Protective Effect of Environmental Enrichment on the Morphology of Neurons in the Motor Cortex of Diabetic and Stressed Rats
Article Information
Narendra Pamidi 1, Christina Gertrude Yap1*, Satheesha Nayak B2
1Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Kuala Lumpur, Malaysia
2Department of Anatomy, Melaka Manipal Medical College, Manipal University, Manipal, Karnataka, India
*Corresponding Author: Christina Gertrude Yap, Jeffrey Cheah School of Medicine and Health Sciences, Monash University, Sunway campus, Kuala Lumpur, Malaysia – 46150
Received: 12 November 2019; Accepted: 09 December 2019; Published: 12 December 2019
Citation: Narendra Pamidi, Christina Gertrude Yap, Satheesha Nayak B. Protective Effect of Environmental Enrichment on the Morphology of Neurons in the Motor Cortex of Diabetic and Stressed Rats. Arch Biochem Mol Biol 10 (2019): 052-070.
View / Download Pdf Share at FacebookAbstract
Background: The present study aimed to evaluate the effects of EE on the morphology of pyramidal neuron at the motor cortex of diabetic and stressed rats.
Methods and materials: Male Wistar rats were grouped into Normal Control (NC), Vehicle Control (VC), Diabetes (D), Diabetes + Stress (D+S), Diabetes + Environmental Enrichment (D+EE) and Diabetes + Stress +Environmental Enrichment (D+S+EE) (n=8). Hyperglycemia was induced in Westar rats using streptozotocin (40mg/kg; ip). Blood sugar levels and body weight was measured at regular intervals to monitor the development of hyperglycemia. All experimental groups were housed in standard cages throughout the experiment. Rats in groups D+S and D+S+EE were transferred into space restrained cages for 6 hours daily. D+S+EE group were transferred into EE cages immediately after the space restrained session for subsequent 6 hours daily. On day 30, all rats were sacrificed and brains were harvested and prepared for rapid Golgi staining protocol. Dendritic branchings and dendritic intersections of the motor cortex neurons were quantitated using a camera lucida attached to Biolux research microscope. Data was analyzed using ANOVA with Bonferroni’s test.
Result: Hyperglycemia was developed in all experimental groups (Blood glucose > 250 mg/dl, p<0.001) on Day 2 post STZ injection. Exposure to EE did not show any evidence in reducing blood sugar levels in D+EE and D+S+EE groups. EE treated groups (D+EE; D+S+EE) exhibited significant prevention (D+EE vs. D: p<0.001; D+S+EE vs. D+S: p<0.001) of body weight reduction on Day 30 in comparison to D and D+S groups. Overall, significantly lower numbers of total apical (p<0.001) and basal (p<0.001) dendritic branching points was observed in groups D and D+S relative to NC group. On the other hand, D+EE and D+S+EE groups demonstrated significantly higher numbers of total apical (p<0
Keywords
Diabetes; Stress; Motor cortex; Neurons; Dendritic branching; Enriched environment
Diabetes articles, Stress articles, Motor cortex articles, Neurons articles, Dendritic branching articles, Enriched environment articles
Article Details
1. Introduction
Diabetes mellitus (DM) is a complex metabolic disorder characterized by hyperglycemia. In general hyperglycemia is preceded by progressive reduction of beta-cell mass which leads to insufficient insulin secretion (type 1 DM) or insulin resistance where the insulin is unable to facilitate glucose entry into target cells (type 2 DM; gestational diabetes) [1]. Persistent insulin resistance will challenge the secretory capacity of beta-cells and subsequently lead to beta-cell dysfunction [1]. The challenge in managing DM patients is the late onset of clinical symptoms and often at the time of diagnosis patients already present with complications [2]. Broadly, the complications of DM can be classified as: (i) Microvascular complications: retinopathy, nephropathy and neuropathy and (ii) Macrovascular complications: cerebrovascular disease (risk for stroke); cardiovascular disease (risk of heart attack); and peripheral vascular disease, (poor circulation to the limbs).
Diabetic neuropathy (DN) is a common complication in prolonged diabetes. In the hyperglycemic milieu excess glucose molecules are metabolized through the polyol pathway that produces excessive reactive oxygen species (ROS), advanced glycation-end (AGEs) and other cytokines, which lead to nerve injury [3]. Since DM is a systemic disease, the entire nervous system is vulnerable to injury and damage, which includes both the somatic and autonomic nervous system. 50% of diabetic peripheral neuropathies are asymptomatic [4] and if not recognized and implement preventive measures early enough patients will be at risk of serious injuries and often lead to lower limbs amputations. Peripheral neuropathies include motor and sensory polyneuropathies. The motor cortex is the region of the brain where neural impulses that control the execution of movements are generated. Motor cortex transmits information of planned movements to other brain areas (cerebellum and basal ganglia) for refined discharge of the movements [5].
Environmental enrichment (EE) exposure has been used as a therapeutic measure for managing fear, abnormal behaviors, and stress in laboratory animals [6]. Studies have provided evidence that EE enhanced CNS activities at the functional, anatomical, and molecular levels, both during the critical period and during adulthood. Enhanced environmental stimuli induced cellular activities in the intricate neuronal network to promote synaptic efficacy and improve signal transmission in motor neurons [7]. Therapeutic effects of EE have also been demonstrated in Alzheimer’s [8] and Parkinson’s [9] diseases.
