Preliminary Identification of the Sex Pheromones of the Citrus Fruit Borer (Citripestis sagittiferella Moore) Inhabiting the Mekong Delta of Vietnam
Article Information
Tran Trong Dung1*, Duong Kieu Hanh1, Trieu Phuong Linh1, Dinh Thi Chi1, Tran Thanh Thy2, Le Van Vang1, Tran Vu Phen1
1College of Agriculture, Can Tho University, Can Tho City, Vietnam
2Department of Science Research, Nam Can Tho University, Nguyen Van Cu Street, Can Tho City, Vietnam
*Corresponding Author: Tran Trong Dung, College of Agriculture, Can Tho University, Can Tho City, Vietnam
Received: 17 February 2021; Accepted: 02 March 2021; Published: 08 March 2021
Citation:
Tran Trong Dung, Duong Kieu Hanh, Trieu Phuong Linh, Dinh Thi Chi, Tran Thanh Thy, Le Van Vang, Tran Vu Phen. Preliminary Identification of the Sex Pheromones of the Citrus Fruit Borer (Citripestis sagittiferella Moore) Inhabiting the Mekong Delta of Vietnam. International Journal of Plant, Animal and Environmental Sciences 11 (2021): 194-205.
View / Download Pdf Share at FacebookAbstract
Abstract
Citripestis sagittiferella is one of the most harmful pest insects of pomelo in the Mekong Delta of Vietnam. In order to utilize sex pheromone as tool for population monitoring by which supplies information for timing control, the sex pheromone of C. sagittiferella was preliminarily identified then tested under field condition. Fruits infected C. sagittiferella larvae were collected from pomelo orchards in Vinh Long and Hau Giang provinces and brought to Can Tho University laboratory for rearing to extract the pheromone. The analysis was conducted by GC-EAD, GC-MS and the double bond position was affirmed by analysis of its DMDS adduct. GC-EAD analysis resulted in three EAG-responses, namely component I (Comp. I), Comp. II and Comp. III. Mass spectra with diagnostic ions at m/z 61, 194 and 192 ([M-60]+ ions) indicated that they were unsaturated 14 carbons (C14) straight chain acetates. Reference to the synthetic standards by both GC-EAD and GC-MS analysis confirmed that the three pheromone components were (E)-11-tetradecenyl acetate (E11-14:OAc), (Z)-11-tetradecenyl acetate (Z11-14:OAc) and (9Z,11E)-9,11-tetradecadienyl acetate (Z9, E11-14:OAc), respectively. Unfortunately, lures prepared from mixtures of synthetic E11-14:OAc, Z11-14:OAc and E9, Z11-14:OAc at several mixing ratios showed no attraction to C. sagittiferella males in field tests.
Keywords
Citripestis sagittiferella; Sex pheromone; (E)-11-tetracenyl acetate; (Z)-11-tetracenyl acetate; (9Z, 11E)-9,11-tetradecadienyl acetate
Citripestis sagittiferella articles; Sex pheromone articles; (E)-11-tetracenyl acetate articles; (Z)-11-tetracenyl acetate articles; (9Z, 11E)-9 articles,11-tetradecadienyl acetate articles
Article Details
1. Introduction
Pomelo (Citrus maxima Merr.; Synonym: C. grandis Osbeck; C. decumana L.) is the largest citrus fruit which was native to South-East Asia [1]. In the Mekong Delta of Vietnam, pomelo is growing predominantly on the alluvial soil in provinces locating along “Tien” and “Hau” rivers, the two main branches of the Mekong River that flows on Vietnamese territory before emptying into the East Sea. The planting area of pomelo in the Mekong Delta in 2019 was 31,900 ha, producing 333,800 tons of fruits [Agriculture and Rural Development of Vietnam (MARD) report].
Although being listed as a major pest of citrus fruits in South East Asia in 1993 [2], the citrus fruit borer, Citripestis sagittiferella (Lepidoptera: Pyralidae, Phycitinae), was considered as a newly emerging insect pest of pomelo by MARD in 2013. C. sagittiferella females lay their eggs on the pock of pomelo fruits, most is at lower half of the fruits. Newly hatching larvae mine and feed inside the fruits, causing consequently fruit loss with the loss ratio could reach to higher than 70% [3]. Moreover, C. sagittiferella larvae have also been recorded attacking all other kinds of citrus fruits [4]. Because most of the larval stage of C. sagittiferella were shielded by pomelo pock, effective control by sprays of insecticides for this species was difficult to obtain. Many pomelo growers in the Mekong Delta of Vietnam had to spray insecticides at 7- to 10-days intervals during the fruiting stage of the trees to prevent the damage of this species [5]. Since pomelo fruits were eaten freshly and used as a gift for occasions and visits among families, furthermore, to adapt to development of sustainable agriculture, management programs which limited the use of insecticides for control of C. sagittiferella are necessary.
