Quercetin Extracted from Trifolium Alexandrinum Enhances wound healing in 1 Streptozotocin-induced Diabetic rats through Antioxidant and Anti-inflammatory effects
Article Information
Heba M Abdou1*, Dohaa M Ahmad2, Fatma A Hamaad2
1Department of Zoology, Faculty of Science, Alexandria University, Alexandria, Egypt
2Department of Biochemistry, Faculty of Science, Alexandria University, Alexandria, Egypt
*Corresponding Author(s): Heba M Abdou, Department of Zoology, Faculty of Science, Alexandria University, Alexandria, Egypt
Received: 24 October 2020; Accepted: 06 November 2020; Published: 24 November 2020
Citation:
Heba M. Abdou, Dohaa M. Ahmad, Fatma A. Hamaad. Quercetin Extracted from Trifolium Alexandrinum Enhances wound healing in 1 Streptozotocin-induced Diabetic rats through Antioxidant and Anti-inflammatory effects. Journal of Pharmacy and Pharmacology Research 4 (2020): 116-138.
View / Download Pdf Share at FacebookAbstract
Abstract
Background: Diabetes-induced abnormal wound healing stimulates the generation of inflammatory mediators, including reactive oxygen species.
Objective: Our study aimed to examine the antioxidant and anti-inflammatory effects of quercetin extracted from Trifolium alexandrinum (TA) on enhancing wound healing in diabetic rats.
Methods: Seventy-five male Wistar albino rats were divided into 5 groups as follows: Gp1 (control wounded nondiabetic group), Gp2 (untreated wounded diabetic group), Gp3 (diabetic wounds treated with quercetin-loaded nanoparticles (Q-LNs) group), Gp4 (wounded diabetic rats treated with quercetin (Q) group) and Gp5 (wounded diabetic rats treated with TA extract group).
Key findings: The wounded diabetic rats were treated orally and topically with Q-LNs, Q and TA extract at doses of 50 mg/kg BW, 50 mg/kg BW and 200 mg/kg BW, respectively, for 15 days. The wound closure percent was calculated on the 5th, 10th and 15th days and was noticeably increased as a result of topical and oral treatment of wounded diabetic rats with Q-LNs, Q and TA. The elevated blood glucose and the reduced serum insulin levels were significantly improved in treated wounded diabetic rats. Oxidative stress markers were improved. The increased levels of cytokines, as well as MIF, were decreased in treated wounded diabetic rats, while the decreased levels of TGF-β and hexosamine were enhanced. Histological examination showed marked amelioration in the treated wounded skin.
Conclusion: It can be concluded that treatment with Q-LNs, Q and TA extract improved the diabetic state, cytokines involved in inflammation and the antioxidant defense system and may play essential roles in promoting the wound healing process.
Keywords
Trifolium alexandrinum; Wound healing; Diabetes mellitus; Quercetin; Cytokines; Macrophage migration inhibitory factor
Trifolium alexandrinum articles; Wound healing articles; Diabetes mellitus articles; Quercetin articles; Cytokines articles; Macrophage migration inhibitory factor articles
Article Details
1. Introduction
Wound healing is a complex process that facilitates the rebuilding of tissue structure and function after damage [1]. Wound healing requires the integration of various signaling molecules and cells in progressive phases that involve hemostasis, inflammation, proliferation and remodeling [2]. Regenerative wound therapy is a new and rapidly developing area in biomedical research that aims to restore skin to its pristine function a reestablish damaged cells and skin tissue without scarring [3]. Diabetes mellitus (DM) is a metabolic disorder of carbohydrate, fat and protein metabolism. Diabetes occurs due to an inadequate level of insulin or insulin resistance, prompting hyperglycemia [4]. Diabetes is related to numerous health complications, including impaired healing [5]. A crucial feature of diabetes that may add to abnormal wound healing is its affiliation with anomalous inflammatory responses that induce the generation of inflammatory mediators, including reactive oxygen species, cytokines and advanced glycation end products [6].
Isoflavones are obtained from numerous palatable plants and have been shown to have notable antioxidant activities that repress oxidative damage. This antioxidative activity has been demonstrated by the capability of isoflavones to scavenge free radicals and diminish oxidative stress [7]. The variety of cellular actions of isoflavones and lignans indicates their valuable potential impacts on different chronic diseases. Quercetin is a flavonoid derived from plants and is found in vegetables, fruits, red wine, other beverages, green tea and nutritional supplements. Quercetin has anti-inflammatory, antiallergic, anticancer, antibacterial and antiviral properties [8]. Quercetin is an effective free radical scavenger and an effective inhibitor of lipid peroxidation in vitro and in vivo. Drugs with free radical scavenging properties have been shown to improve wound healing in treated individuals [9].
Indeed, even with these numerous pharmacological properties, the utilization of quercetin in the pharmaceutical field is restricted by its low aqueous solubility and chemical instability [8]. Nanoparticles have received noteworthy consideration as potential medication delivery vehicles. Chitosan (CS) is a polysaccharide that has been recognized as an ideal hydrophilic carrier system due to its exceptional physicochemical properties and reported low toxicity. Thus, the addition of quercetin to chitosan nanoparticles may be valuable in enhancing the bioavailability of quercetin [10].
Egyptian clover (Trifolium alexandrinum, TA) belongs to the family Fabaceae. Previous phytochemical examinations of TA have uncovered the presence of flavonoids and their glycosides, isoflavonoids, amino acids and their derivatives, proteins, terpenoid glycosides and fatty acids in various parts of the plant. TA has diverse biological activities, including antioxidant and antidiabetic activities [4].
The present study aimed to investigate Quercetin extracted from Trifolium alexandrinum enhances wound healing in streptozotocin-induced diabetic rats through antioxidant and anti-inflammatory effects.
2. Materials and methods
2.1. Chemicals
Streptozotocin (STZ), quercetin, genistein, daidzein, hespirtine, formononetin and biochanin A were obtained from Sigma-Aldrich Chemical Company (www.sigmaldrich.com, St. 4 Louis, MO, USA). All other chemicals used were of analytical grade.
2.2. Preparation of Trifolium alexandrinum extract
Aerial parts of the plant Trifolium alexandrinum (TA) Linn. (Family: Fabaceae) were collected during the vegetative and flowering growth stages from different localities of Alexandria, Egypt. The plant extract was prepared according to previous work by Mohamed et al. [11]. The resulting dried TA extracts were kept in a sterile labeled container and refrigerated at 4 °C for further assessment.
2.3. Determination of total phenolic and flavonoid content of Trifolium alexandrinum extract
Total phenolic content of the TA extracts was determined by the Folin-Ciocalteu assay according to Limmongkon et al. [12]. Total flavonoids were determined colorimetrically by using the aluminum chloride assay as described by Kiranmai et al. [13].