Since DM is an incurable metabolic disorder people leaving with DM are often dependent on varying extent of pharmacological interventions depending on their individual health condition. In addition to the later, DM patients are strictly required to adapt into healthy lifestyle modifications which might even require alterations to their cultural norms [10]. Such changes may initiate psychological issues and will develop perception of poor quality of life in affected individuals. Common psychological issues associated with DM are stress, depression and anxiety [11]. Stress alters metabolic processes therefore, will impact on exacerbation of hyperglycemia and its complications [12].
Hyperglycemia and stress are two inevitable events in diabetes that contribute to the pathogenesis of diabetic neuropathy. Theoretically, if EE is able to enhance CNS activities, similar principles could also apply to neuroprotection of the neurons at the motor cortex. To date the therapeutic effects of EE on neuronal morphology in DM and stress have not been conclusively demonstrated.
Therefore, the aim of this study is to evaluate the effects of EE on changes of motor neuron morphology in diabetic and stressed rats.
2. Materials and methods
2.1. Animals and experimental groups
5 week old male albino rats (Wistar strain) were used. Animals were housed in a 12/12 hour dark/light environment at our animal holding facility. All the animals were allowed water ad libitum and fed with standard rat pellet (Hindustan lever, India). Research protocol was approved by our institutional animal ethical committee for experimental procedures (IAEC/KMC/07/2007–2008). Animals were divided into Normal Control (NC), Vehicle Control (VC), Diabetes (D), Diabetes + Stress (D + S), Diabetes + Environmental enrichment (D + EE), Diabetes + Stress + Environmental enrichment (D + S + EE) groups (n=8). NC group rats were continued in their home cage and VC group rats were fed with citric acid buffer solution (pH 4.5) as vehicle for STZ
2.2. Experimental induction of diabetes with STZ
STZ solution (10 mg/ml) was prepared by dissolving STZ powder (Sigma, St, Louis Mo., USA), in ice-cold citrate buffer (pH 4.5). STZ solution was kept chilled in a tray of ice cubes to prevent decomposition of STZ at room temperature. Diabetes was induced in D, D+S, D+EE, D+S+EE groups with a injection of STZ (40 mg/kg body weight; ip). STZ induces destruction of beta cells in the pancreas. Thereafter, diabetic rats were fed with 5% glucose solution to sustain drug-induced hyperglycemia after STZ injection.
2.3. Blood glucose and body weight measurement
To confirm and maintain the consistency of diabetic status, blood glucose levels and body weight of rats were measured on Day 0 (before STZ injection), Day 2, Day 15 and Day 30 (before sacrifice) respectively after STZ injection. Blood samples were collected from tail vein and glucose levels were estimated using ‘Accu-Chek Advantage Glucose Monitor’. The normal reference limits for non-fasted blood glucose levels in rats are 86 –162 mg/dl [13]. Rats having blood glucose levels above 225 mg/dl after the injection of STZ were considered being diabetic and were used for the experiment.
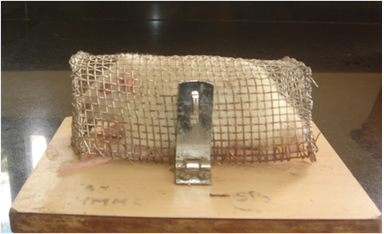
2.4. Restraint stress protocol (Figure 1)
Restraint stress was introduced on Day 2 post STZ injection after confirming the rats are diabetes by measuring blood glucose levels. A wire mesh restrainer cage constructed with 12 cm (l) x 5.5 cm (h) x 5.5 cm (w) dimensions. It has a wooden base and a stainless steel wire mesh restrainer provided with air holes for ventilation. Rats in D+S and D+S+EE groups were exposed to 6 hours of restraint stress daily at a consistent time during the day from Day 2 till Day 30 of experiment. On completion of the stress session daily, rats were returned to their home cages. Rats were given access to food before the stress and after the stress sessions. After the final stress session (Day 30), rats were weighed and blood glucose levels were assessed to ensure that hyperglycemia had been maintained in both the groups.
This is a photograph of an experimental rat in the wire mesh restrainer cage. Rats in groups. On Day 2 till Day 30 post STZ injection, rats in groups D+S and D+S+EE were placed individually in this cage at a consistent time for 6 hours per day to induced stress. D+S group will be returned into their normal housing cage after “restrained stress” sessions, while the D+S+EE group will be transferred into the EE cage for 6 hours before returning into their normal housing cage.
2.5. Enriched environment/ Environmental enrichment (EE) protocol (Figure 2)
EE protocol was also introduced on Day 2 post STZ injection after confirming the diabetic status in the rats. EE was created in a large wooden cage with dimensions: 50 cm Length (l) x 50 cm (w) x 29 cm (h). EE cages were fitted with a variety of objects, including rotating wheels, plastic tubes and objects of different dimensions, which allowed the rats to explore and interact with different objects. The orientations and types of objects in the EE cages were different each day in order to induce novel stimulation in rats. Rats in D+EE and D+S+EE groups were housed in EE cages for 6 hours daily until the end of the experiment (Day 30). Animals were placed in the enriched cages in groups of 3-4 rats at each session. After EE exposure they were returned into their standard cages.
