:1 |
98.5 |
40 |
|
P |
3.2:1 |
98.5 |
20-25 |
|
Q |
3.2:1 |
98.5 |
40 |
|
R* |
3.0:1 |
92.0 |
20-25 |
|
S* |
3.0:1 |
92.0 |
40 |
Samples R* and S* were the controls
Table 8: Composition for the Second Set of Dressing Samples.
|
System |
Property |
Day |
||
|
0 |
3 |
9 |
||
|
J |
τo (Pa) |
2.02 |
1.41 |
1.48 |
|
K (Pa.sn) |
6.70 |
4.65 |
5.36 |
|
|
n |
0.36 |
0.52 |
0.51 |
|
|
L |
τo (Pa) |
2.01 |
1.40 |
1.22 |
|
K (Pa.sn) |
7.10 |
5.07 |
4.68 |
|
|
n |
0.38 |
0.53 |
0.59 |
|
|
N |
τo (Pa) |
1.93 |
1.51 |
1.42 |
|
K (Pa.sn) |
7.27 |
5.90 |
5.89 |
|
|
n |
0.41 |
0.51 |
0.54 |
|
|
P |
τo (Pa) |
1.78 |
1.50 |
1.53 |
|
K (Pa.sn) |
6.12 |
6.05 |
6.03 |
|
|
n |
0.41 |
0.53 |
0.52 |
|
|
R |
τo (Pa) |
1.81 |
1.39 |
1.57 |
|
K (Pa.sn) |
5.99 |
5.27 |
5.03 |
|
|
n |
0.38 |
0.55 |
0.46 |
|
Table 9: Flow Properties for the Second Set of Dressing Samples at 20-25°C.
|
System |
Parameter |
Day |
|||
|
0 |
3 |
9 |
15 |
||
|
K |
τo (Pa) |
1.70 |
1.70 |
1.70 |
1.70 |
|
K (Pa.sn) |
6.99 |
4.73 |
4.03 |
2.18 |
|
|
n |
0.35 |
0.53 |
0.57 |
0.72 |
|
|
M |
τo (Pa) |
8.41 |
7.28 |
5.25 |
4.70 |
|
K (Pa.sn) |
1.58 |
2.11 |
1.35 |
1.33 |
|
|
n |
0.81 |
0.76 |
0.92 |
0.92 |
|
|
O |
τo (Pa) |
8.42 |
7.93 |
6.32 |
4.67 |
|
K (Pa.sn) |
1.75 |
2.04 |
1.60 |
1.22 |
|
|
n |
0.83 |
0.78 |
0.86 |
0.94 |
|
|
Q |
τo (Pa) |
7.15 |
8.52 |
6.43 |
5.34 |
|
K (Pa.sn) |
1.49 |
1.95 |
1.73 |
1.44 |
|
|
n |
0.83 |
0.77 |
0.89 |
0.90 |
|
|
S |
τo (Pa) |
6.99 |
7.41 |
6.16 |
4.29 |
|
K (Pa.sn) |
1.45 |
1.98 |
1.42 |
1.36 |
|
|
n |
0.79 |
0.76 |
0.89 |
0.95 |
|
Table 10: Herschel and Bulkley´s Flow Properties for the Second Set of Dressing Samples at 40°C.
As expected, the influence of temperature was notable on the three flow parameters, decreasing magnitude of the yield stress and consistency coefficient and increasing the flow behavior index. That in general terms, it means that this set of dressings flow easier, in comparison with the other samples (first set and second set at 20°C). The ANOVA indicated that yield stress and flow index were significantly affected by the storage time, whereas the consistency coefficient was significantly affected by the oil concentration and storage time.
4. Conclusions
A new formulation of mustard dressing was characterized and the effect of four process variables on this vinnaigrete type was studied. The oil content, mustard concentration, storage time and temperature are variables with evident influence on most of the characteristics of a salad dressings. The study and knowledge help to get a better formulation, handling, control and process design in the dressing industry. The storage time was found to be the most influencing factor for the prepared mustard emulsions, notably on the flow properties, stability and peroxide content. Its effect can be overcome with different combinations of oil and mustard, formulation modifications and novel emulsions with a longer shelf life and good acceptance by consumers.
References
- Sherman P. Textural characteristics of food emulsions. In Rheology and Texture in Food Quality. Eds.: DeMan JM, Voisey PW, Rasper VF, et al. The AVI. Publishing Company, Inc. Boca Raton, FL., USA (1975): 487.
- Ma L and Barbosa-Cánovas GV. Rheological characterization of mayonnaise. Part II: Flow and viscoelastic properties at different oil and xanthan gum concentrations. Journal of Food Engineering 25 (1995): 409-425.
- Sotoyama K, Asano Y, Ihara K, et al. Water/oil emulsions prepared by the membrane emulsification method and their stability. Journal of Food Science 64 (1999): 211-215.
- Juszczak L, Witczak M, Fortuna F, et al. Rheological properties of commercial mustards. Journal of Food Engineering 63 (2004): 209-217.
- Sikora M, Badrie N, Anil K Deisingh, et al. Sauces and dressings: A review of properties and applications. Critical Reviews in Food Science and Nutrition 48 (2008): 50-77.
- Pero M, Emam-Djomeh Z, Yarmand M, et al. Stability and rheological properties of model low-fat salad dressing stabilized by salep. Journal of Dispersion Science and Technology 35 (2014): 215-222.
- Sanmartín B, Díaz O, Rodríguez-Turienzo L, et al. Emulsion characteristics of salad dressings as affected by caprine whey protein concentrates. International Journal of Food Properties 21 (2018): 12-20.