2.4. Determination of the total antioxidant capacity of Trifolium alexandrinum extract
The antioxidant activity of TA extracts was evaluated by the phosphomolybdenum method according to the method described by Prieto et al. [14].
2.5. HPLC analysis of Trifolium alexandrinum extract
Initially, 30 mg of the dry TA extract was extracted with 4 ml of 6 M HCl and incubated at 100 °C for 15 min in a water bath with magnetic stirring. After cooling, the residue was filtered and extracted with 15 ml of dichloromethane (three times). The extract was concentrated under reduced pressure and dissolved in 10 ml of methanol. The extract was filtered through a 0.45 μm membrane before injection into the HPLC system [15].
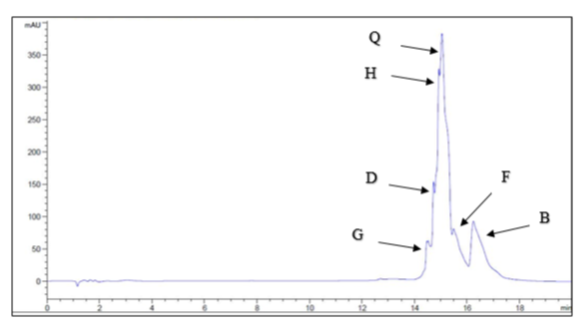
HPLC analyses were performed as described by Ramos et al. [15] on an Agilent Technologies instrument (Santa Clara, CA, USA, 5C18, 4.6*150 mm, 300° A) equipped with a DAD detector. Elution of isoflavones was performed using a linear gradient system, and the mobile phase consisted of acetonitrile:water:trifluoroacetic acid (20:80:0.01 (v/v/v)) (A) and acetonitrile:trifluorocetic acid (100:0.1 (v/v)) (B). The gradient profile was 0 to 40% B from 0–10 min, 40% B from 10–11 min, and 40 to 100% B from 11–12 min. At the end of each run, 100% mobile phase A was used for 6 min to restore the initial conditions. The flow rate was 0.7 ml/min. The detection wavelength was 260 nm. The identification of isoflavones was performed by comparing the UV profiles and retention times with chemical reference substances. The area under the curve of each isoflavone in the extract was determined, and these areas were used to calculate the percent weights of isoflavones in the samples.
2.6. Isolation of quercetin from Trifolium alexandrinum extract
The dried TA extract was resuspended in distilled water and defatted with petroleum ether. The residue was diluted with H2O and extracted using ethyl acetate (EtOAc) in triplicate. Subsequently, the EtOAc fractions were chromatographed over silica gel (n-hexane/EtOAc/MeOH) to obtain 8 fractions. Fraction 6, which was eluted with EtOAc/MeOH (8:1), was further separated and purified by capillary electrophoresis using silica gel column chromatography to yield quercetin. The structure of the isolate was determined by HPLC [16].
2.7. Preparation of chitosan nanoparticles and quercetin-loaded nanoparticles
Chitosan nanoparticles were prepared according to the literature based on the ionic gelation of chitosan with tripolyphosphate (TPP) anions. Quercetin-loaded nanoparticles were formed upon the incorporation of 4 mL of TPP solution containing quercetin into 10 mL of chitosan solution. The nanoparticles were separated from aqueous medium containing unassociated quercetin by ultracentrifugation at 40,000 g and 48 °C for 30 min and lyophilized [10].
2.8. Induction of diabetes
Sixty adult Wistar albino male rats were injected intraperitoneally (i.p.) with a single dose of 40 mg/kg BW streptozotocin (STZ) that was freshly dissolved in cold sodium citrate buffer (0.1 M/L; pH 4.5) [17]. Three days after the STZ injection, blood was withdrawn from the tail vein, and the glucose level was measured. Rats were considered diabetic when their fasting blood glucose levels were more than 200 mg/dL.
2.9. Experimental animals
Seventy-five male Wistar albino rats weighing 160–180 g were obtained from the Animal Care Unit, Department of Home Economics, Faculty of Agriculture, Alexandria University, Egypt. The rats were housed in stainless steel wire cages placed in a well-ventilated animal house and were maintained on basal diet and tap water ad libitum under constant laboratory conditions (temperature: 22±3 °C, photoperiod: 12/12-h light/dark cycle). The handling of rats and the experimental procedures were executed according to the ethical guidelines approved by the Alexandria University Egypt Institutional Animal Care and Use Committee (ALEXU-IACUC) (Approval number: AU 04 20 09 26 3 03).
2.10. Wounding operation
Rats were anaesthetized by intraperitoneal administration of 60 mg/kg BW ketamine [18]. The dorsal fur of the anaesthetized rats was shaved with an electric clipper, and the area of the wound was outlined on the back with a marker. A full thickness wound of approximately 1 cm was made on the dorsal side by removing the epidermal and dermal layers with a sterile surgical scalpel.
2.11. Animal groupings
Gp1: Control wounded nondiabetic group (Cont group): Fifteen rats received distilled water orally by gavage and were topically administered sterile normal saline on the wounds once daily for 15 days.
After the induction of diabetes mellitus (3 days post-STZ injection) and wounding, the rats were allocated into 1 of 4 groups, each with 15 rats.
Gp2: untreated wounded diabetic group; Diabetic group (40 mg/kg BW i.p.).
Gp3: treated wounded diabetic group; Diabetic+ Quercetin-loaded nanoparticle (Q-LN)-treated group, treated with 50 mg/kg BW /day [19].
Gp4: treated wounded diabetic group; Diabetic+ Quercetin (Q)-treated group, treated with 50 mg/kg BW/ day [19].
Gp5: treated wounded diabetic group; Diabetic+Trifolium alexandrinum (TA) extract-treated group, treated with 200 mg/kg BW / day [11].
Rats in groups 3, 4 and 5 were treated orally by gavage and topically on the wounds once daily for 15 days.
2.12. Collection of blood samples
After 5, 10 and 15 days of treatment, blood samples were collected from the jugular veins of fasted rats under diethyl ether anesthesia. The blood was allowed to coagulate at room temperature and then centrifuged at 3000 rpm for 15 min. The supernatant sera were aspirated and fractioned into three Eppendorf tubes.
2.13. Measurement of wound diameter and closure
The wound diameter was measured on days 5, 10 and 15 after incision, and the wound closure percentage was calculated using the following equation: wound closure rate on day X (%)= [(wound diameter on day 0 – wound diameter on day X)/ (wound diameter on day 0)] ×100, as described by Ahmed et al. [20].
