Profile of Intestinal Barrier Functional Markers in Italian Patients with Diarrhea-Predominant IBS: Preliminary Data from a Low-FODMAPs diet Trial
Article Information
Riezzo Giuseppe1, Orlando Antonella1, Tutino Valeria2, Clemente Caterina1, Linsalata Michele1, Prospero Laura1, D’Attoma Benedetta1, Martulli Manuela1, Russo Francesco1*
1Laboratory of Nutritional Pathophysiology, National Institute of Gastroenterology “S. de Bellis” Research Hospital, IRCCS “Saverio de Bellis” Castellana Grotte (BA), Italy
2Laboratory of Nutritional Biochemistry, National Institute of Gastroenterology “S. de Bellis” Research Hospital, IRCCS “Saverio de Bellis” Castellana Grotte (BA), Italy
*Corresponding author: Dr. Francesco Russo, Laboratory of Nutritional Pathophysiology, National Institute of Gastroenterology “S. de Bellis” Research Hospital, IRCCS “Saverio de Bellis” Castellana Grotte (BA), Italy
Received: 14 January 2020; Accepted: 23 January 2020; Published: 03 February 2020
Citation: Riezzo Giuseppe, Orlando Antonella, Tutino Valeria, Clemente Caterina, Linsalata Michele, Prospero Laura, D’Attoma Benedetta, Martulli Manuela, Russo Francesco. Profile of Intestinal Barrier Functional Markers in Italian Patients with Diarrhea-Predominant IBS: Preliminary Data from a Low-FODMAPs diet Trial. Archives of Clinical and Biomedical Research 4 (2020): 017-031.
View / Download Pdf Share at FacebookAbstract
The intake of fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) is linked to IBS symptoms. Thus, reduced FODMAPs content in the diet may improve symptoms in IBS-D patients, mainly abdominal distension and diarrhea. The mechanism by which the low-FODMAPs diet improves symptoms is still an unsolved matter. On this basis, the study aimed to evaluate variations in the circulating levels of molecules involved in the epithelial barrier integrity and function following a low FODMAPs diet to find new diagnostic markers of IBS. In this exploratory study on the low FODMAP diet, patients suffering from IBS-D according to Rome III criteria were recruited. They performed a validated questionnaire, the Gastrointestinal Symptom Rating Scale (GSRS), the evaluation of the intestinal permeability (lactulose and mannitol absorption test), the measurement of intestinal barrier integrity using Zonulin, Intestinal fatty acid-binding proteins (I-FABP) and Diamine oxidase (DAO) concentrations, as well the skatole and indican evaluation as measure of dysbiosis. A low FODMAPs diet in patients with IBS-D reduced “abdominal pain”, “indigestion syndrome”, and “diarrhea syndrome” GSRS symptom clusters after 90 days of treatment. Besides, this diet improves intestinal functions, possibly moderating the excessive presence of serum and fecal zonulin, regulating mucosal inflammation, and imbalance in the dysbiosis fermentation processes.
Keywords
Iritable bowel syndrome; FODMAPs; Intestinal permeability; Intestinal barrier function; Zonulin; Dysbiosis
Article Details
Introduction
Irritable Bowel Syndrome (IBS) is a functional gastrointestinal (GI) disease affecting 10% -20% of the population and is composed of abdominal pain/discomfort, in combination with alterations of the stool habit [1]. IBS represents one of the most frequent referrals to a GI specialist. The diagnosis of IBS is still mainly based on the specific GI symptom questionnaires and stool characteristics and the concomitant exclusion of organic GI diseases [1]. There are at least three IBS subtypes according to the stool characteristics, namely: diarrhea (IBS-D), constipation (IBS-C), and mixed variant (IBS-M) [2]. The pathophysiology of IBS involves low-grade inflammation, abnormal motility, alterations in gut-brain communication, psychosocial factors, and possible modifications in the intestinal barrier [3].
The intestinal barrier is a complex and sophisticated structure at the border between the intestinal medium and the lumen of the gut. It protects against pathogenic microorganisms and toxins but allows the absorption of nutrients and fluids [4]. A dysfunctional gut barrier may lead to variations of small intestinal permeability (s-IP) also in functional diseases such as the IBS diarrhea (IBS-D) subgroup other than celiac disease (CD) and inflammatory bowel disease (IBD) [5,6]. The s-IP evaluation uses sugar molecules of different sizes, such as lactulose (La) and mannitol (Ma) [7]. Their excretion in the urine expressed as the La/Ma ratio provides information on the tight junction (TJ) integrity and the complete epithelial absorptive area [8]. Different proteins are involved in the integrity of the intestinal barrier. Zonulin reversibly regulates s-IP by changing the TJ protein-protein interaction [9,10]. The role of the protein has been demonstrated in CD [11] but not in IBS [12]. Intestinal fatty acid-binding proteins (I-FABP) [13,14] and diamine oxidase (DAO) [15,16] can enter the blood through a dysfunctional barrier, and they are considered markers of mucosal injury. Lastly, an imbalance in the microbiota (dysbiosis) may contribute to intestinal barrier dysfunction. Urinary quantitation of skatole (3-methyl-indole) and indican are used to diagnose the presence of a putrefactive and fermentative intestinal dysbiosis, respectively [17,18].