Pheromone are cell type special signals used for communication between individuals of the same species [6]. According to Ando and Yamakawa (2011) [7], pheromone was classified as 3 type: Type I, Type II and other Type. Most of pheromone depended on Type I and Type II [8].
Type I mostly belongs to primary alcohol with straight chain 10-18 carbon (C10 – C18) with the derivative of acetate and aldehyde. It is the most popular type [8].
Type II includes polyenyl hydrocarbon containing 17-23 carbon with epoxy and ketone functional group.
Besides, there are some cases that contain both Type I and Type II characteristics. According to Yan et al. (2014) [9], pheromone of some species on Pyraloidae bring both unsaturated hydrocarbon and epoxy group. According to Kanno et al. (2010) [10], pheromone of Amyelois transitella have both Type I (aldehyde, alcohol group) and Type II (epoxy and ketone group).
Pyralidae is one of the two families of Pyraloidea, the third largest supper family in Lepidoptera with more than 16.000 species have been described [11]. As a consequence of comprising of many agricultural pests, researches on sex pheromone identification has been carried on 39 pyralid and 62 crambid species [12], most of which aimed to utilize sex pheromone as tool for pest management. In the Mekong Delta of Vietnam, correlation between number of captured males by pheromone traps and damage ratios which could be used as reference to determine the action threshold for control of the citrus pock caterpillar (Prays endocarpa) and the diamond back moth (Plutella xylostella) has been reported [13,14].
Until now, there is no published data on sex pheromone of C. sagittiferela yet. Moreover, in order to utilize sex pheromone as tool for population monitoring by which supplies information for timing control, the sex pheromone of C. sagittiferella inhabiting the Mekong Delta of Vietnam was identified by using GC-EAD and GC-MS analysis.
2. Materials and Methods
2.1 Materials
Chemicals: (E)-11-tetradecen-1-ol (E11-14:OH), (Z)-11-tetradecen-1-ol (Z11-14:OH) and (3Z,6Z,9Z)-3,6,9-Tricosatriene (Z3,Z6,Z9-23:H) were supplied by Shin-Etsu Chemical Company (Japan). Corresponding (E)-11-tetradecenyl acetate (E11-14:OAc) and (Z)-11-tetradecenyl acetate (Z11-14:OAc) were prepared by acetylation of E11-14:OH and Z11-14:OH. 9-Bromo-1-nonanol, (E)-2-pentanal, tetracyanoethylene and Sodium bis (trimethylsilyl) amide were purchased from Sigma-Aldrich (America). Dimethoxymethane (DMM), Lithium bromide (LiBr), p-tolunenesulfonic acid monohydrate (p-TsOH), Triphenylphosphine (PPh3), Sodiumsulfate (Na2SO4) were purchased from Wako Chemicals (Japan); silica gel was a product of Kanto Chemical Company (Japan). Solvents including n-hexane, benzene, ethyl acetate, tetrahydrofuran (THF), acetic anhydride, and pyridine were products of Merck (Germany). Purity of the chemicals (>98%) were checked by a gas chromatography (GC) analysis before using for field tests.
2.2 Methods
2.2.1 Insects and pheromone extracts
Insects: Pomelo fruits infected C. sagittiferella larvae were collected from pomelo orchards in Vinh Long and Hau Giang provinces and brought to Can Tho University (Can Tho city, Vietnam). In laboratory, the C. sagittiferella was described and identified by Dung et al. (2017) [9] before pheromone extraction. The fruits were placed in plastic trays (480 mm x 392 mm x 215 mm) containing a layer of sterile soil (about 5.0 cm). The trays were covered with cheesecloth and placed in room conditions (around 12L:12D photo regime; 27-30°C). After pupation, each pupa which enclosed in a soil cocoon was placed individually in a small plastic cup. After eclosion, male and female adults were checked by inspecting their abdominal tips. The pheromone glands of 2- or 3-day-old virgin females were excised 2-3 h after sunlight off and soaked in hexane (10 μl/female) for 15 min to extract the pheromone components [15].