2.14. Biochemical parameters
Serum glucose levels were measured using a commercial diagnostic kit (Biodiagnostics, Egypt). Serum insulin levels were measured by sandwich ELISA according to the manufacturer's instructions using kits purchased from Linco Research, USA. Tissue homogenate was prepared for the measurement of total protein levels in the wound as described by Lowry et al. [21]. The concentration of malondialdehyde (MDA), a product of lipid peroxidation, was measured as an indicator of oxidative stress in wound tissue according to the method described by Varshney and Kale [22]. Reduced glutathione (GSH) was measured spectrophotometrically at 412 nm according to the method described by Jollow et al. [23]. Superoxide dismutase (SOD; EC: 1.15.1.1) and glutathione peroxidase (GPx; EC: 1.11.1.9) activities were determined in wound tissue by the methods described by Nishikim et al. [24] and Rotruck et al. [25], respectively. Collagen and hexosamine were measured by the methods described by Woessner [26] and Elson and Morgon [27], respectively. Tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), transforming growth factor β (TGF- β) and macrophage migration inhibitory factor (MIF) concentrations were estimated using ELISA kits according to the manufacturer’s protocol.
2.15. Histopathological examination
After sacrifice and decapitation, the rats were dissected, and skin in the wound regions was rapidly excised and fixed in 10% neutral buffered formalin for 48 h. The fixed skin of each rat was then processed for paraffin-embedding and sectioning. The sections were prepared at a thickness of 5 μm using a microtome, processed in a xylene alcohol series and stained with alum hematoxylin and eosin (H&E). The sections were examined microscopically to evaluate histopathological changes [28].
2.15. Statistical analysis
The data were analyzed using one-way analysis of variance (ANOVA) [29] followed by the LSD test to compare various groups with each other. The results are expressed as the mean ± SE, and values of P > 0.05 were not considered significantly different, whereas values of P < 0.05 were considered significant.
3. Results
3.1. Phytochemical Analyses
As shown in Table (1), the total phenolic content in TA extract is expressed in terms of the gallic acid equivalent (68.8±0.2 mg/g). The total flavonoid content in the TA extract is expressed in terms of the catechin equivalent (40.7±0.22 mg/g). On the other hand, the total antioxidant capacity of TA extract is expressed in terms of the gallic acid equivalent (210.5±0.45 mg/g).
3.2. Concentrations of isoflavones in Trifolium alexandrinum
HPLC analysis was used to determine the concentrations of the six investigated isoflavones, which are shown in Figure (1). The highest concentration was quercetin (2091.67 μg/g TA extract), followed by biochanin A (975 μg/g TA extract), hespirtine (74.167 μg/g TA extract), formononetin (20.83 μg/g TA extract), and daidzein (19.44 μg/g TA extract), and the lowest concentration was genistein (16.52 μg/g TA extract).
Table 1: Total phenolic, total flavonoid contents and total antioxidant capacity of TA extract
|
Parameters |
TA extract |
|
Total phenolic content (mg GAE/g)A |
68.8±0.2 |
|
Total flavonoid content (mg CE/g)B |
40.7±0.22 |
|
Total antioxidant capacity (mg GAE/g)A |
210.5±0.45 |
AValue is expressed as mean ± SE mg of Gallic acid equivalent per gram of dry weight of the extract.
BValue is expressed as mean ± SE mg of Catechin equivalent per gram of dry weight of the extract.
GAE: Gallic acid, CE: Catechin, TA: Trifolium alexandrinum
3.3 Wound Closure and Healing Process
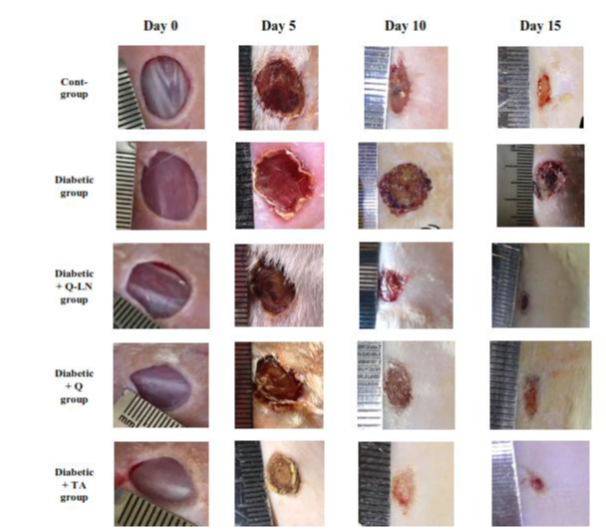
Wound closure was measured on the 5th, 10th and 15th days after injury, and the results are shown in Figure (2) and Table (2). In the control rats, the wound closure percentage reached 28.03% on day 5 after injury, 52.30% on day 10 after injury and 80.50% on day 15 after injury. On the other hand, in the untreated diabetic rats, wound closure was more severely affected than in the control rats, and the wound closure percentage reached 24.33% on day 5 and increased to 31.76% on day 10 and 49.13% on day 15 after injury. In diabetic rats treated with Q-LNs, the wound closure percentage reached 33.43% on day 5 after injury and increased to 63.43% on day 10 and 88.36% on day 15 after injury. In diabetic rats treated with quercetin (Q), the wound closure percentage reached 28.70% on day 5 after injury and increased to 61.96% on day 10 and 86.77% on day 15 after injury. In diabetic rats treated with Trifolium alexandrinum (TA) extract, the wound closure percentage reached 34.50% on day 5 after injury and increased to 71.03% on day 10 and 92.37% on day 15 after injury. Treatment with Q-LNs, quercetin and TA extract produced significant effects on the wound closure percentage compared to that of untreated diabetic rats. Furthermore, treatment with TA extract produced the most potent effect on wound closure compared to treatment with either Q-LNs or Q.
Table 2: wound closure percent in different experimental groups
|
Groups |
Wound closure percent |
||
|
Day 5 |
Day 10 |
Day15 |
|
|
Cont-group |
28.03±0.50a |
52.30±0.50a |
80.50±0.81a |
|
Diabetic group |
24.33±0.52b |
31.76±0.50b |
49.13±0.75b |
|
Diabetic + Q-LN group |
33.43±0.40c |
63.43±0.52c |
88.36±0.84c |
|
Diabetic + Q group |
28.70±0.49a |
61.96±0.99c |
86.77±0.55c |
|
Diabetic + TA group |
34.50±0.67c |
71.03±0.70d |
92.37±0.77d |
Values are expressed as means ± SE (n=5).
Mean values, in the same column for each parameter, having different superscript letters a, b, c and d are significantly different from each other (P < 0.05).