An altered intestinal barrier may be considered as a consequence of an imbalanced diet, and the majority of IBS subjects believe that some foods are responsible for their symptoms, tending to exclude them without, however, compromising their nutritional status[19]. Recent evidence suggests that the intake of fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) may activate a sequence of symptoms in IBS patients [20]. These peculiar characteristics of FODMAPs rely on their poor absorption in the small intestine. They reach intact the colon increasing the endoluminal water content since they have osmotic activity and inducing gas production due to their fermentation by intestinal microbiota. These events are responsible for abdominal distension and diarrhea. Available data indicate that reducing the FODMAPs content in the diet may improve symptoms complained by IBS-D patients [21].
On this basis, the present study aimed at evaluating possible variations in the circulating levels of the above-cited mediators of regulation and maintenance of epithelial barrier integrity and function following a low FODMAPs diet in an attempt to find new useful markers for the diagnosis IBS.
Materials and Methods
Patient recruitment
In this exploratory study on the low FODMAP diet, patients suffering from IBS-D according to Rome III criteria were recruited from January 2018 to August 2019, from among the outpatients of the National Institute of Gastroenterology “S. de Bellis” Research Hospital, Castellana Grotte (Bari), Italy.
All patients completed a validated questionnaire for gastrointestinal (GI) symptoms (see the “Symptom assessment” section). Besides, they underwent a physical examination, a blood withdrawal (for whole blood count, liver function tests, C-reactive protein, thyroid function test), stool culture, stool examination for parasites, fecal occult blood test. The availability of a recent gastroscopy and colonoscopy to avoid the enrolment of patients with organic diseases was also requested.
The inclusion criteria were as follows: (a) age more than 18 years; (b) a symptom profile resembling IBS-D (with active symptoms for at least two weeks) and a stool pattern, as described by Schmulson et al. [22]; (c) a minimum average of 3.0 on the seven-point Likert scale of the GSRS composite symptom [23]; (d) a diet without any restriction on eating and drinking [in particular, no previous period of gluten-free diet before examination]. Age, body mass index (BMI), alcohol intake, smoking, and use of medication were checked to obtain a homogeneous group of IBS-D.
Exclusion criteria included: pregnancy, constipation, giardiasis, post-infectious IBS, hepatic, renal, or cardiovascular disease, metabolic and endocrine disorders, a history of SSRIs and other antidepressant therapy, fever, intense physical activity, previous abdominal surgery, a history of malignancy, secondary causes of intestinal atrophy, no consumption of drugs for treating IBS in the last two weeks before evaluation, antibiotic therapy or probiotic agents, and other drugs known to cause abdominal pain. For excluding CD, a combination of tissue transglutaminase and anti-endomysium antibodies was used. Additionally, only the HLA-DQ2/HLADQ8-negative/negative IBS-D patients were recruited in this study to avoid the possible presence of gluten-sensitive diarrhea without CD that has been observed in IBS patients positive for HLA-DQ2 or HLA-DQ8 [24].
The reasons for study discontinuation were recorded in the case report form and could include lost to follow-up, withdrew consent, adverse event (speci?ed), ineligibility to continue the study, death, and other (including the administrative closure of trial).
Female patients were examined during the follicular phase of the menstrual cycle. All the subjects were compliant and were willing to participate in the study. Informed consent was obtained from all the participants for blood testing and clinical data collection. This study was part of a research project approved by the local Scienti?c and Ethics Committees of IRCCS “Saverio de Bellis”, Castellana Grotte, Bari, Italy, and it was registered on clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/NCT03423069).
Symptom Assessment
The symptom profile was investigated by administering to IBS-D patients a validated questionnaire for GI symptoms, the Gastrointestinal Symptom Rating Scale (GSRS) [23]. This questionnaire utilizes a 7-level Likert scale (1-7), based on the intensity and frequency of GI symptoms recorded during the previous week. A higher score indicates the main symptoms complained about by the patients. The 7-level scores were merged to obtain a four-level score of intensity/frequency: absent, mild, moderate, and severe. Combination scores among the items were calculated for the following domains: “abdominal pain” (abdominal pain, gastric hunger pain, and nausea), “indigestion syndrome” (abdominal distension, borborygmi, burping and flatulence), and “diarrhea syndrome” (increased frequency of evacuation, loose stools, and urgent bowel movement). The questionnaire was administered before the start of the diet and after 90 days of low FODMAPs diet administration.