Photograph showing experimental rats in EE simulation cages. The orientation and types items in each cage will be changed every day to induce cognitive stimulation. Day 2 till Day 30 post STZ injection rats in groups D+EE and D+S+EE will be placed in these cages (3-4 rats per cage) at a consistent time for 6 hours per day. After 6 hours exposure to EE all rats were returned into their normal housing cages.
2.6. Rapid Golgi staining [14]
Chemicals and material used
Potassium dichromate (MERCK-INDIA), Chloral hydrate (Sigma,USA), Glutaraldehyde (SD Fine chemicals, India), Formaldehyde (SD Fine chemicals, India), Dimethyl sulphoxide (SD Fine chemicals, India), Silver nitrate (SD Fine chemicals, India), Paraffin wax (Melting point 58o-60oC), Ethyl alcohol (NICE chemicals, India), Xylene (NICE chemicals, India), Glass slides (75x25mm- Blue star, India), Cover slips (22x60 mm - Blue star, India).
Preparation of staining reagents
Golgi fixative and silver nitrate solutions were freshly prepared before use. Golgi fixative was prepared by dissolving the following chemicals in 100 ml of distilled water: Potassium dichromate - 5g; Chloral hydrate - 5g; Glutaraldehyde - 8 ml; Formaldehyde - 6 ml; Dimethyl sulphoxide - 10 drops; Silver nitrate solution (1.5%) was prepared by dissolving 1.5 g of silver nitrate crystals in 100 ml of distilled water, just before use.
2.7. Tissue collection
Rats in all the groups were sacrificed on Day 30 by anesthetic (ether) overdose. The brains were dissected from the experimented animals and were divided into cranial and caudal halves. Each cranial and caudal half was further divided into left and right halves with a mid-line cut (Sagittal plane). The cranial brain pieces were used for investigation of the morphology of the motor cortex neurons.
2.8. Tissue processing protocol
Day 1
Freshly prepared Golgi fixative was divided into two halves. Brain tissues were placed in a bottle containing one-half of freshly prepared fixative (Day 1 of tissue processing protocol). The other half was kept in refrigerator to be used on Day 2. Sample jar containing tissue samples were kept in a dark chamber to avoid any photochemical reaction
Day 2
Brain tissues fixed on Day 1 were transferred into the other sample jar containing fresh refrigerated fixative, which was prepared and stored in the refrigerator on Day 1. Sample jar containing tissue samples were kept in a dark chamber again to avoid any photochemical reaction.
Day 3
Fresh Golgi fixative was prepared as explained above. Brain tissues fixed on Day 2 were removed from the sample jar, rinsed with fresh fixative and transferred into a new sample jar containing freshly prepared Golgi fixative. The sample jars were kept again in a dark chamber.
Day 4
The brain tissues were allowed to be immersed in Golgi fixative.
Day 5
Impregnation with silver nitrate solution
1.5% aqueous solution of silver nitrate was prepared fresh. The brain tissues were removed from the Golgi fixative and rinsed in the 1.5% aqueous solution of silver nitrate till the reddish brown color of the potassium dichromate silver complex disappeared. Brain tissues were then impregnated in the silver nitrate solution in a dark chamber for a minimum period of 48 hours to maximize impregnation of silver nitrate into the tissues.
Tissue embedding
After impregnation, brain tissues were immersed in the absolute alcohol for 5 minutes. Silver chromate complex deposited on the tissue pieces was cleared with a soft brush. After that, the tissues were mounted onto a tissue block holder and embedded with a low melting paraffin wax (58°- 60° C).
Sectioning
Tissue sections were prepared using a base sledge microtome. Tissues from the motor cortex were sectioned in a coronal plane with 120µm thickness. The sections were transferred to a petri dish filled with absolute alcohol. Between each section, the cut end of the tissue block was moistened with absolute alcohol using a fine soft brush.
Clearing
The 120µm sections were transferred from the absolute alcohol onto a blotting paper using a fine brush. Subsequently, the sections were transferred to a petri dish containing xylene. Xylene is a clearing agent. Once the sections appeared transparent and begin to sink to the bottom of the petri dish, they will be transferred gently onto glass slides and allowed to air dry.
Mounting
All air dried sections were mounted serially using DPX and cover slips. Care was taken to avoid inclusion of air bubbles. Slides were permanently coded prior to the quantification, to avoid observer bias.
2.9. Camera lucida tracing of neuron dendritic branching
10 well-stained neurons from each rat in all experimental groups were selected to be traced at 400x magnification using a camera lucida attached to a Biolux research microscope. The later was modified to fit in a large beam-splitting prism and large sized mirror. In addition, we modified the drawing pencil by attaching a red indicator light parallel to the pencil tip which enabled clear visualization through the prism in a dark room.
2.10 Criteria for selection of neurons
The dendritic quantification of neurons of the motor cortex tissues were carried out. Criteria for the selection of neurons for dendritic quantification are:
- Pyramidal neurons confined to motor cortex tissues at layers II & III.
- Neurons which were well stained and homogenously impregnated with silver nitrate
throughout their arborization.
- Only non-overlapping neurons were selected.
- Dendritic quantification was done by counting the numbers of:
- a) Dendritic intersections
- b) Dendritic branching points.