3.4. Effects of quercetin-loaded nanoparticles (Q-LNs), quercetin (Q) and Trifolium alexandrinum extract (TA) on serum glucose and insulin levels in rats
As shown in Table 3, the serum glucose levels of the untreated diabetic group were significantly (p≤0.05) increased compared to those of the control group. In contrast, there were significant (p≤0.05) decreases in the levels of serum insulin compared to those of the control group. On the other hand, the administration of Q-LNs, Q and TA extract caused significant (p≤0.05) reductions in glucose levels on the 5th, 10th and 15th days in diabetic rats compared to untreated diabetic rats. Q-LNs, Q and TA extract treatment caused significant (p≤0.05) increases in the levels of insulin compared to those of the untreated diabetic group.
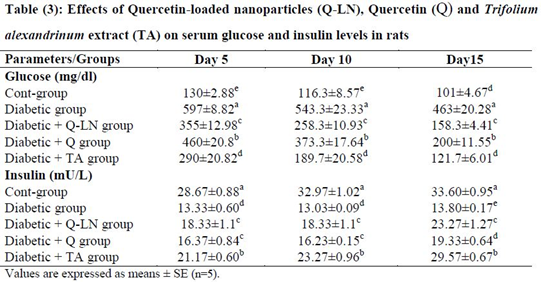
Mean values, in the same column for each parameter, having different superscript letters a, b, c, d and e are significantly different from each other (P < 0.05).
3.5 Effects of quercetin-loaded nanoparticles (Q-LNs), quercetin (Q) and Trifolium alexandrinum extract (TA) on the oxidant and antioxidant profiles in wounded tissue
The obtained results showed a significant (p≤0.05) increase in the level of MDA in wounded tissue in the untreated diabetic group in comparison with the control group. The tissue levels of reduced glutathione (GSH) and activities of the antioxidant enzymes GPX and SOD in the untreated diabetic group were significantly (p≤0.05) reduced compared to those in the control group at the three stages (Table 4). On the other hand, Q-LNs, Q and TA extract administration caused a significant (p≤0.05) reduction in the level of MDA and a significant (p≤0.05) enhancement in the GSH level and GPX and SOD activities in wounded tissue when compared to those of the untreated diabetic group on the 5th, 10th and 15th days. TA extract was effective in preventing lipid peroxidation, as observed by reductions in the levels of MDA in the diabetic groups.
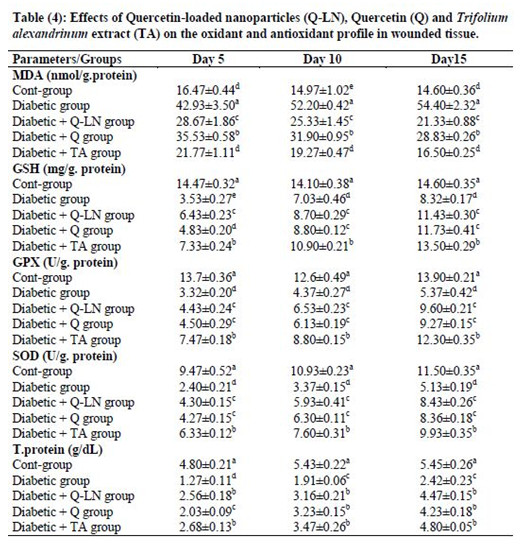
Values are expressed as means ± SE (n=5).
Mean values, in the same column for each parameter, having different superscript letters a, b, c, d and e are significantly different from each other (P < 0.05).
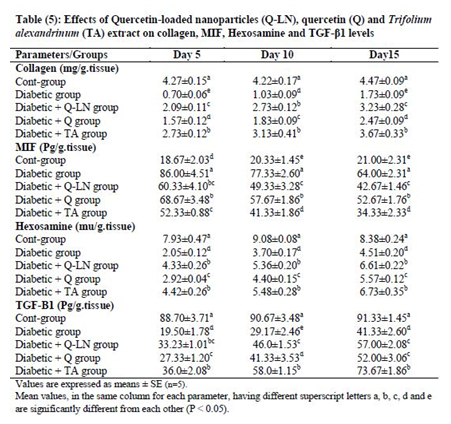
3.6. Effects of quercetin-loaded nanoparticles (Q-LNs), quercetin (Q) and Trifolium alexandrinum (TA) extract on collagen, MIF, hexosamine and TGF-β1 levels
The obtained results showed significant (p≤0.05) decreases in the levels of collagen, hexosamine, and TGF-β1 and a significant (p≤0.05) increase in the MIF level in wounded tissue in the untreated diabetic group in comparison with the control group (Table 5). On the other hand, Q-LNs, Q and TA extract administration caused significant (p≤0.05) increases in the levels of collagen, hexosamine, and TGF-β1 and significant (p≤0.05) reductions in the MIF level in wounded tissue compared to those of the untreated diabetic group on the 5th, 10th and 15th days. TA was effective in ameliorating these parameters, as observed in the diabetic groups.
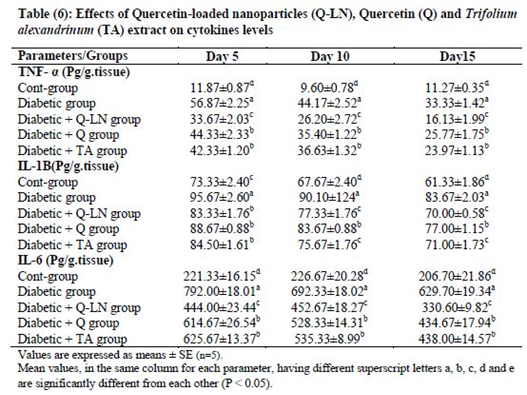
3.7. Effects of quercetin-loaded nanoparticles (Q-LNs), quercetin (Q) and Trifolium alexandrinum (TA) extract on cytokine levels
Tissue levels of TNF-α, IL-1β and IL-6 were significantly (p≤0.05) elevated in wounded diabetic rats on the 5th, 10th and 15th days compared to the corresponding wounded control rats. Treatment of wounded diabetic rats with Q-LNs, Q and TA extract produced significant reductions (p≤0.05) in the levels of TNF-α, IL-1β and IL-6 compared to those of the untreated diabetic group. Q-LNs was the most effective treatment in ameliorating the elevated TNF-α and IL-6 levels in wounded diabetic rats (Table 6).