GC-EAD analysis: Electroantennographic responses (EAG-response) of male antennae to pheromone components in the pheromone gland extracts and synthetic standards were measured by a GC-EAD system with an HP-5890 Series II gas chromatograph (Hewlett Packard) equipped with a DB-23 capillary column (0.25 mm ID, 30 m length, 0.25 μm film thickness; J & W Scientific, Folsom, CA, USA). The inlet was set up at 220oC and splitless mode. An Y connector at near the end of the column split the gas effluent into two lines at a ratio of 1:1 before leading to flame ionization detector (FID) and Electroantennogram detector (EAD) [16]. The program temperature for GC running was maintained at 80°C for 1 min, ramped at 8°C/min to 210°C, and kept at 210°C for 10 min. The EAG activities of synthetic standards were measured under the same GC conditions and analyzed three times on three male antennae.
GC-MS analysis: A Hewlett Packard 6900 Series gas chromatograph equipped with a Hewlett Packard 5973 mass selective detector (Agilent Technologies Inc., Palo Alto, CA, USA), a split/splitless injector, and a DB-23 column were used for recording mass spectra of EAG active components in the extracts and synthetic standards. Helium (He) was used as carrier gas at a flow rate of 1.0 mL/min. The GC conditions using for the analysis were set up in the same manner as that for the GC-EAD analysis. The content amount of pheromone components was determined by calculation of peak area on a total ion chromatogram (TIC) using a calibration curve prepared with a synthetic standard. For analysis of DMDS adducts, the HP-5 column (0.25 mm ID, 30 m length, 0.25 μm film thickness; J & W Scientific, Folsom, CA, USA) was used. The temperature program was maintained at 100°C for 1 min and then programmed at 15°C/min to 280°C.
DMDS Derivatization of Pheromone Components: To determine the double bond position in the pheromone component, the crude pheromone extract (thirteen female equivalence, 13 FE) was dissolved in 50 μl of dimethydisulfide (DMDS) and 5 μl of iodine solution in diethyl ether (60 mg/ml). The mixture was kept warming at 40°C overnight [17]. After that a 10% sodium thiosulfate solution (0.2 ml) was added and the DMDS adducts were extracted with hexane.
2.3 Field examinations
Field attraction of the synthetic sex pheromones were examined at pomelo orchards in Vinh Long and Hau Giang provinces where the infested pomelo fruits were collected. The lure was a rubber septum (white rubber, 8 mm OD; Sigma-Aldrich) impregnated with a hexane solution of synthetic compounds. Each lure was placed at the center of a sticky board trap (30×27 cm bottom plate with roof; Takeda Chemical Ind., Ltd., Osaka, Japan), which was hung in pomelo canopy at approximately 1.2 - 1.5 m above the ground and distanced about 25 m far from each other. Traps baited with empty septa were used as a control. The males captured by traps were counted every week. In order to avoid a positional effect, position of traps in each experimental block was randomly changed after counting captured males. The trial was designed as Randomized Completed Block Design (RCBD) with 3 replicates. Each replicate corresponded with 1 pheromone trap. Trial included 5 treatments of E11-14:OAc, Z11-14:OAc; Z9, E11-14:OAc and T23 (Z3,Z6,Z9-23:H) with different mixing scale. 1 positive check and if 1 virgin female adult and 1 negative check with 10 µl n-hexan were included also.
Data analysis: Data obtained in each field test were analyzed by one-way ANOVA, and pairwise comparisons among traps were performed with Tukey-Kramer Test with P-values adjusted for multiple comparisons. In order to homogenize the variance, means were transformed by using log (x+0.5) transformation. Treatments with zero catches were omitted from the ANOVA. All statistical analysis were performed with R version 3.0.1 (R Development Core Team 2015).