2.11. Dendritic quantification
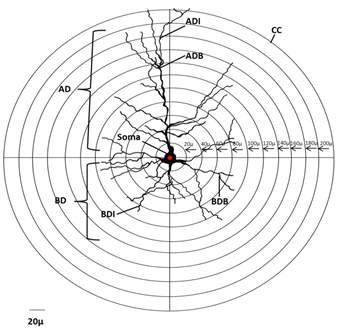
Dendritic intersections and branching points were counted with reference to the concentric circle method [15] (Figure 3). The measurements were calibrated using a stage micrometer at 400x magnification. Concentric circles were drawn on a transparent sheet with the radial distance between two adjacent concentric circles being equivalent to 20 µm. During dendritic quantification, the sheet with concentric circles was placed on the camera lucida traced neuron in such a way that the approximate center of the cell body of the neuron coincides with the center of concentric circles. The number of branching points between the two successive concentric circles (i.e., within each successive 20μm radial spheres) as well as the total number of branching points was counted. The dendritic intersection is the point where a dendrite touches or intersects the given concentric circle. The numbers of dendritic intersections at each concentric circle were counted by placing the transparent sheet with concentric circles on the camera lucida traced neurons. Both branching points and intersections of the total dendritic tree of all the neurons were counted from the center of the soma. Data was collected for numbers of apical dendritic intersections, apical branching points, basal dendritic intersections and basal branching points.
Concentric circles with the radial distance between two adjacent concentric circles being equivalent to 20 µm. Dendritic quantification is performed by placing the traced neuron aligning the center of the cell body with the center of concentric circles as seen. AD = apical branching; BD = basal branching.
2.12. Data analysis
Results were statistically analyzed using analysis of variance (ANOVA) with Bonferroni’s multiple comparison test as post hoc test (Graph Pad Prism 2.01 software, Inc. USA). Values are expressed as mean ± SEM. A comparison relative to a reference group is reported as demonstrating a statistical difference when p<0.05.
3. Results
3.1. Blood glucose levels (mg/dl) (Table 1)
At Day 0 blood glucose levels of rats in NC, VC, D, D+S, D+EE, D+S+EE were all within normal reference limits and there was no significance difference between all groups (Group means = 92.12mg/dl). Blood glucose levels for rats in NC and VC groups were consistent and within normal reference limits throughout the experimental period. On Day 2 (2 days post STZ injection) rats in D, D+S, D+EE, D+S+EE groups had become hyperglycemic (Blood glucose > 250 mg/dl, p<0.001) and the blood glucose levels were significantly increased (Group mean = 291.64 mg/dl) relative to NC rats (Mean = 94.25mg/dl). On Day 15 blood glucose levels were elevated significantly (p<0.001) in all the experimental groups (D, D+S, D+EE, D+S+EE) groups (Group mean = 374.95mg/dl) relative to NC (Mean = 91.17mg/dl). On Day 30 (Before sacrificing) blood glucose levels were significantly high (p<0.001) in D, D+S, D+EE, D+S+EE group (Group mean=470.2mg/dl) relative to NC rats (Mean = 93.33mg/dl). Exposure to EE did not show any affect in reducing blood sugar levels in rat in (D+EE) and (D+S+EE) groups.
|
Groups |
Day 0 |
Day 2 |
Day 30 |
|
Normal Control (NC, n=8) |
95.17±2.023 |
99.33±3.18 |
91.17±1.249 |
|
Vehicle Control (VC, n=8) |
91.67±1.892 |
94.5±3.041 |
95.5±2.553 |
|
Diabetes (D, n=8) |
96.33±2.789 |
295.5±19.51* |
389.5±41.56* |
|
Diabtes+Stress (D+S, n=8) |
99.5±2.156 |
321.5±26.43** |
405.7±28.51** |
|
Diabetes+ Environmental Enrichment (D+EE, n=8) |
93.33±1.542 |
276.8±19.67*** |
372.8±31.79*** |
|
Diabetes+Stress+ Environmental Enrichment (D+S+EE, n=8) |
96.5±3.519 |
289.8±11.06**** |
463.8±32.23**** |
Table 1: Blood glucose levels of rats.
Blood glucose levels in STZ induced diabetic rats with and without treatment with EE. Data represents mean ± SEM. One way ANOVA with Bonferroni’s multiple comparison tests showed blood glucose levels in D, D+S, D+S+EE groups were significantly higher relative to NC group on Day 2 (p<0.0001) and Day 30 (p<0.0001). NC vs. VC: p>0.05; *D vs. NC: p<0.0001; **D+S vs. NC: p<0.0001; ***D+EE vs. D: p< 0.0001; ****D+S+EE vs. D+S: p<0.0001.
3.2. Body weight (g) (Table 2)
There were no significant differences in body weight of rats in all experimental groups (NC, VC, D, D+S, D+EE, D+S+EE groups; Groups mean = 92.93g) on Day 0 (p>0.05) and Day 2 (p>0.05). Mean body weight for NC group was 108.22g and groups mean for all diabetic groups (D, D+S, D+EE, D+S+EE) was 100.78g. On Day 30 a highly significant decrease in body weight (p<0.001) was observed in D, D+S, D+EE, D+S+EE groups (Groups mean= 79.94g) compared to NC (Mean=218g). However, the EE treated groups (D+EE; D+S+EE)) exhibited significantly less (D+EE vs. D: p<0.001; D+S+EE vs. D+S: p<0.001) weight reduction relative to groups which were not exposed to EE (D and D+S).
|
Groups |
Day 0 |
Day 2 |
Day 30 |
|
Normal Control (NC, n=8) |
98.33±3.303 |
117.2±1.014 |
220.3±6.059 |
|
Vehicle Control (VC, n=8) |
96.83±2.574 |
113.2±1.973 |
218.5±5.169 |
|
Diabetes (D, n=8) |
97.5±3.433 |
107.8±3.859 |
82.83±7.534* |
|
Diabtes+Stress (D+S, n=8) |
102.3±2.155 |
112.2±1.887 |
58.17±4.679** |
|
Diabetes+ Environmental Enrichment (D+EE, n=8) |
99.83±1.222 |
109.8±2.626 |
86±4.442*** |
|
Diabetes+Stress+ Environmental Enrichment (D+S+EE, n=8) |
93±1.438 |
108.7±3.93 |
64.67±5.044**** |
Table 2: Body weight of rats.