3.6. Effects of quercetin-loaded nanoparticles (Q-LNs), quercetin (Q) and Trifolium alexandrinum extract (TA) on the histopathological results
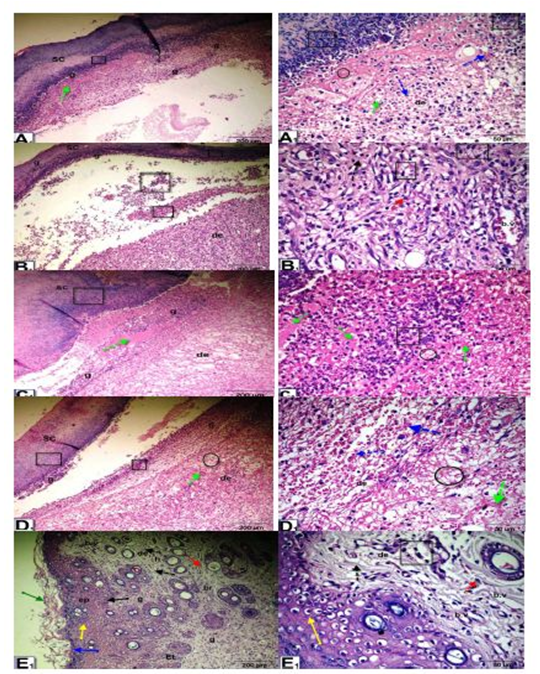
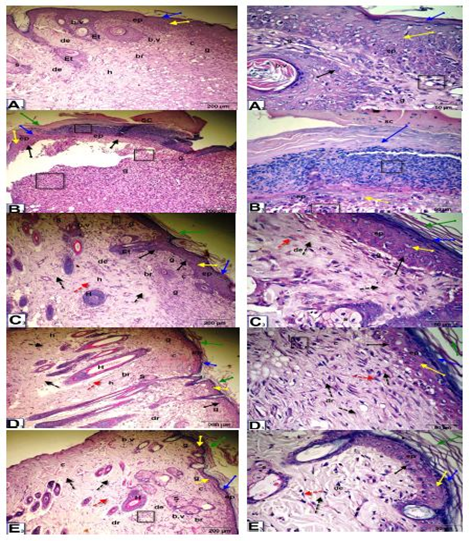
Histopathological observation of wound tissue in the experimental groups at day 5 after wounding showed changes in the different groups during the wound-healing process (Figure 3). The histological changes in the epidermis included scabs, inflammatory cell infiltration, re-epithelialization, epithelial and dermal damage, and increased fibrous connective tissue, fibroblasts, vacuolations, granulation, congestion, blood vessels and red blood cells. The untreated wounded diabetic group exhibited delayed wound healing compared to the control groups. On the other hand, Q-LNs, Q and TA extract treatment ameliorated these histological changes compared to those of the untreated diabetic group.
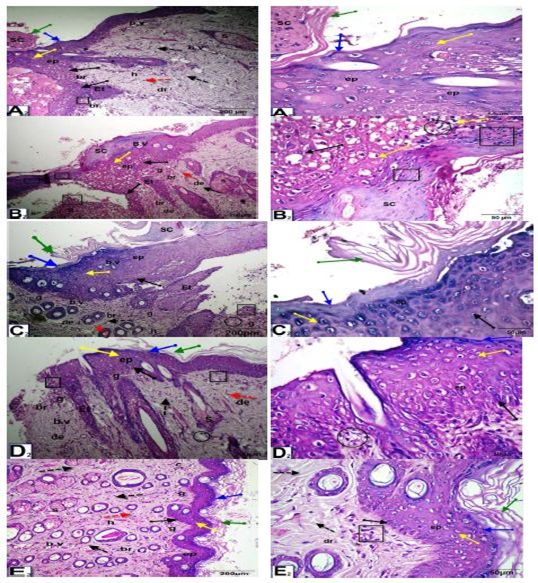
As shown in Figure (4), at 10 days after wounding, untreated wounded diabetic rats exhibited scabs, a thinner epidermis, inflammatory cell infiltration, vacuoles, and disorganization of the dermal matrix with increased granular cells and collagen. In the control nondiabetic wounded group and groups treated with Q-LNs, Q and TA extract, the wound space was almost closed, with newly formed epithelium with disappearance of scabs. The epidermal layers, stratum corneum, stratum lucidum, stratum granulosum and stratum spinosum appeared. The epithelial cells appeared mostly normal. The dermis contained numerous blood vessels, a granular layer with inflammatory cell infiltration, a healed area with multiple layers of fibrous connective tissue, fibroblasts, and a boundary between unhealed and healed tissue.
As shown in Figure (5), at 15 days after wounding, untreated wounded diabetic rats exhibited of remnants of scabs, the skin included a thinner epidermis with granular cells, the dermis contained dense granular cells, reduced collagen and fibroblasts and increased inflammatory cell infiltrate and reduced blood vessels. On the other hand, in the control wounded nondiabetic group and groups treated with Q-LNs, Q and TA extract, complete re-epithelialization was observed. Organized layers of epidermis, stratum corneum, stratum lucidum, stratum granulosum and stratum spinosum were present. The dermis contained numerous blood vessels, a granular layer with few inflammatory cell infiltration, a healed area with multiple layers of fibrous connective tissue with more collagen fibers, fibroblasts, and a boundary between unhealed and healed tissue.
Figure 3: Micrographs of wound tissue of the experimental groups at day 5 after wounding: showing histological changes in different groups during the wound-healing process; group control non diabetic wounded (A1), group diabetic untreated wounded (B1), group diabetic wounded orally and topically treated with Q-LNPs (C1), group diabetic wounded orally & topically treated with Q (D1), group diabetic wounded orally & topically treated with TA extract (E1) respectively, the histological changes include Scab (SC), inflammatory cells infiltrate (black square), re-epithelialization (black arrow), epithelial tongue (Et), stratum Corneum (green arrow), stratum Lucidum ( blue arrow), Stratum Granulosum (yellow arrow) and Stratum Spinosum (black arrow), dermis (de), fibrous connective tissue (black dotted arrow), fibroblast (red dotted arrow), vacuolations (black circle), granulation (g), Congestion (green dotted arrow), red blood cells (blue dotted arrow), blood vessels (b. v) (H&E stain, X 200& 420).
Figure 4: Photomicrographs of skin sections of male rats, 10 days after wounding; control un diabetic wounded (A2), group diabetic wounded orally and topically treated with Q-LNPs (C2), group diabetic wounded orally and topically treated with Q (D2) and group diabetic wounded orally and topically treated with TA extract (E2) respectively, showing that the wound space is almost closed with newly formed epithelium (ep) with disappearance of scap. Appearance of the epidermis layers, stratum Corneum (green arrow), stratum Lucidum (blue arrow), stratum granulosum (yellow arrow) and stratum spinosum (black arrow). The epithelial cells appeared more or less normal. The dermis (D) contained numerous blood vessels (b.v), granular layer (g) with inflammatory cells infiltrate (black square), (h) healed area with multiple layers of fibrous connective tissue (black dotted arrow), fibroblasts (red dotted arrow), boundary between unhealed and healed tissue (br). While, in diabetic untreated wounded (B2) presence of scap, skin included a thinner epidermis, inflammatory cells infiltrate (black square), vacuoles (black circle), disorganization of the dermal matrix with more granular cells (g) & collagen (c) (H&E x 200 & 400).