- Chantrapornchai W, Clydesdale FM, McClements DJ. Influence of flocculation on optical properties of emulsions. Journal of Food Science 66 (2001): 464-469.
- Solano Hurtado A. and Vélez Ruiz J.F. Evaluation of flow and physicochemical properties of dressing formulated with avocado oil. Proceedings of the 3rd International Symposium on Food Rheology and Structure. Zurich, Switzerland (2003): 441-442.
- Alvarez A, Cancela MA, Maceiras R. Comparison of rheological behavior of sweet and salad sauces. International Journal of Food Properties 7 (2004): 511-518.
- Dolz M, Hernández MJ, Delegido J, et al. Influence of xanthan gum and locus bean upon flow and thixotropic behavior of food emulsions containing modified starch. Journal of Food Engineering 81 (2007): 179-186.
- Waite JG, Jones M, Turek JM, et al. Production of shelf-stable ranch dressing using high-pressure processing. Journal of Food Science 74 (2009): 83-93.
- Perrechil F, Santana R, Fasolin L, et al. Rheological and structural evaluations of commercial Italian salad dressings. Food Science and Technology, Campinas 30 (2010): 477-488.
- Ma Z and Boye J. Microstructure, physical stability, and rheological properties of salad dressing emulsion supplemented with various pulse flours. Journal of Food Research 2 (2013): 167-181.
- Bortnowska G, Balejko J, Schube V, et al. Stability and physicochemical properties of model salad dressings prepared with pregelatinized potato starch. Carbohydrate Polymers 111 (2014): 624-632.
- Kaltsas O, Yanniotis M, Polissioul M. Stability, physical properties and acceptance of salad dressings containing saffron (Crocus sativus) or pomegranate juice powder as affected by high shear (HS) and ultrasonication (US) process. LWT-Food Science and Technology 97 (2018): 404-413.
- Dirección General de Normas. Mayonesa. NMX-F-102-S-1978 Mexico. In Spanish (1979).
- Norma Mexicana (NMX-F-102-S-1978). Acidity determination in manufactured products from fruits and vegetables. DIRECCIÓN GENERAL DE NORMAS. Mexico. In Spanish (1978).
- Official Methods of Analysis. Association of Official Analytical Chemist, Inc. Virginia, USA (1984).
- Yang S and Cotterill OJ. Physical and functional properties of 10% salted yolk in mayonnaise. Journal of Food Science 54 (1989): 210-214.
- DeCindio B and Cacace D. Formulation and rheological characterization of reduced-calorie food emulsions. International Journal of Food Science and Technology 30 (1995): 505-514.
- Ford LD, Borwankar R, Martin Jr RW, et al. Dressings and Sauces. In Food Emulsions. Eds.: Friberg SE, Larsson K. Marcel Dekker, Inc. New York, USA (1997): 361.
- Chirife J, Vigo MS, Gómez RG, et al. Water activity and chemical composition of mayonnaises. Journal of Food Science 54 (1989): 1658-1659.
- Damodaran S, Parkin K, Fennema OR. Food Chemistry. CRC Press. Boca Raton, FL. USA (2008).
- Fischbach R and Kokini JL. Effect of aging mustard flour on rheological properties of model O/W emulsions. Journal of Food Science 52 (1989): 1748-1749.
- Carrillo AR and Kokini JL. Effect of egg yolk and egg yolk + salt on rheological properties and particle size distribution of model oil-in-water salad dressing emulsions. Journal of Food Science 53 (1988): 1352-1354.
- Tesch S, Gerhards Ch, Schubert H. Stabilization of emulsions by OSA starches. Journal of Food Engineering 54 (2002): 167-174.
- Coupland JN and Mc Clements DJ. Droplet size determination in food emulsions: comparison of ultrasonic and light scattering methods. Journal of Food Engineering 50 (2001): 117-120.
- Mendenhall W, Scheaffer RL, Wackerly DD. Mathematical Statisitics with Applications. (6th). Thomson. USA (2010).
- Asano Y and Sotoyama K. Viscosity change in oil/water food emulsions prepared using a membrane emulsification system. Food Chemistry 66 (1999): 327-331.
- Moros JE, Franco JM, Gallegos C. Rheology of spray-dried egg yolk-stabilized emulsions. International Journal of Food Science and Technology 37 (2002): 297-307.
- Min S, Mistry BS, Lee HO. Improvement of oxidative and emulsión stability of model salad dressing by glucosa oxidase-catalase. Journal of Food Science 68 (2003): 1272-1275.
- Paredes MD, Rao MA, Bourne MC. Rheological characterization of salad dressings. 1. Steady shear, thixotropy and effect of temperature. Journal of Texture Studies 19 (1988): 247-258.
- Paredes MD, Rao MA, Bourne MC. Rheological characterization of salad dressings 2: Effect of storage. Journal of Texture Studies 20 (1989): 235-250.
- Campanella OH, Dorward NM, Singh H. A study of the rheological properties of concentrated food emulsions. Journal of Food Engineering 25 (1995): 427-440.
- Floury J, Desrumaux A, Axelos MAV, et al. Effect of high pressure homogenization on methylcellulose as food emulsifier. Journal of Food Engineering 58 (2003): 227-238.
- Martínez I, Riscardo MA and Franco JM. Effect of salt content on the rheological properties of salad dressing-type emulsion stabilized by emulsifier blends. Journal of Food Engineering 80 (2007): 1272-1281.


 Impact Factor: * 3.8
Impact Factor: * 3.8 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 