3. Results
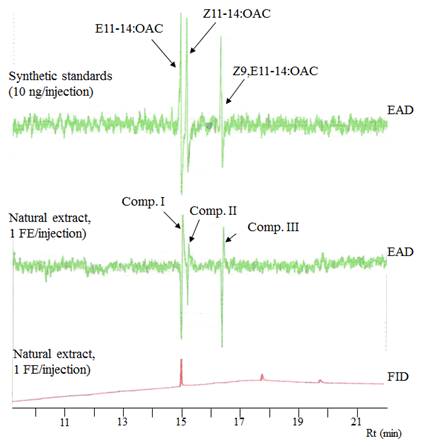
3.1 GC-EAD analysis
Figure 1 showed the results of GC-EAD analysis of the pheromone extract and synthetic standards. Citrus fruit borer (CFB) male antenna responded clearly to three components in the pheromone extract (1 FE), namely component I (Comp. I), Comp. II and Comp. III, at retention times (Rt) of 15.0 min (intensity of response: 155 µV), 15.20 min (81 µV) and 16.45 min (175 µV), respectively (Figure 1, lower). Expectedly, GC-EAD analysis of synthetic standards, E11-14:OAc, Z11-14:OAc and Z9, E11-14:OAc compounds showed the same Rts and EAG-responses from the male antenna as those of the three components in the pheromone extract (Figure 1, upper).
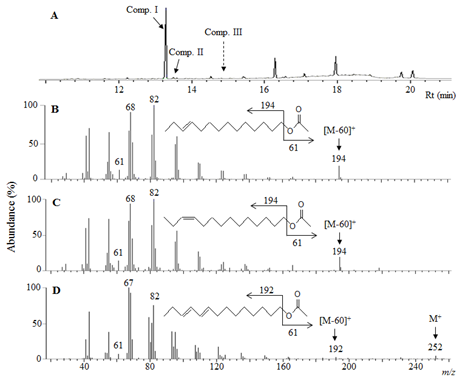
3.2. GC-MS analysis
Although GC-EAD analysis of 1 FE extract obtained strong three EAG-responses (Figure 1), GC-MS analysis of 6 FE detected only Comp. I and Comp. II on the total ion chromatogram (TIC) at Rts of 13.292 min and 13.497 min with a ratio of 99:1, while the peak of Comp. III was not detected (Figure 2A). Trace of the Comp. III was observed on TIC of the analysis of 23 FE extract at Rt 14.867 min (data was not showed). Mass spectrum of the Comp. I was basically similar to that of the Comp. II with diagnostic ions at m/z 61 and 194 ([M-60]+) (Figure 2B and 2C) indicated that they were tetradecenyl acetates [8]. Additionally, with 0.21 min difference in Rts between the two components, while the DB-23 capillary column was used for the analysis, it was suggested that Comp. I and Comp. II were E- and Z-isomers of each other. Comp. III eluted later than Comp. II 1.37 min, its mass spectrum with clear molecular ion (M+) at m/z 252, diagnostic ions at m/z 61 and 192 (ion [M-60]+), two units less than those of Comp. I and Comp. II, revealed a conjugated tetradecadienyl acetate (Figure 2D).
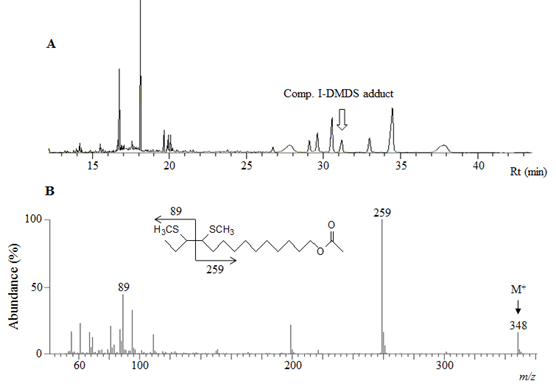
The double bond position in Comp. I was determined by GC-MS analysis of its DMDS derivative and the result was showed in the Figure 3. Mass spectrum of the adduct (at Rt 31.12 min on TIC, Figure 3A) with the M+ ion at m/z 348 and two fragmentary ions at m/z 259 and 89 (Figure 3B) affirmed that the double bond position of the Comp. I was at the carbon 11th (C11).
Since Comp. I was identified as E11-14:OAc, Comp. II and Comp. III were consecutively interpreted to be Z11-14:OAc and Z9, E11-14:OAc. Expectedly, Rts and mass spectra of Comp. I, Comp. II and Comp. III coincided well with those of synthetic E11-14:OAc, Z11-14:OAc and Z9, E11-14:OAc, respectively. Result of titer experiment showed that content of E11-14:OAc in a female extract was about 7 ng.
3.3 Field attraction of C. sagittiferella males
Tests for examination of the field attraction by synthetic lures were carried out at pomelo orchards in Hau Giang and Vinh Long provinces where C. sagittiferella larvae were collected. Unexpectedly, only one CFB male was captured in one test, the other test captured no CFB male (Table 1).