Figure 5: Photomicrographs of skin section of male rats, 15 days after wounding; control un treated wounded (A3), group diabetic wounded orally and topically treated with Q-LNP (C3), group diabetic wounded orally and topically treated with Q (D3) and group diabetic wounded orally and topically treated with TA extract (E3) respectively, showing complete re-epithelialization. Presence of layers of epidermis; stratum corneum (green arrow), stratum lucidum (blue arrow), stratum granulosum (yellow arrow) and stratum spinosum (black arrow). Dermis (de) contained numerous blood vessels (b.v), granular layer (g) with less inflammatory cells infiltrate (black square), (h) healed area with multiple layers of fibrous connective tissue (black dotted arrow) with more collagen fibers (c), fibroblasts (red dotted arrow), boundary between unhealed and healed tissue (br). While, in diabetic untreated wounded (B3) presence of remnant of scap (SC), skin included a thinner epidermis with granular cells (g) (black square), dermis with dense granular cells(g), less collagen(c), fibroblasts and more inflammatory cells infiltrate (black square) and less blood vessels (H&E stain, X 200& 400).
4. Discussion
Wound healing has been depicted as a process that requires efficient integration and interactions among various processes, growth factors and diverse types of cells and tissues [20]. Impaired wound healing has been established in diabetes mellitus. Despite the essential need for controlled inflammation, granulation and re-epithelialization, diabetes-induced impaired wound healing seem to be associated with dysfunctions in processes that lead to rapid healing [30]. Hyperglycemia is the key culprit responsible for impaired angiogenesis and proper wound healing [31].
In the current study, wound healing, as examined by morphological assessment and quantified by the wound closure percent, was impaired in diabetic rats; the wound closure reached 24.33% on day 5 and increased to 31.76% on day 10 and 49.13% on day 15 after injury compared to those of the control group. STZ-induced diabetes slowed wound healing and reduced perfusion due to the presence of peripheral arterial disease and diminished sensory nerve function caused by peripheral neuropathy, which may lead to impeded healing [32]. Treatment of wounded diabetic rats with Q-LNs, Q and TA extract promoted wound closure. The hypoglycemic effect of quercetin in the current study is in accordance with the findings of previous investigations, which indicated that quercetin exerted an antihyperglycemic effect in diabetic rats [20]. Furthermore, the phytochemical constituents present in TA extract may be responsible for the improvements in wound healing due to their antimicrobial properties, resulting in wound contraction and an increased rate of epithelialization [33]. Moreover, TA supports wound healing via numerous cellular mechanisms, such as chelating free radicals and reactive oxygen species, promoting wound contraction and enhancing capillary vessel and fibroblast formation [33]. The increased wound contraction induced by TA extract treatment might be due to the enhanced activity of fibroblasts in restoring wound tissue. Myofibroblasts are supposed to play a crucial role in wound contraction by applying tension on the surrounding extracellular matrix (ECM) and secreting proteins such as collagen to stabilize the contraction [34].
In the present study, the untreated diabetic group showed significant increases in glucose levels accompanied by decreases in insulin levels. STZ causes severe damage to both β-cells and pancreatic islets. Hyperglycemia results from the destruction of pancreatic β-cells, leading to deficiencies in insulin secretion [35]. Supplementation with Q-LNs, Q and TA extracts modulated glucose and insulin levels on the 5th, 10th and 15th days of treatment. These results may be due to the hypoglycemic effect of quercetin, which regulates the enzymes involved in glucose metabolism, changes β-cell functions and insulin activity and improves other diabetes-related parameters [36].
In the present study, there were significant increases in MDA levels and decreases in GSH levels and GPX and SOD activities in wound tissue homogenates from untreated diabetic wounds. The increased oxidative stress and diminished antioxidant activities during inflammation induce marked cytotoxic and degradative effects within the wound area, leading to delayed wound healing and wound closure in diabetes patients [37]. Consequently, the exclusion of ROS may be a vital strategy to improve wound healing [38]. However, tissue homogenates from diabetic wounds treated with Q-LNs, Q and TA extract showed significant antioxidant activity, as evidenced by the reduced levels of MDA and elevated levels of GSH, GPX and SOD activities in response to oxidative stress due to diabetes. These results could be explained by the antioxidant activities of quercetin and TA extract [33, 35]. Flavonoids are effective antioxidants that are able to scavenge free radical species and have been reported to play key roles in augmenting the wound-healing process [39]. Wound healing correlated with the efficient management of oxidative stress in response to TA extract treatment. Moreover, the inhibition of lipid peroxidation and free radical scavenging abilities aid in rapid wound repair by enhancing the transport of oxygen towards the wound and promoting other cellular repair mechanisms [33]. TA extract modulates the expression of certain enzymes involved in scavenging ROS and decreases myeloperoxidase and free radical-induced tissue damage, promoting antioxidant status, increased collagen deposition, the formation of other connective tissue constituents and antibacterial activity [33, 40].
The obtained results showed decreases in the levels of collagen, hexosamine and TGF-β1 and an increase in the MIF level in wounded tissue in the untreated diabetic group. Wound healing deficits associated with diabetes are complex and interrelated and are believed to be caused by impaired blood flow and impaired oxygen release due to increased blood sugar, decreased collagen and fibronectin synthesis owing to protein malnutrition, impaired local immunity and decreased anabolic activity, with decreased levels of insulin and growth hormone [41]. Many studies support the role of MIF in insulin resistance and have correlated type 2 diabetes with increased circulating MIF levels [42]. Hexosamine functions as a physiological glucose sensor that serves as an adaptor to divert excess calories towards storage as fat. In accordance with a previous report, Veeramani et al. [43] showed that diabetic rats had elevated levels of hexosamine, which could be due to increased expression of glutamine fructose-6-phosphate aminotransferase (GFA), which catalyzes the amination of fructose-6-phosphate to glucosamine-6-phosphate, which is rate limiting, and increased plasma glucose. Glycosaminoglycans are known to stabilize collagen fibers by enhancing electrostatic and ionic interactions and possibly control their ultimate alignment and characteristic size [44]. On the other hand, Q-LNs, Q and TA extract administration caused significant increases in the levels of collagen, hexosamine and TGF-β1 and a significant reduction in the MIF level in wounded tissue when compared to those of the untreated diabetic group on the 5th, 10th and 15th days of treatment. TA extract was effective in ameliorating these parameters. The wound healing potential of this herbal extract could be attributed to the high content of flavonoids, which impart astringent and antiseptic properties that are responsible for the increased rate of epithelialization and collagen synthesis, ultimately resulting in wound contraction [33]. TA extract treatment exerts its beneficial effects on wound healing by stimulating the collagen deposition and angiogenesis. Collagen is the predominant extracellular protein in the granulation tissue of healing wounds, and it is a principal component of connective tissue, providing a structural framework for the regenerating tissue. In addition, the stimulation of epithelial cell proliferation and angiogenesis is essential for wound healing [41]. Collagen synthesis by fibroblasts is TGF-β1-dependent, which is important for proper wound healing. Early studies have reported that TNF-α application decreased the expression of collagen and reduced the tensile strength of the wound. TNF-α inhibits Smad phosphorylation through the c-Jun N-terminal kinase pathway and reduces the transcription of TGF-β1, collagen 1A, fibronectin and alpha-smooth muscle actin [37]. Gopalakrishnan et al. [45] reported that quercetin could enhance TGF-β1 expression in healing wounds in the early proliferative phase, thereby favoring fibroblast activity and improved ECM deposition and granulation tissue formation, which might balance ECM deposition and degradation. TGF-β1 has various functions in wound healing and is likewise involved in upregulating the angiogenic growth factor vascular endothelial growth factor (VEGF). TGF-β1 can be both inhibitory and stimulatory in nature. TGF-β1 is supposed to act selectively as a negative regulator on early hematopoietic progenitor cells, thereby inhibiting angiogenesis and tumor progression [45]. Flavonoids inhibit lipid peroxidation and are supposed to increase collagen fibril viability by activating DNA synthesis and preventing cell damage [46]. Consequently, the wound healing effects of TA may be attributed to its phytochemicals. These phytochemicals might have additive effects that accelerate this progression, probably during the proliferation phase of wound healing [47].