Changes in body weight with and without exposure to EE. Data presented represents mean ±SEM. One way ANOVA with Bonferroni’s multiple comparison tests demonstrated there was significant reduction in body weight in D, D+S, D+S+EE groups relative to NC group respectively on Day 30. NC vs. VC: p>0.05; *D vs. NC: p<0.0001; ** D+S vs. NC: p<0.0001; ***D+EE vs. D: p<0.0001; **** D+S+EE vs. D+S: p<0.0001. EE showed prevention of body weight gain in STZ induced diabetic and stressed rats.
3.3. Dendritic morphology of Motor cortex neurons
Apical dendritic intersections
Apical dendritic intersections were significantly decreased in D group rats at 40µm (p<0.001), 60 µm (p<0.01), 80µm, 100µm, 120µm (p<0.001), 160µm, 180µm and 200µm (p<0.001) circles relative to the NC. Rats in D+S group showed a significant decrease in the apical dendritic intersections at 40µm, 60µm, 80µm, 100µm, 120µm, 140µm, and 160µm, 180µm and at 200µm (p<0.001) circles relative to NC. A significant increase in the apical dendritic intersections was also observed in the D+EE rats at 40 µm (p<0.05), 60 µm (p<0.01), 80 µm (p<0.05), 100µm, 120µm (p<0.05), 160µm (p< 0.01), 180µm and at 200µm circles (p<0.001) in comparison to the D group rats. D+S+EE rats also showed a significant increase in the apical dendritic intersections at 40µm, 60µm, 80µm, 100µm, 120µm, 140µm (p<0.01), and at 200µm circles (p<0.001) relative to rats in the D+S group (Table 3).
Table 3: Number of apical dendritic intersections on motor cortex neurons in STZ induced diabetic and diabetic stressed rats with and without exposure to enriched environment.
Data are represented as mean ± SEM. One way ANOVA with Bonferroni’s multiple comparison tests were used to analyze the data. Number of apical dendritic intersections on Day 30: D vs. NC: **p<0.01; ***p<0.001; D+S vs. NC: ### p< 0.001; D+EE vs. D: a p<0.05; aa p<0.001; aaap<0.001; D+S+EE vs. D+S: bbp <0.01, bbbp <0.001. VC vs. NC: p>0.05. n = number of rats per group.
Basal dendritic intersections
Diabetic rats showed a significant decrease in the basal dendritic intersections at the 20µm (p<0.01), 60µm, 80µm, 100µm circles (p<0.001) relative to the NC. D+S group rats showed a significant decrease in the basal dendritic intersections at the 20µm, 40µm, 60µm, 80µm, 100µm circles (p<0.001) relative to NC group. D+EE rats showed a significant increase in the basal dendritic intersections at 20µm (p<0.05), 60µm, 80µm (p<0.01) and 100µm (p<0.001) circles relative to rats in the D group. D+S+EE rats also showed a significant increase, in the basal dendritic intersections at the 20µm (p<0.05), 40µm (p<0.001), 60µm, 80µm (p<0.01) and at 100µm circles (p<0.001) relative to rats in the D+S group (Table 4).
Table 4: Number of basal dendritic intersections of motor cortex neurons in STZ induced diabetic and diabetic +
stressed rats with and without exposure to enriched environment.
Data are represented as mean ± SEM. One way ANOVA, Bonferroni’s multiple comparison tests Number of apical dendritic intersections on Day 30: NC vs.VC: p>0.05; D vs.NC: **p<0.01, ***p<0.001; D+S vs. NC: ###p< 0.001; D+EE vs. D: ap<0.05, aap<0.001, aaap<0.001; D+S+EE vs. D+S: bbp<0.01, bbbp<0.001. n = number of rats in each experimental group
Apical dendritic branching points
Pyramidal neurons in the motor cortex of the D group rats showed significant decrease in the numbers of apical dendritic branching points at the 40-60µm, 60-80µm (p<0.001), 80-100µm (p<0.01), 100-120µm, 120-140µm (p<0.001), 140-160µm (p<0.01), 160-180µm and 180-200µm (p<0.001) zones relative to rats in the NC group. Also, significant reduction in the apical dendritic branching points at the 20-40 µm (p<0.01), 40-60µm (p<0.001), 80-100µm, 100-120µm, 120-140µm, 140-160µm, 160-180µm and 180-200µm (p<0.001) zones were observed in D+S group rats relative to NC rats. On the contrary, D+EE group rats showed a significant increase in the apical dendritic branching points at the 40-60 µm (p<0.01), 100-120 µm, 120-140 µm, 140-160 µm (p<0.05), 160-180 µm and 180-200 µm (p< 0.001) zones relative to the D group rats. At 20µm, 40µm (p<0.01), 80-100µm, 100-120µm (p<0.01), 140-160µm (p<0.05), 160-180µm and 180-200µm (p<0.001) zones D+S+EE rats showed a significant increase in the apical dendritic branching points relative to D+S group (Table 5).