Table 1: Field examinations for synthetic pheromone lures of C. sagittiferella at pomelo orchrads in Hau Giang and Vinh Long provinces. Test -1 was conducted at Tan Phu Thanh village, Chau Thanh A district, Hau Giang province from 8th - 29th April 2018; Test -2 was conducted at My Hoa village, Binh Minh district, Vinh Long province from 14th June – 5th July 2018
|
Lure component (mg/septum) |
Captured male/week |
|||
|
E11-14:OAc |
Z11-14:OAc |
Z9,E11-14:OAc |
T23 |
|
|
Test-1 |
||||
|
0 |
0.0 |
0.5 |
0 |
|
|
0.5 |
0.0 |
0 |
0 |
|
|
0.5 |
0.05 |
0 |
0 |
|
|
0.5 |
0.05 |
0.05 |
0 |
|
|
0.5 |
0 |
0.05 |
0 |
|
|
0 |
0 |
0 |
0 |
|
|
1 virgin female 0 |
||||
|
Tes-2 |
||||
|
0 |
0.0 |
0.5 |
0.05 |
0 |
|
0.5 |
0.0 |
0 |
0.05 |
0 |
|
0.5 |
0.05 |
0 |
0.05 |
1 |
|
0.5 |
0.05 |
0.05 |
0.05 |
0 |
|
0.5 |
0 |
0.05 |
0.0 |
0 |
|
0 |
0 |
0 |
0.0 |
0 |
|
1 virgin female 0 |
||||
Lures prepared with synthetic E11-14:OAc or Z9, E11-14:OAc as major components showed no attraction to C. sagittiferella males in field. According to Dung et al. (2017) [5], C. sagittiferella was very popular in this location (frequency of occurrence was more than 50%). Moreover, the result from virgin female treatment showed no attraction also. The mating mechanism of insect is very complicated [18]. This is the strain of many consecutive and interactive behaviors such as finding the location of female, coupling…[19]. According to Deisig et al. (2014) [20], excreted pheromone from female adult must contact and correlate with some component on the hosted plant to form some compounds which attract the male adult. Similarly, sex pheromone of Cestsia flavipes showed some similar result as contacting the leaf and branch tissue of hosted plant to amplify the attraction, then to attract the male adult [21]. According to Romero et al. (2014) [22], before mating, one of the most important behaviors of Dalbulus maidis are to move for a distance and to clap the wings. However, in this trial, the C. sagittiferella female was locked up in the small cage with the roof. It was therefore proposed that the cage did not fully imitate some essential condition (if any) for mating behavior under natural condition. Thus, it may lead to low attraction of sex pheromone from virgin female on this trial.
4. Discussion
The sex pheromone of C. sagittiferella was preliminarily identified by using GC-EAD and GC-MS analysis. Although, double bond position was affirmed only for the Comp. I by DMDS derivative experiment due to low content of the other pheromone components, by reference to GC-EAD and GC-MS analysis of synthetic standards, the sex pheromone of C. sagittiferella was identified as a composition of E11-14:OAc, Z11-14:OAc and Z9, E11-14:OAc at a ratio of 99:1:trace, respectively.
Pyralidae is one of the two families of Pyraloidea with about 5.000 described species [11]. To date, sex pheromones from 39 pyralid species have been identified [12]. Most of the pheromone components were unsaturated straight chain compounds with a terminal functional group (Type I pheromone). In that, Z9, E11-14:OAc was identified as the major pheromone component of Dioryctria abietella, D. abietivorella and D. mendacella [23-25] while E11-14:OAc and Z11-14:OAc were pheromone components of Etiella behrii and E. zinckenella [26,27]. Sex pheromones of C. sagittiferella was a new identification.