Low FODMAPs diet
All patients completed a 7?day food record before the consultation, then they were submitted to a first nutritional visit with the measurement of weight, height, the calculation of BMI, and the anthropometric evaluation. During the first consultation, patients received the low FODMAPs diet, individualized for each of them using information from the food record. The low FODMAPs diet is based on limited intake of foods containing fermentable oligosaccharides, disaccharides, monosaccharides, and polyols [21]. Patients were also provided with a brochure reporting detailed information on the permitted products, which foods to avoid and which to diminish, as well as information where to buy specific foods. The dieticians guaranteed an adequate intake of fiber and calcium and provided advice on how to cook without onion and garlic. Patients were evaluated every 30 days, also by filling a food diary for checking the compliance with the diet. Lastly, patients were allowed to phone the dieticians during the diet period for all the needed information.
Sugar Absorption Tests
For the s-IP evaluation, the participants drank a sugar test solution containing 10 g of La, 5 g of Ma, and 40 g of Sucralose as an osmotic filler in 100 mL water in the morning after an overnight fast. Participants then collected urine for the subsequent ?ve hours. Samples of urine were stored at −80°C until analysis. The urinary detection and measurement of each sugar was performed by chromatographic analysis, as described previously [25]. High-performance anion-exchange chromatography coupled with pulsed amperometric detection was performed on a Dionex Model ICS-5000 with a gold working electrode and a 25μl peek sample loop (Dionex Corp., Sunnyvale, California, USA). The carbohydrate separation was conducted by CarboPac PA-10 pellicular anion-exchange resin connected to a CarboPac PA-10 guard column (Thermo?sher Scienti?c, Waltham, Massachusetts, USA) at 30°C. The samples were eluted with 50mmol/l NaOH at a ?ow rate of 1ml/min. The percentages of ingested sugars in urine were evaluated, and the La/Ma ratio was calculated for each sample. Based on data from controls in our laboratory, La/Ma≥0.035 is indicative of increased intestinal permeability [26].
Measurements of zonulin, I-FABP, and DAO
All the analytical measurements were performed at the time of enrolment and the end of treatment after 90 days of diet in blind coded samples (no name or personal identi?ers). Peripheral venous blood samples were obtained from participants in the study in the fasting state at least twelve hours after the last meal. After allowing clotting for at least 30min, the samples were centrifuged at 1600 ×g for 15min. Zonulin was assayed in serum and stool samples by using a competitive ELISA kit (Immunodiagnostik AG, Bensheim, Germany). Serum levels of I-FABP were evaluated by enzyme-link immunosorbent assay (ELISA) using a specific anti-human I-FABP antibody (Thermo Fisher Scientific, Waltham, Massachusetts, USA). A commercially available ELISA Kit (Cloud-Clone Corp. Houston, USA) was used to determine DAO levels.
Skatole and indican evaluation
All patients collected a sample of urine in the morning. The detection and measurement of skatole in urine were performed by the 3-methylindole kit (Eureka Lab Division, Chiaravalle, AN, Italy) on a Thermo Scientific model Dionex HPLC system (Sunnyvale, CA, USA) consisting of an UltiMate 3000 pump and a Rheodyne injector with a 20-µL loop. Samples, calibrators, and quality controls were prepared according to the manufacturer's instructions. In detail, 950 μL of buffer reagent and 20 μL of the internal standard were added to 50 μL of a urinary sample. Samples were vortexed, and 20 μL was injected into the HPLC system. Skatole separation was carried out by a Poroshell 120 EC-C18 column (2.7µm, 50 x 4,6 mm; Agilent, Santa Clara, CA, USA) and a buffer flow rate of 1.0 ml/min. The sample run was 15 min, and spectrofluorimetric detector wavelengths were set at 280 nm (excitation) and 360 nm (emission). For urinary indican determination, a standard colorimetric assay kit was used according to the procedures indicated by the manufacturer. Urinary skatole and indican were considered normal for values lower than 10 µg/l and 10 mg/l, respectively [27].
Statistical Analysis
All results are expressed as mean± SEM or median and range in the case of continuous or discrete variables, respectively. Wilcoxon matched-pairs signed-rank test was performed for all the variables to avoid the assumption of the normal distribution. All the di?erences were considered signi?cant at a 5% level. A speci?c statistical package for exact nonparametric inference (2005 Stata Statistical Software Release 9; Stata Corp., College Station, Texas, USA) was used.
Results
Figure 1 shows the flow of participants through the study. Twenty D-IBS patients (3 men and 17 women; mean age = 32.3± 9.7yrs.; BMI= 23.4±4.1) completed the study.