Table 5: Number of apical dendritic branching points of motor cortex neurons in STZ induced diabetic and diabetic +
stressed rats with and without exposure to enriched environment.
Data are presented as mean ± SEM. One way ANOVA with Bonferroni’s multiple comparison tests were performed to analyzed the data. Number of apical dendritic branching points on Day 30: VC vs. NC: p>0.05; D vs. NC: **p<0.01, ***p<0.001; D+S vs. NC: ##p< 0.01, ### p< 0.001; D+EE vs. D: ap<0.05, aap<0.001, aaap<0.00; D+S+EE vs. D+S: bp<0.05, bbp<0.01, bbbp<0.001. n = number of rats in each experimental group.
Basal dendritic branching points
Basal dendritic branching points were significantly reduced in D group rats at 0-20µm (p<0.01), 40-60µm (p<0.001) and 80-100µm (p<0.001) zones relative to the rats in NC group. D+S group also demonstrated significantly less basal dendritic branching points at the 80-100µm (p<0.001) zones relative to NC. On the contrary, significantly increased basal dendritic branching points were observed at the 0-20µm (p<0.05), 60-80µm (p<0.01) and 80-100µm (p<0.001) zones in the motor cortex of D+E group relative to D group. Also, significantly more basal dendritic branching points were observed at 0-20µm (p<0.05), 20-40µm (p< 0.001), 40-60µm (p< 0.01), 60-80µm (p<0.01) and 80-100µm (p<0.001) zones in group D+S+EE rats relative to the D+S group (Table 6).
|
Groups |
Concentric zones (µm) |
||||
|
0 - 20 |
20-40 |
40-60 |
60-80 |
80-100 |
|
|
Normal Control (NC, n=8) |
1.780 ±0.228 |
4.099 ±0.273 |
5.224 ±0.264 |
3.910 ±0.161 |
1.472 ±0.170 |
|
Vehicle Control (VC, n=8) |
1.739 ±0.089 |
3.672 ±0.255 |
5.215 ±0.155 |
3.818 ±0 .273 |
1.512 ±0.031 |
|
Diabetes (D, n=8) |
0.927 ±0.237** |
3.062 ±0.159 |
2.769 ±0.306*** |
2.004 ±0.275*** |
0.411 ±0.079*** |
|
Diabetes+ Stress (D+S, n=8) |
0.742 ±0.100### |
1.535 ±0.280### |
2.048 ±0.359### |
1.243 ±0.279### |
0.225 ±0.051### |
|
Diabetes+ Environmental Enrichment (D+EE, n=8) |
1.668 ±0.107a |
3.249 ±0.276 |
4.273 ±0.247aa |
3.484 ±0.244aa |
1.346 ±0.151aaa |
|
Diabetes+ Stress+ Environmental Enrichment (D+S+EE, n=8) |
1.512 ±0.110b |
3.439 ±0.2576bbb |
3.548 ±0.178bb |
2.718 ±0.239bb |
1.093 ±0.201bbb |
Table 6: Number of basal dendritic branching points of motor cortex neurons in STZ induced diabetic and diabetic +
stressed rats with and without exposure to enriched environment
Data are presented as mean ± SEM. Data was analyzed using one way ANOVA with Bonferroni’s multiple comparison tests. Number of basal dendritic branching points: D vs. NC: **p<0.01, ***p<0.00; D+S vs. NC: ##p< 0.01, ###p< 0.001; D+EE vs. D: a p<0.05, aap<0.001, aaap<0.00; D+S+EE vs. D+S: bbp <0.01, bbbp<0.001. n = number of rats.
Total number of dendritic branching points
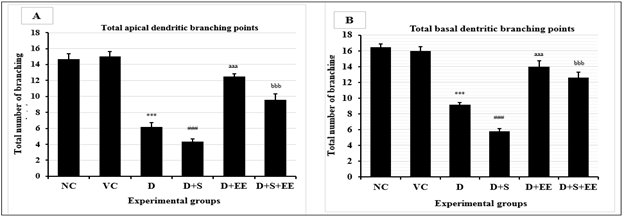
Overall, groups D and D+S demonstrated significantly less total apical (p<0.001) and basal (p<0.001) dendritic branching points in the motor cortex relative to NC group. D+EE and D+S+EE groups demonstrated significantly higher numbers of total apical (p<0.001) and basal (p<0.001) dendritic branching points in the motor cortex relative to D and D+S groups (Figure 4).
Data is presented as mean±SEM. One way ANOVA with Bonferroni’s multiple comparison tests was used to analyze the data. Total apical (A) and basal (B) dendritic branching points of motor cortex neurons in rat brain from experimental groups. (A) NC vs.VC: p>0.05; ***D vs.NC: p<0.001: ###D+S vs. NC: p<0.001, aaaD+EE vs. D: p<0.001; bbbD+S+EE vs. D+S: p<0.001. (B) NC vs.VC: p>0.05; ***D vs.NC: p<0.001: ###D+S vs. NC: p<0.001, aaaD+EE vs. D: p<0.001; bbbD+S+EE vs. D+S: p<0.001.