Field attraction of C. sagittiferella by synthetic lures was tested at pomelo orchards where C. sagittiferella larvae were collected. Unexpectedly, lures prepared with synthetic E11-14:OAc or Z9, E11-14:OAc as major components showed no attraction to C. sagittiferella males in field (Table 1, Test 1). Moreover, addition of (3Z,6Z,9Z)-3,6,9-tricosatriene (Z3,Z6,Z9-23:H) compound as a minor component into the lures did not support the attraction (Table 1, Test 2). Sex pheromones from 36 species in Phycitinae have been identified. In most of cases, field examination of the synthetic pheromones often resulted in weak attractiveness. Since the study of Cabrera et al. (2001) [28] on the sex pheromone of Neoleucinodes elegantalis, hybrid pheromones which comprised of Type I component(s) and a polyunsaturated hydrocarbon (Type II component) have been reported for 14 species in Pyraloidea. While Z3,Z6,Z9-23:H is a common Type II component of crambid species, (Z,Z,Z,Z,Z)-3,6,9,12,15-tricosapentaene (Z3,Z6,Z9,Z12,Z15-23:H) and (Z,Z,Z,Z,Z)-3,6,9,12,15-pentacosapentaene (Z3,Z6,Z9,Z12,Z15-25:H) were recorded as active pheromone components of 7 pyralid species including Amyelois transitella and Pyralis farinalis [29], Dioryctria abietella [30], D. abietivorella [31], D. amatella [32], D. mendacella [25], Orthaga achatina [33]. A future study would clarify whether C. sagittiferella females produce Type II components by a detailed analysis of extracts of not only pheromone glands but also body surfaces, in addition to test of their role in field attraction of C. sagittiferella in the Mekong Delta of Vietnam.
Acknowledgement
This study is funded in part by the Can Tho University Improvement Project VN14-P6, supported by a Japanese Official Development Assistance (ODA) loan.
References
- Morton JF. Pummelo. In: Fruits of warm climates. Florida Flair Books, Miami (1987): 147-151.
- Waterhouse DF. The major arthropod pests and weeds of agriculture in Southeast Asia: distribution, importance and origin (1993).
- Quan VB, Vu LH, You TV. Current Situation, Consequences and Prevention Measures Citripestis sagittiferella (Lepidoptera: Pyralidae) at The Book District, Soc Trang Province. Science Journal of Can Tho University (2014): 142-148.
- Cuc NTT. Insects and mites damaging fruit trees and their natural enemies in Vietnam. Can Tho University Publisher (2015): 623pages (in Vietnamese).
- Dung TT, Pham TV, Le Van Vang CN, et al. Harm situation, morphological and biological characteristics of the pomelo fruit borer Citripestis sagittiferalis in the Mekong Delta. Science Journal of Can Tho University (2017): 64-69.
- Jeong PY, Jung M, Yim YH, et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature 433 (2005): 541-545.
- Ando T, Yamakawa R. Analysis of lepidopteran sex pheromones by mass spectrometry. TrAC Trends in Analytical Chemistry 30 (2011): 990-1002.
- Ando T, Inomata SI, Yamamoto M. Lepidopteran sex pheromones. The Chemistry of Pheromones and Other Semiochemicals I (2004): 51-96.
- Yan Q, Khanh CN, Naka H, et al. Reexamination of the female sex pheromone of the sweet potato vine borer moth: identification and field evaluation of a tricosatriene. Journal of Chemical Ecology 40 (2014): 590-598.
- Kanno H, Kuenen LP, Klingler KA, et al. Attractiveness of a four-component pheromone blend to male navel orangeworm moths. Journal of Chemical Ecology 36 (2010): 584-591.
- Solis M. Phylogenetic studies and modern classification of the Pyraloidea (Lepidoptera). Revista Colombiana de Entomología 33 (2007): 1-8.
- Ando, T. and M. Yamamoto. Internet database: https://lepipheromone.sakura.ne.jp/pdb_top.html (2020).
- Chau NQ, Pham KS. Monitoring population dynamics of the citrus pock caterpillar (Prays endocarpa) by sex pheromone traps in the Mekong Delta of Vietnam. Can Tho University Journal of Science 54 (2018): 35-39.
- Chi DT, Khanh CNQ, Nghia HT, et al. Correlation between Numbers of Captured Males by Sex Pheromone Trap and Damage Ratio of the Diamond Back moth (Plutella xylostella) in Cruciferous Vegetable Fields. Journal of Plant Protection 282 (2019): 23-31 (in Vietnamese).
- Thuy HN, Khanh CN, Son PK, et al. Sex pheromones of three citrus leafrollers, Archips atrolucens, Adoxophyes privatana, and Homona sp., inhabiting the Mekong Delta of Vietnam. Journal of Chemical Ecology 39 (2013): 783-789.