Table 1 shows the symptom score calculated with GSRS as single symptoms and symptom clusters in the IBS-D patients before and after diet.
|
Parameters |
IBS-D before diet |
IBS-D after diet |
P |
|
GSRS single items |
|||
|
Nausea/vomiting |
2 [1-3] |
1 [1-3] |
n.s. |
|
Abdominal pain (colic pain) |
3 [1-4] |
2 [1-4] |
0.033 |
|
Gastric hunger pain |
2 [1-4] |
2 [1-3] |
n.s. |
|
Abdominal distension |
3 [1-4] |
2 [1-3] |
0.024 |
|
Burping |
2 [1-4] |
2 [1-3] |
n.s. |
|
Borborygmi |
3 [2-4] |
2 [2-3] |
0.006 |
|
Flatulence |
3 [2-4] |
2 [2-3] |
0.020 |
|
Increased passage of stools |
2 [1-3] |
1 [1-3] |
0.021 |
|
Loose stools |
3 [1-3.5] |
2 [1-4] |
0.019 |
|
Urgent bowel movement |
3 [1-3] |
2 [1-3] |
0.006 |
|
Total score |
35[21-45] |
27 [16-37] |
0.001 |
|
GSRS combination scores |
|||
|
Abdominal pain |
6 [3-9.5] |
5 [3-8] |
0.012 |
|
Indigestion syndrome |
10 [7-15.0] |
8 [4-21.0] |
0.005 |
|
Diarrhea syndrome |
7 [4-9.5] |
5 [3-7] |
0.001 |
IBS-D = diarrhea-predominant IBS. Data are expressed as Median and range. All data were analyzes by Wilcoxon test.
Table 1: Clinical data (GSRS items) of IBS-D patients before and after lowFODMAPs diet
When the before-after diet comparison was performed, the GSRS items abdominal pain, abdominal distension, borborygmi, and flatulence, as well as the increased passage of stools, loose stools, and urgent bowel movement significantly reduced after 90 days of low FODMAPs diet. As expected, the GSRS total score and symptom clusters, namely “abdominal pain”, “indigestion syndrome “, and “diarrhea syndrome” were found significantly reduced after diet.
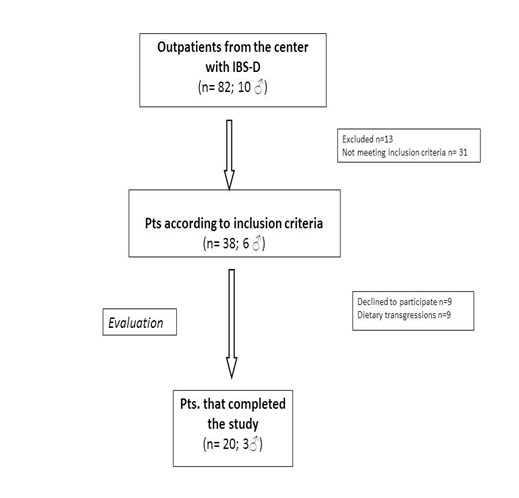
The IBS-D patients underwent s-IP testing before and after the low FODMAPs diet (Figure 2). As for the percentage of lactulose urinary recovery (%La) (Figure 2A), no significant modifications (p=0.061) were present in IBS-D patients after the diet compared to their values at the beginning of the study, although a slight %La decrease was observed (0.36±0.08 vs. 0.29±0.04). By opposite, patients in the study showed significantly (p= 0.0137) lower %Ma at the end of treatment (14.29±0.76 vs. 13.34±0.77) (Figure 2B). Consequently, the La/Ma ratio changed after 90 days of low FODMAPS diet, even if without statistical significance (0.026±0.005 vs. 0.024±0.004; p= 0.78) (Figure 2C).
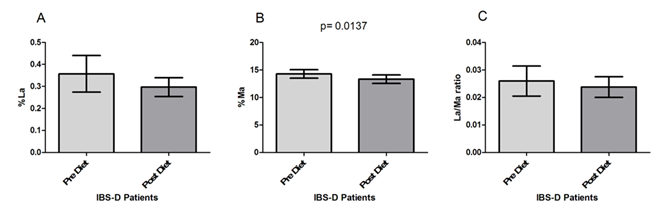
Figure 3 reports the serum and fecal levels of Zonulin. As for serum concentration (Fig. 3, Panel A), zonulin decreased significantly at the end of the diet period (63.14±3.43 ng/ml vs. 52.06±2.78 ng/ml; p= 0.0046). The fecal concentrations of Zonulin levels also decreased significantly at the end of the diet (139.7±19.89 ng/ml vs. 96.18±12.76 ng/ml; p= 0.013), (Fig. 3, Panel B).
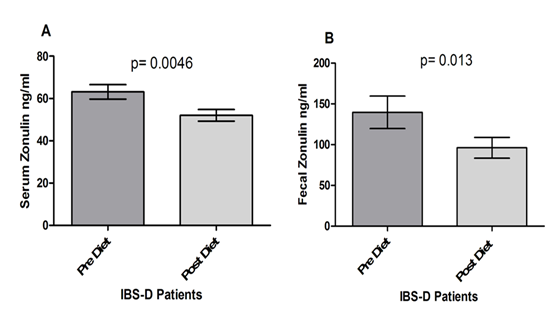
Figure 4 shows the serum levels of DAO and I-FABP. DAO levels at the end of the diet period were significantly lower compared to values at the start of the treatment (40.39±2.573 vs. 35.66±1.706; p= 0.0349) (Fig. 4 Panel A). I-FABP levels were also reduced by the FODMAPs diet in IBS-D patients, although without reaching statistical significance (1.589±0.214 vs. 1.382±0.177; p= 0.204), (Figure 4 Panel B).