4. Discussion
STZ-induced diabetes simulate hyperglycemia in established type 1 and type 2 diabetes. In both conditions there will be significant reduction of pancreatic beta-cells. Diabetes state (frank hyperglycemia) was developed in all experimental groups (D, D+S, D+EE and D+S+EE) on Day 2 post STZ injection (40mg/kg body weight; ip). Blood glucose levels of all animals in groups D, D+S, D+EE and D+S+EE was significantly increased relative to the control groups (NC and VC) (Table 1). As the diabetes state is maintained, significant body weight reduction was observed in the diabetic rats (groups: D, D+S, D+S+EE) relative to the control groups (NC and VC) (Table 2). Weight loss in diabetes is a normal phenomenon (type 1 and uncontrolled type 2) because in the diabetes state, insufficient insulin prevents glucose from the blood from entering into the cells to be used as energy. During this state, gluconeogenesis is initiated where the body starts burning fat and muscle for energy, causing a reduction in overall body mass [16]. However, our results shows rats in D+EE and D+S+EE (p<0.0001) groups had significantly higher body eights relative to D and D+S (p<0.0001) respectively. The later finding suggests that EE improved insulin sensitivity and therefore, the body did not need to breakdown fat and muscles for cellular energy.
“Stress” (physiological, mental and pathological) is referred to a state when these are activated sympathetic activity [17]. Sympathetic hormones play crucial role in elevation of glucose levels, reduces insulin secretion rate and therefore, exacerbates hyperglycemia [15]. Restrained stress in rats [18] employed in this study stimulate stress experienced by diabetic patients. Diabetes is more than hyperglycemia alone because unhealthy lifestyle and physical inactivity play important roles in the progression, prognosis and exacerbation of the disease. Often diabetic patients are managed with anti-glycemic drugs, advice to follow strict diet control, lifestyle modification and an exercise regime. Some patients who are religiously compliant to the proposed management plans still were not able to achieve normoglycemia. Stress management is a factor that is usually left out in planning interventions for diabetic patients. Stress is inevitable in diabetes. Patients may be experiencing stress due to day-to-day issues like financial issues, family and relationships let alone the stress of coping with the disease itself and the fear (anxiety) of developing complications. If stress level is controlled, dysregulation of metabolic processes can be prevented and subsequently normoglycemia can be better managed.
Our results shows EE prevented morphological damage of neurons at the motor cortex of STZ induced diabetic and stressed rats which were not exposed to EE (D; D+S). Healthy dendritic arborizations of motor neurons seen in the D+EE suggest that EE can preserve peripheral motor functions in diabetes, and what we observed in the D+S+EE group suggest that EE eliminated the destructive effects of stress on the motor neurons. The catastrophic outcome of hyperglycemia arise from the positive turnover of ROS production (Oxidative stress). ROS oxidizes cellular components, causes cell death, and triggers inflammatory process during the pathogenesis of diabetes [19].
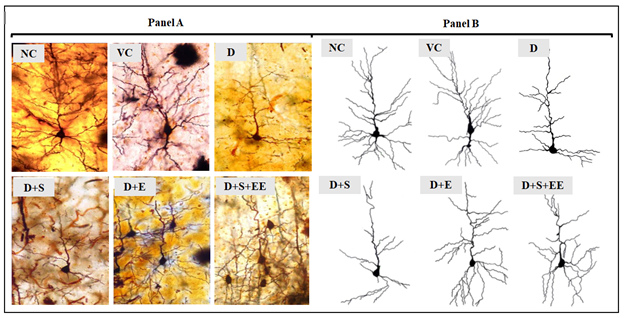
Motor cortex consists of the primary motor cortex, premotor cortex and the supplementary motor area. At cellular level motor neurons at the motor cortex are responsible for communication across the motor cortex and subsequently convey signals to the motor neurons in the spinal cord in order to coordinate preparation and control of normal musculature movements in the periphery [18]. Therefore, maintenance of normal neuronal morphology (dendritic arborization) at the central nervous system (CNS) is crucial for the intricate signaling network to function effectively [20]. Our findings showed evidence that the numbers of apical (Table 3) and basal (Table 4) dendritic intersections and apical (Table 5) and basal (Table 6) dendritic branching points were significantly reduced in D and D+S groups relative to control groups (NC and VC) respectively. However, in diabetic groups exposed to EE (D+EE; D+S+EE) the numbers of apical (Table 3) and basal (Table 4) dendritic intersections and apical (Table 5) and basal (Table 6) dendritic branching points were significantly higher relative to groups which were not exposed to EE. Overall, the total branching points of pyramidal neurons at the motor cortex (Figure 4) showed diabetic (D+EE) and stress (D+S+EE) groups which was exposed to EE had significantly higher numbers of arborizations relative to diabetes and stress groups which were not exposed to EE. Figure 5 shows representative tracings of pyramidal neuronal arborization from NC, VC, D, D+S, D+EE and D+S+EE groups. Our findings show diabetes and stress significantly caused neuronal damage evident by the reduced counts of dendritic arborizations and intersections in the motor cortex and that EE significantly prevented neuronal damage in the motor cortex of STZ-induced diabetes and stressed rats.