- Inomata SI, Kinjo M, Komai F, et al. Sex pheromones of five olethreutine species (Lepidoptera: Tortricidae) associated with the seedlings and fruits of mangrove plants in the Ryukyu Islands, Japan: identification and field evaluation. Journal of Chemical Ecology 31 (2005): 859-878.
- Buser HR, Arn H, Guerin P, et al. Determination of double bond position in mono-unsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Analytical Chemistry 55 (1983): 818-822.
- Xu P, Hooper AM, Pickett JA, et al. Specificity determinants of the silkworm moth sex pheromone. PloS One 7 (2012): e44190.
- Jervis MA, editor. Insects as natural enemies: a practical perspective. Springer Science & Business Media (2007).
- Deisig N, Dupuy F, Anton S, et al. Responses to pheromones in a complex odor world: sensory processing and behavior. Insects 5 (2014): 399-422.
- Joyce AL, White WH, Medina RF. Host plants impact courtship vibration transmission and mating success of a parasitoid wasp, Cotesia flavipes (Hymenoptera: Braconidae). Evolutionary Ecology 28 (2014): 361-372.
- Ramirez-Romero R, Perez-Ascencio D, Garibay-Benítez D. Courtship behavior of the corn leafhopper Dalbulus maidis (DeLong & Wolcott)(Hemiptera: Cicadellidae). Journal of Insect Behavior 27 (2014): 804-815.
- Löfstedt C, Van der Pers JN, Löfqvist J, et al. Sex pheromone of the cone pyralid Dioryctria abietella. Entomologia Experimentalis et Applicata 34 (1983): 20-26.
- Millar JG, Grant GG, McElfresh JS, et al. (3 Z, 6 Z, 9 Z, 12 Z, 15 Z)-Pentacosapentaene, a key pheromone component of the fir coneworm moth, Dioryctria abietivorella. Journal of Chemical Ecology 31 (2005): 1229-1234.
- Hall DR, Farman D, Domínguez JC, et al. Female sex pheromone of the cone moth, Dioryctria mendacella: investigation of synergism between Type I and Type II pheromone components. Journal of Chemical Ecology 43 (2017): 433-442.
- Wakamura S, Hattori M, Igita K, et al. Sex pheromone of Etiella behrii, a pod borer of soybean in Indonesia: identification and field attraction. Entomologia Experimentalis et Applicata 91 (1999): 413-420.
- Tabata J, Yokosuka T, Hattori M, et al. Sex attractant pheromone of the limabean pod borer, Etiella zinckenella (Treitschke)(Lepidoptera: Pyralidae), in Japan. Applied Entomology and Zoology 43 (2008): 351-358.
- Cabrera A, Eiras AE, Gries G, et al. Sex pheromone of tomato fruit borer, Neoleucinodes elegantalis. Journal of Chemical Ecology 27 (2001): 2097-2107.
- Leal WS, Parra-Pedrazzoli AL, Kaissling KE, et al. Unusual pheromone chemistry in the navel orangeworm: novel sex attractants and a behavioral antagonist. Naturwissenschaften 92 (2005): 139-146.
- Löfstedt C, Svensson GP, Jirle EV, et al. (3Z, 6Z, 9Z, 12Z, 15Z)-pentacosapentaene and (9Z, 11E)-tetradecadienyl acetate: sex pheromone of the spruce coneworm Dioryctria abietella (Lepidoptera: Pyralidae). Journal of Applied Entomology 136 (2012): 70-78.
- Strong WB, Millar JG, Grant GG, et al. Optimization of pheromone lure and trap design for monitoring the fir coneworm, Dioryctria abietivorella. Entomologia Experimentalis et Applicata 126 (2008): 67-77.
- Miller DR, Millar JG, Mangini A, et al. (3 Z, 6 Z, 9 Z, 12 Z, 15 Z)-Pentacosapentaene and (Z)-11-Hexadecenyl Acetate: Sex Attractant Blend for Dioryctria amatella (Lepidoptera: Pyralidae). Journal of Economic Entomology 103 (2010): 1216-1221.
- Yan Q, Li HD, Chen Y, et al. Identification and field evaluation of the sex pheromone of Orthaga achatina (Lepidoptera: Pyralidae). Journal of Chemical Ecology 44 (2018): 886-893.
- Uehara T, Naka H, Matsuyama S, et al. Identification of conjugated pentadecadienals as sex pheromone components of the sphingid moth, Dolbina tancrei. Journal of Chemical Ecology 39 (2013): 1441-1447.

 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 