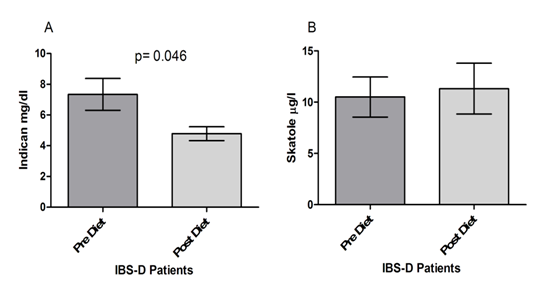
Finally, as concerns the dysbiosis test (Figure 5), patients in the study showed significantly lower urinary concentrations of indican at the end of treatment than those at the start of the trial (7.338±1.037 mg/dl vs. 4.787±0.453 mg/dl; p=0.046) (Fig. 5, Panel A). By opposite, no significant differences were found as concerns skatole in IBS-D before and after the low FODMAPs diet (10.50±1.961 µg/l vs. 11.32±2.474 µg/l; p=0.046) (Figure 5, Panel B).
Discussion
Our study demonstrated that a long lasting low FODMAPs diet significantly reduced symptoms in patients with IBS-D. The mechanism by which the low-FODMAPs diet improves symptoms ameliorating the intestinal barrier function via the reduction in serum and fecal zonulin, reduced mucosal inflammation and dysbiosis fermentation processes. In the last years, several data have demonstrated that a diet low in FODMAPs is useful in the management of functional GI symptoms, but there is limited evidence of its efficacy in Italian IBS-D patients. A recent meta-analysis [21] reported that patients receiving a low-FODMAP diet experienced statistically significant pain and bloating reduction compared with those receiving a traditional diet, while there was no significant difference regarding stool consistency. In this preliminary study, “abdominal pain”, “indigestion syndrome”, and “diarrhea syndrome” clusters were found to be significantly reduced after 90 days of a low-FODMAPs diet. This datum was probably due to different reasons such as a) a different diet in Italy compared to other western diets, b) the symptoms in the basal state reported by the IBS-D patients, and lastly c) the choice to perform the comparison among IBS-D patients before and after a long-lasting dietician-controlled low-FODMAPs diet. The decision to evaluate the efficacy of the low-FODMAPs diet in a long-lasting period in IBS-D patients is certainly more representative of the magnitude of effect on GI symptoms than just a 30-day diet, due to symptoms fluctuations that occur naturally in all the IBS subtypes irrespective of medications.
The mechanism by which the low-FODMAPs diet improves symptoms is still an unsolved matter. In this field, the exact role of the gut barrier function as part of the pathophysiology of IBS continues to be debated [28]. A recent experimental study described an increase in gut permeability in mice fed with a 2-week high FODMAP diet compared to mice fed a standard diet [29]. The same study also showed that a 4-week low FODMAP diet normalized IP in rodents undergoing high-stress levels. In humans, some authors found altered barrier with increased permeability (or “leakiness”) only in a proportion of IBS-D patients, while others failed in finding significant differences between IBS patients and healthy people [26,30]. In this context, also in our study, a few number of patients (4 out 20) had an altered s-IP at the start of the study.
Interestingly, our IBS-D patients showed a significantly lower Ma%, although the La/Ma ratio did not at the end of the diet. Typically, Ma is considered a marker of permeability of the small intestine (loss of absorbent area), while lactulose is a marker of permeability of the damaged small intestine [31]. The reduction of mannitol, therefore, could represent an improvement in food intolerance/malabsorption in the small intestine after the low FODMAPs diet. The absence of significant modifications in the lactulose concentration could probably either be due to the low number of patients, so far studied or the moderate s-IP alterations.
Among the proteins involved in the integrity of the intestinal barrier function, zonulin is considered as a biomarker of poor health, as in the case of obesity and insulin resistance [32]. However, the degree of correlation between zonulin and inflammation and between zonulin and epithelial permeability remains to be determined, even if serum zonulin is elevated in IBD patients [33]. As regards IBS-D, the serum concentrations of zonulin in patients were similar to healthy controls (HC) [34], even grouping IBS-D patients according to a normal or altered s-IP [26]. In another paper, serum levels of zonulin from IBS patients were similar to those observed in CD patients and higher than those in HC [12, 25]. The discrepancy among authors on the role of serum zonulin in IBS-D patients could be due to a high number of proteins participating in the regulation of TJs and IP, or the specificity of the ELISA test. Recent studies suggest a high level of cross-reactivity between zonulin and the serum protein properdin, which may impair the assay sensitivity [35]. Serum zonulin is a product originating from several different tissues apart from the GI tract. Therefore fecal zonulin can be considered a more specific marker for impaired IP because it is the result of its leaking into the lumen in the case of weakened intestinal barrier [33]. Besides, fecal zonulin induces the passage of luminal contents across the epithelial barrier, causing the release of pro-inflammatory cytokines [9]. Therefore, reduction in zonulin concentration could not only reflect ameliorated IP but could also indicate a reduced mucosal inflammation after the low FODMAPs diet.