Tissue TNF-α, IL-6 and IL-1β levels were significantly elevated in untreated wounded diabetic rats on the 5th, 10th and 15th days compared to the corresponding wounded controls. The treatment of wounded diabetic rats with Q-LNs, Q and TA extract abrogated the elevated TNF-α, IL-6 and IL-1β levels. Tissue destruction causes an acute inflammatory response, in which neutrophils, monocytes and mast cells infiltrate the site of injury and produce cytokines [48]. The invasion of monocytes and their differentiation into macrophages are essential for normal repair. Activated macrophages can produce several proinflammatory cytokines, including TNF-α, IL-6 and IL-1β. These cytokines exert a series of biological effects that might be important for inflammation and damaged tissue repair, including wound healing [49]. Q-LNs was the most effective treatment in ameliorating the elevated TNF-α and IL-6 levels in wounded diabetic rats. The levels of these cytokines are profoundly elevated in chronic wounds. This result is in accordance with that of Lee et al. [49], who suggested that proinflammatory cytokine production and subsequent inflammation should be inhibited by Q-LNs, as well as free quercetin, causing significant reductions in TNF-α, IL-6 and IL-1β. Treatment with quercetin hastens the switch from inflammatory to anti-inflammatory responses and the dominance of T helper 2 (Th2) on T helper 1 (Th1) cells during the process of wound healing [20]. Li et al. [50] reported that quercetin exerted its anti-inflammatory effects through inhibiting the production of TNF-α in macrophages and blocking TNF-α mediated inflammation. This effect may be directly mediated by preventing the activation of extracellular signal-related kinase (ERK), c-Jun NH2-terminal kinase (JNK), c-Jun, and nuclear factor-κB (NF-κB), which are potent inducers of inflammatory gene expression and protein secretion, or indirectly by stimulating peroxisome proliferator-activated receptor-γ (PPARγ) activity, thereby antagonizing NF-κB or activator protein-1 (AP-1) transcriptional activation of inflammatory genes. Peranteau et al. [51] reported that overexpression of anti-inflammatory cytokines decreased the inflammatory response to injury and created an environment that was conducive to regenerative wound healing. Based on these previously described ideas, treatment with quercetin limits prolonged inflammation and modulates the immune response during the progress of wound healing in diabetic animals [50].
The process of wound healing can be divided into five phases, including the cellular phase (granulation), wound contraction (narrowing of the wound area), collagenation (collagen deposition or formation of collagenous glue), epithelialization (epithelial covering) and cicatrization (scar remodeling). These phases overlap, and any agent that enhances these processes can promote wound healing. DM delays wound healing by affecting these processes [20]. Histopathological examination of the steps of the wound healing process (inflammation, proliferation, and remodeling) were observed within the experimental groups. Delayed wound healing processes were observed in the untreated diabetic group compared to the control group. Moreover, the untreated diabetic group showed marked epidermal necrosis and increased inflammatory cell migration, indicating an impaired wound healing process in the untreated diabetic group. However, treatment with Q-LNs, Q and TA extract showed mature granulation tissue formation and epidermal regeneration, followed by hyperkeratosis, thus improving the healing of diabetic wounds. These histological changes in the skin of wounded rats were accompanied by increases serum levels of the proinflammatory cytokines TNF-α, IL-6 and IL-1β. These cytokine levels were worse in wounded diabetic rats than in wounded nondiabetic rats; therefore, the worst deleterious effects suggest that these inflammatory cytokines may play crucial roles in delaying wound healing in diabetic rats compared to nondiabetic rats. This evidence was supported by Ebaid et al. [32].
5. Conclusion
The current study revealed that the treatment of diabetic wounds with Q-LNs, Q and TA extract orally and topically could significantly accelerate the wound healing process. Additionally, TA extract is an important source of many healthy components, such as antioxidant and antimicrobial agents. Furthermore, the presence of isoflavones, of which quercetin exhibited the highest concentration, makes TA a superior treatment in wound healing, providing protection of wound healing. This finding is supported by the accelerated rate of wound contraction, as well as by enhanced epithelization. Therefore, the present study showed that one of the mechanisms by which the isolated constituents Q-LNs, Q and TA extract improve healing may be due to their capacity to enhance tissue antioxidant levels and their anti-inflammatory effects. The use of TA extract and its bioactive components is recommended to enhance the wound healing process in excision wounds. In addition, additional studies of the bioactive components of the TA extract would contribute to other medicinal applications.
Acknowledgments
The authors are thankful to histological analysis by Prof. Dr. M.A.H. Yehia lab at the, Medical Research Institute, Alexandria University, Egypt.
Funding: This research is funded by (STDF) Science & Technology Development Fund, Ministry of Scientific Research, Egypt. Project number (22895).
Competing interests: The authors declare that they have no competing interests.
References
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Science Translational Medicine 6 (2014): 265sr6.
- Barman PK, Koh TJ. Macrophage dysregulation and impaired skin wound healing in diabetes. Frontiers in Cell and Developmental Biology 8 (2020).
- Boyce ST, Lalley AL. Tissue engineering of skin and regenerative medicine for wound care. Burns & Trauma 6 (2018).
- Amer M, El-Habibi ES, El-Gendy A. Effects of Trifolium alexandrinum extracts on streptozotocin-induced diabetes in male rats. Annals of Nutrition and Metabolism 48 (2004): 343-347.