The illustrations in Figure 5 demonstrates the dendritic arborization patterns observed in all experimental groups. Panel A: Photomicrographs of motor cortex (Layer II and III) stained with Rapid Golgi staining. Panel B: Corresponding pencil tracings of motor cortex neurons in Panel A drawn under the Camera Lucida. EE=Environmental Enrichment; NC=Normal control; VC=Vehicle control; D=Diabetic; D+S=Diabetes+Stress; D+EE=Diabetes+Environmental Enrichment; D+S+EE=Diabetes+Stress+Environmental Enrichment. Total counts of dendritic branching points and dendritic intersections were statistically analyzed using ANOVA with Bonferroni’s tests. Data demonstrated significant increase in dendritic arborizations in D+EE and D+S+EE groups relative to D and D+S groups respectively. D and D+S groups demonstrated significantly lower number of dendritic arborizations relative to NC.
5. Conclusion
Environmental enrichment (EE) can be implemented as an alternative therapeutic choice for eliminating stress, preventing neuronal damage at the motor cortex and facilitating effective glycemic control in diabetes along with anti-hyperglycemic agents, lifestyle modifications and frequent physical activities.
Acknowledgements
The authors wish to expresses sincere thanks to Manipal University (MU), Manipal, India for permitting to conduct the work and for providing the laboratory facilities to perform this work.
Conflict of interest
The authors declare no conflict of interest to report.
References
- Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 66 (2017): 241-255.
- Phillips LS, Ratner R, Buse JB and Kahn SE. We Can Change the Natural History of Type 2 Diabetes. Diabetes Care 37 (2014): 2668-2676.
- Mathew J, Mohan M, and Menon A. Multiple Cranial Neuropathies in a Patient with Diabetes Mellitus. Ann Indian Acad Neurol 22 (2019): 353–355.
- Pop-Busui R, Boulton AJM, Feldman EL, Bril V, Freeman R, et al. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 40 (2017): 136–154.
- Sanes JN. Neocortical mechanisms in motor learning. Curr Opin Neurobiol 13 (2003): 225-231.
- Fairhurst GD, Frey MD, Reichert JF, Szelest I, Kelly DM, Bortolotti GR. Does environmental enrichment reduce stress? An integrated measure of corticosterone from feathers provides a novel perspective. PLoS One 6 (2011): e17663.
- Baroncelli L, Braschi C, Spolidoro M, Begenisic T, Sale A, Maffei L. Nurturing brain plasticity: Impact of environmental enrichment. Cell Death Differ 17 (2010): 1092?1
- Rodriguez JJ, Noristani HN, Olabarria M, Fletcher J, Somerville TD, et al. Voluntary Running and Environmental Enrichment Restores Impaired Hippocampal Neurogenesis in a Triple Transgenic Mouse Model of Alzheimer's Disease. Curr Alzheimer Res 8 (2011): 707-717.
- Steiner B, Winter C, Hosman K, Siebert E, Kempermann G, Petrus D S, Kupsch A. Enriched environment induces cellular plasticity in the adult substantia nigra and improves motor behavior function in the 6-OHDA rat model of Parkinson's disease. Exp Neurol 199 (2006): 291-300.
- Pera PI. Living with diabetes: quality of care and quality of life. Patient Preference and Adherence 5 (2011): 65–72.
- Badescu SV, Tataru C, Kobylinska L, Georgescu EL, Zahiu DM, Zagrean AM, and Zagrean L. The association between Diabetes mellitus and Depression. J Med Life 9 (2016): 120–125.
- Surwit RS, Schneider MS and Feinglos MN. Stress and Diabetes Mellitus. Diabetes Care 15 (1992): 1413-1422.
- Grant CW, Duclos SK, Moran-Paul CM, Yahalom B, Tirabassi RS, Arreaza-Rubin G, Spain LM, Guberski DL. Development of standardized insulin treatment protocols for spontaneous rodent models of type 1 diabetes. Comp Med 62 (2012): 381-390.
- Winer JA, Morest DK. The neuronal architecture of the dorsal division of the medial geniculate body of the cat. A study with the rapid Golgi method. The Journal of Comparative Neurology 221 (1983): 1-30.
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87 (1953): 387–406.
- Kahanovitz L, Sluss PM, and Russell SJ. Type 1 Diabetes – A Clinical Perspective. Point Care 16 (2017): 37–40.
- Wong H, Singh J, Go RM, Ahluwalia N, Guerrero-Go MA. The Effects of Mental Stress on Non- insulin-dependent Diabetes: Determining the Relationship Between Catecholamine and Adrenergic Signals from Stress, Anxiety, and Depression on the Physiological Changes in the Pancreatic Hormone Secretion. Cureus 11(2019): e5474.
- Campos AC, Fogac MV, Aguia DC, Guimara FS. Animal models of anxiety disorders and stress. Revista Brasileira de Psiquiatria 35 (2013): S101–S111.
- Ma X, Chen Z, Wang L, Wang G, Wang Z, Dong X, Wen B, Zhang Z. The Pathogenesis of Diabetes Mellitus by Oxidative Stress and Inflammation: Its Inhibition by Berberine. Front Pharmacol 9 (2018): 782.
- Meier JD, Aflalo TN, Kastner S, Graziano MS. Complex organization of human primary motor cortex: a high-resolution fMRI study. J. Neurophysiol 100 (2008): 1800-1812.



 Impact Factor: * 3.0
Impact Factor: * 3.0 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