Serum I-FABP has been related to several GI conditions [36-38], particularly CD. I-FABP can detect gluten-induced damage in the early CD diagnosis and follow-up [39, 40], as well in patients with non-celiac wheat sensitivity [41]. As for IBS, a recent paper calculated s-IP by the La/Ma ratio in IBS-D patients [26] putting in evidence an I-FABP concentration similar to those found in CD in the IBS-D subgroup with altered s-IP, and significantly different from those found in the IBS-D patients with normal s-IP and HC. This evidence suggests the IBS-D patients are a heterogeneous group for etiology (post-infective or not) and pathogenesis (loss of integrity of the intestinal epithelium or not). As regards the role of a low FODMAPs diet, a short-term low FODMAPs diet improved GI symptoms and mucosal function in runners [42]. The low FODMAPs diet improved the symptom profile even though differences in I-FABP concentrations were not evident. The same was true in our study since symptoms improved without clear differences in the I-FABP levels before and after diet. The absence of change in I-FABP has to be considered with caution being I-FABP just one of the several factors that play a role in the integrity of barrier function, independently of direct enterocyte damage [43].
Serum levels of DAO correlate inversely with s-IP [16]. Serum DAO activity is significantly decreased in patients with GI and extra-GI diseases, such as malabsorption syndromes [44], CD [45], Crohn's disease [46] as well as IBS-D patients [47]. Interestingly, IBS-D patients with altered s-IP showed similar values of DAO to those found in CD patients, and significantly higher than those observed in the IBS-D patients with normal s-IP [26]. In the present study, DAO levels were reduced considerably by the low-FODMAPs diet. These findings are in agreement with data from IBS-D patients but are in contrast with increased DAO correlated to symptom improvement observed after diet in patients with histamine intolerance (HIT) [48]. Clearly, IBS and HIT do not share the same pathophysiology since HIT is linked to a reduced serum DAO, while IBS-D shows an elevated serum DAO respect to controls. Besides, serum DAO values are not fully established to reflect DAO located in the gut, and the evaluation of the serum DAO by currently available assays has several limitations [49]. Therefore, the diagnostic value of the serum DAO has yet to be confirmed.
Lastly, it has been proposed that alterations in the balance between microbiome composition and function (dysbiosis) could have broad impacts on the functions of the gut in IBS [50]. The presence of a putrefactive and fermentative intestinal dysbiosis is currently diagnosed by the measure of skatole and indican, respectively [17, 18]. Fermentative dysbiosis is due to small intestinal bacterial overgrowth linked to an unbalanced dietary intake of fibers, proteins, and fats. Putrefactive dysbiosis is due to non-absorbed sugar hydrolyzation by several bacteria strains in the colon [27]. The low FODMAPS diet excludes the non-absorbable foods from the diet, and it has been proven useful in the treatment of IBS-D symptoms, namely abdominal pain, abdominal distension, and diarrhea [20]. Our data put in evidence a significantly different concentration of indican but not skatole, suggesting an improved bacterial composition in the small intestine of the IBS-D patients induced by the low FODMAPs diet.
The results of our study further confirm the concept that the intestinal barrier is a dynamic system that responds to humoral signals and various types of molecules. An unbalanced diet rich in FODMAP in patients with IBS-D can be responsible for symptoms linked to altered IP (probably in the transcellular pathway), malabsorption, minimal inflammation, and fermentative intestinal dysbiosis. In this perspective, a low FODMAPs diet seems to improve intestinal functions, possibly stimulating a bacterial flora moderating the excessive presence of fecal zonulin and regulating imbalance in the dysbiosis fermentation processes.
Authorship and contributorship
Russo F and Riezzo G were responsible for data analysis and interpretation, wrote the manuscript and contributed to the intellectual content of the article; Orlando A and Tutino V performed the nutritional visits, administered the diet, collected and interpreted the data Linsalata M and Clemente C performed the experiments; Prospero L, D’Attoma B, and Martulli M collected and interpreted the data and made intellectual contributions to the article. All authors participated in the study to a significant extent and read and approved the submitted manuscript.
Funding information / Competing interest
This study was supported by the Italian Ministry of Health (RC26-2019). All authors do not report any relevant affiliation or financial involvement with any Organization or entity with financial interest or in financial conflict with the subject matter or materials discussed in the manuscript . No writing assistance was utilized in the production of manuscript.
References
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. Jama 313 (2015): 949-58.
- Bai T, Xia J, Jiang Y, Cao H, Zhao Y, Zhang L, et al. Comparison of the Rome IV and Rome III criteria for IBS diagnosis: A cross-sectional survey. Journal of gastroenterology and hepatology 32 (2017): 1018-10125.
- Akiho H, Ihara E, Nakamura K. (2010) Low-grade inflammation plays a pivotal role in gastrointestinal dysfunction in irritable bowel syndrome. World journal of gastrointestinal pathophysiology 1 (2010): 97-105.
- Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, et al. Homeostasis of the gut barrier and potential biomarkers. American journal of physiology. Gastrointestinal and liver physiology 312 (2017): G171-G93.
- Khan I, Ullah N, Zha L, Bai Y, Khan A, et al. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 8 (2019): 126-129.
- Schumann M, Siegmund B, Schulzke JD, Fromm M. Celiac Disease: Role of the Epithelial Barrier. Cellular and molecular gastroenterology and hepatology 3 (2017): 150-62.
- Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, et al. Intestinal permeability--a new target for disease prevention and therapy. BMC gastroenterology 14 (2014): 189.
- Aguirre Valadez JM, Rivera-Espinosa L, Mendez-Guerrero O, Chavez-Pacheco JL, Garcia Juarez I, Torre A. Intestinal permeability in a patient with liver cirrhosis. Therapeutics and clinical risk management 12 (2016): 1729-1748.
- Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiological reviews 91 (2011): 151-175.
- Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue barriers 4 (2016): e1251384.
- Fasano A, Not T, Wang W, Uzzau S, Berti I, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 355 (2000): 1518-1519.
- Singh P, Silvester J, Chen X, Xu H, Sawhney V, et al. Serum zonulin is elevated in IBS and correlates with stool frequency in IBS-D. United European gastroenterology journal 7 (2019): 709-715.
- Pelsers MM, Hermens WT, Glatz JF. (2005) Fatty acid-binding proteins as plasma markers of tissue injury. Clinica chimica acta; international journal of clinical chemistry 352 (2019): 15-35.
- Besnard P, Niot I, Poirier H, Clement L, Bernard A. New insights into the fatty acid-binding protein (FABP) family in the small intestine. Molecular and cellular biochemistry 239 (2002): 139-147.
- Song WB, Lv YH, Zhang ZS, Li YN, Xiao LP, et al. Soluble intercellular adhesion molecule-1, D-lactate and diamine oxidase in patients with inflammatory bowel disease. World journal of gastroenterology 15 (2009): 3916-3919.
- Luk GD, Bayless TM, Baylin SB. Diamine oxidase (histaminase). A circulating marker for rat intestinal mucosal maturation and integrity. The Journal of clinical investigation 66 (1980): 66-70.
- Gao J, Xu K, Liu H, Liu G, Bai M, et al. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Frontiers in cellular and infection microbiology 8 (2018): 13.
- Hawrelak JA, Myers SP. The causes of intestinal dysbiosis: a review. Alternative medicine review : a journal of clinical therapeutic 9 (2004): 180-197.
- Camilleri M, Lyle BJ, Madsen KL, Sonnenburg J, Verbeke K, Wu GD. Role for diet in normal gut barrier function: developing guidance within the framework of food-labeling regulations. American journal of physiology. Gastrointestinal and liver physiology 317 (2019): G17-G39.
- Tuck CJ, Reed DE, Muir JG, Vanner SJ. Implementation of the low FODMAP diet in functional gastrointestinal symptoms: A real-world experience. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 32 (2020): e13730.
- Altobelli E, Del Negro V, Angeletti PM, Latella G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients 9 (2017): 940
- Schmulson MJ, Drossman DA. What Is New in Rome IV. Journal of neurogastroenterology and motility 23 (2017): 151-163.
- Kulich KR, Madisch A, Pacini F, Pique JM, Regula J, et al. Reliability and validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in dyspepsia: a six-country study. Health and quality of life outcomes 6 (2008): 12.
- Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the "no man's land" of gluten sensitivity. The American journal of gastroenterology 104 (2009): 1587-1594.
- Linsalata M, D'Attoma B, Orlando A, Guerra V, Russo F. Comparison of an enzymatic assay with liquid chromatography-pulsed amperometric detection for the determination of lactulose and mannitol in urine of healthy subjects and patients with active celiac disease. Clinical chemistry and laboratory medicine 52 (2014): e61-e64.
- Linsalata M, Riezzo G, D'Attoma B, Clemente C, Orlando A, Russo F. Noninvasive biomarkers of gut barrier function identify two subtypes of patients suffering from diarrhoea predominant-IBS: a case-control study. BMC gastroenterology 18 (2018): 167.
- Simeoni M, Citraro ML, Cerantonio A, Deodato F, Provenzano M, et al. An open-label, randomized, placebo-controlled study on the effectiveness of a novel probiotics administration protocol (ProbiotiCKD) in patients with mild renal insufficiency (stage 3a of CKD). European journal of nutrition 58 (2019): 2145-2156.
- Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut 66 (2017): 1517-1527.
- Zhou SY, Gillilland M, 3rd, Wu X, Leelasinjaroen P, Zhang G, et al. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. The Journal of clinical investigation 128 (2018): 267-280.
- Martinez C, Gonzalez-Castro A, Vicario M, Santos J. Cellular and molecular basis of intestinal barrier dysfunction in the irritable bowel syndrome. Gut and liver 6 (2012): 305-315.
- Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut 55 (2006): 1512-1520.
- Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernandez-Real JM. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PloS one 7 (2012): e37160.
- Malickova K, Francova I, Lukas M, Kolar M, Kralikova E, et al. Fecal zonulin is elevated in Crohn's disease and in cigarette smokers. Practical laboratory medicine 9 (2017): 39-44.
- Barbaro MR, Cremon C, Caio G, De Giorgio R, Volta U, et al. Zonulin serum levels are increased in non-celiac gluten sensitivity and irritable bowel syndrome with diarrhea. Gastroenterology 148(suppl.1) (2015): S-56
- Scheffler L, Crane A, Heyne H, Tonjes A, Schleinitz D, et al. Widely Used Commercial ELISA Does Not Detect Precursor of Haptoglobin2, but Recognizes Properdin as a Potential Second Member of the Zonulin Family. Frontiers in endocrinology 9 (2018): 22.
- Sarikaya M, Ergul B, Dogan Z, Filik L, Can M, Arslan L. Intestinal fatty acid binding protein (I-FABP) as a promising test for Crohn's disease: a preliminary study. Clinical laboratory 61 (2015): 87-91.
- Vogt A, Reuken PA, Stengel S, Stallmach A, Bruns T. Dual-sugar tests of small intestinal permeability are poor predictors of bacterial infections and mortality in cirrhosis: A prospective study. World journal of gastroenterology 22 (2016): 3275-3284.
- Abu Faddan NH, Sherif TM, Mohammed OA, Nasif KA, El Gezawy EM. Intestinal barrier integrity and function in infants with cholestasis. Intestinal research 15 (2017): 118-123.
- Adriaanse MP, Leffler DA, Kelly CP, Schuppan D, Najarian RM, et al. Serum I-FABP Detects Gluten Responsiveness in Adult Celiac Disease Patients on a Short-Term Gluten Challenge. The American journal of gastroenterology 111 (2016): 1014-1022.
- Adriaanse MP, Leffler DA, Kelly CP, Schuppan D, Najarian RM, et al. Serum I-FABP Detects Gluten Responsiveness in Adult Celiac Disease Patients on a Short-Term Gluten Challenge. The American journal of gastroenterology 111 (2016): 1014-1022.
- Uhde M, Ajamian M, Caio G, De Giorgio R, Indart A, et al. (2016) Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 65 (2016): 1930-1937.
- Wiffin M, Smith L, Antonio J, Johnstone J, Beasley L, Roberts J. Effect of a short-term low fermentable oligiosaccharide, disaccharide, monosaccharide and polyol (FODMAP) diet on exercise-related gastrointestinal symptoms. Journal of the International Society of Sports Nutrition 16 (2019): 1.
- March DS, Marchbank T, Playford RJ, Jones AW, Thatcher R, Davison G. Intestinal fatty acid-binding protein and gut permeability responses to exercise. European journal of applied physiology 117 (2017): 931-941.
- D'Agostino L, Ciacci C, Daniele B, Barone MV, Sollazzo R, Mazzacca G. Postheparin plasma diamine oxidase in subjects with small bowel mucosal atrophy. Digestive diseases and sciences 32 (1987):313-317.
- Corazza GR, Falasca A, Strocchi A, Rossi CA, Gasbarrini G. Decreased plasma postheparin diamine oxidase levels in celiac disease. Digestive diseases and sciences 33 (1988): 956-961.
- Honzawa Y, Nakase H, Matsuura M, Chiba T. Clinical significance of serum diamine oxidase activity in inflammatory bowel disease: Importance of evaluation of small intestinal permeability. Inflamm Bowel Dis 17 (2011): E23-E25.
- Xu XJ, Zhang YL, Liu L, Pan L, Yao SK. Increased expression of nerve growth factor correlates with visceral hypersensitivity and impaired gut barrier function in diarrhoea-predominant irritable bowel syndrome: a preliminary explorative study. Alimentary pharmacology & therapeutics 45 (2017): 100-114.
- Lackner S, Malcher V, Enko D, Mangge H, Holasek SJ, Schnedl WJ. Histamine-reduced diet and increase of serum diamine oxidase correlating to diet compliance in histamine intolerance. European journal of clinical nutrition 73 (2019): 102-104.
- Boehm T, Pils S, Gludovacz E, Szoelloesi H, Petroczi K, et al. Quantification of human diamine oxidase. Clinical biochemistry 50 (2017): 444-451
- Casen C, Vebo HC, Sekelja M, Hegge FT, Karlsson MK, et al. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Alimentary pharmacology & therapeutics 42 (2015): 71-83.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 