- Barman PK, Urao N, Koh TJ. Diabetes induces myeloid bias in bone marrow progenitors associated with enhanced wound macrophage accumulation and impaired healing. The Journal of Pathology 249 (2019): 435-446.
- Hu SC, Lan CC. High-glucose environment disturbs the physiologic functions of keratinocytes: focusing on diabetic wound healing. Journal of Dermatological Science 84 (2016): 121-127.
- Umeno A, Horie M, Murotomi K, et al. Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules 21 (2016): 708.
- Wang W, Sun C, Mao L, et al. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends in Food Science & Technology 56 (2016): 21-38.
- Vedakumari WS, Ayaz N, Karthick AS, et al. Quercetin impregnated chitosan–fibrin composite scaffolds as potential wound dressing materials—Fabrication, characterization and in vivo analysis. European Journal of Pharmaceutical Sciences 97 (2017): 106-112.
- Zhang Y, Yang Y, Tang K, et al. Physicochemical characterization and antioxidant activity of quercetin-loaded chitosan nanoparticles. Journal of Applied Polymer Science 107 (2008): 891-897.
- Mohamed AH, Abd El-Hameed MN, Khamis HH, et al. Antidiabetic and anti-inflammatory effects of two Fabaceae extracts against streptozotocin induced diabetic impairment in male rats. World Journal of Advanced Research and Reviews 6 (2020): 12-29.
- Limmongkon A, Janhom P, Amthong A, et al. Antioxidant activity, total phenolic, and resveratrol content in five cultivars of peanut sprouts. Asian Pacific Journal of Tropical Biomedicine 7 (2017): 332-338.
- Kiranmai M, Kumar CM, Mohammed I. Comparison of total flavanoid content of Azadirachta indica root bark extracts prepared by different methods of extraction. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2 (2011): 254-261.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical Biochemistry 269 (1999): 337-341.
- Ramos GP, Dias PM, Morais CB, et al. LC determination of four isoflavone aglycones in red clover (Trifolium pratense L.). Chromatographia 67 (2008): 125-129.
- Zhang Y, Dong H, Wang M, et al. Quercetin isolated from Toona sinensis leaves attenuates hyperglycemia and protects hepatocytes in high-carbohydrate/high-fat diet and alloxan induced experimental diabetic mice. Journal of Diabetes Research 2016 (2016).
- Ekperikpe US, Owolabi OJ, Olapeju BI. Effects of Parkia biglobosa aqueous seed extract on some biochemical, haematological and histopathological parameters in streptozotocin induced diabetic rats. Journal of Ethnopharmacology 228 (2019): 1-10.
- Youth RA, Simmerman SJ, Newell R, et al. Ketamine anesthesia for rats. Physiology & Behavior 10 (1973): 633-636.
- Zhang W, Wang Y, Yang Z, et al. Antioxidant treatment with quercetin ameliorates erectile dysfunction in streptozotocin-induced diabetic rats. Journal of Bioscience and Bioengineering 112 (2011): 215-218.
- Ahmed OM, Mohamed T, Moustafa H, et al. Quercetin and low level laser therapy promote wound healing process in diabetic rats via structural reorganization and modulatory effects on inflammation and oxidative stress. Biomedicine & Pharmacotherapy 101 (2018): 58-73.
- Lowry OH, Rosebrough NJ, Farr AL, et al. Estimation of Total Protein Content. J Biological Chem 193 (1951): 265.
- Varshney R, Kale RK. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. International Journal of Radiation Biology 58 (1990): 733-743.
- Jollow DJ, Mitchell JR, Zampaglione NA, et al. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11 (1974): 151-169.
- Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochemical and Biophysical Research Communications 46 (1972): 849-854.
- Rotruck JT, Pope AL, Ganther HE, et al. Selenium: biochemical role as a component of glutathione peroxidase. Science 179 (1973): 588-590.
- Woessner Jr JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Archives of Biochemistry and Biophysics 93 (1961): 440-447.
- Elson LA, Morgan WT. A colorimetric method for the determination of glucosamine and chondrosamine. Biochemical Journal 27 (1933): 1824-1828.
- Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. Elsevier Health Sciences (2008).
- Roa M, Blane K, Zonneberg M. One way analysis of variance. Version IA (C). PC-STAT, Program coded by University of Georgia, USA (1985).
- Seitz O, Schürmann C, Hermes N, et al. Wound healing in mice with high-fat diet-or ob gene-induced diabetes-obesity syndromes: a comparative study. Experimental Diabetes Research (2010) 2010.
- Ren J, Yang M, Xu F, et al. Acceleration of wound healing activity with syringic acid in streptozotocin induced diabetic rats. Life Sciences 233 (2019): 116728.
- Ebaid H, Abdel-Salam B, Hassan I, et al. Camel milk peptide improves wound healing in diabetic rats by orchestrating the redox status and immune response. Lipids in Health and Disease 14 (2015): 132.
- Shah AS, Ahmed M, Alkreathy HM, et al. Phytochemical screening and protective effects of Trifolium alexandrinum (L.) against free radical-induced stress in rats. Food Science & Nutrition 2 (2014): 751-757.
- Wang L, Yang L, Yuan G, et al. Wound-healing effect of Platycodon grandiflorus (Jacq.) extract in rats. Tropical Journal of Pharmaceutical Research 18 (2019): 1909-1912.
- Yang DK, Kang HS. Anti-diabetic effect of cotreatment with quercetin and resveratrol in streptozotocin-induced diabetic rats. Biomolecules & Therapeutics 26 (2018): 130.
- Ahmad M, Sultana M, Raina R, et al. Hypoglycemic, hypolipidemic, and wound healing potential of quercetin in streptozotocin-induced diabetic rats. Pharmacognosy Magazine 13 (2017): S633.
- Kant V, Gopal A, Pathak NN, et al. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. International Immunopharmacology 20 (2014): 322-330.
- Shetty SB. Wound healing and indigenous drugs: Role as antioxidants: A Review. Research and Reviews: Journal of Medical and Health Sciences 2 (2013): 05-16.
- Arunachalam KD, Subhashini S, Annamalai SK. Wound healing and antigenotoxic activities of Aegle marmelos with relation to its antioxidant properties. J Pharm Res 5 (2012): 1492-1502.
- Murthy S, Gautam MK, Goel S, et al. Evaluation of in vivo wound healing activity of Bacopa monniera on different wound model in rats. BioMed Research International (2013) 2013.
- Al-Bayaty F, Abdulla MA. A comparison of wound healing rate following treatment with aftamed and chlorine dioxide gels in streptozotocin-induced diabetic rats. Evidence-Based Complementary and Alternative Medicine (2012) 2012.
- Sánchez-Zamora YI, Rodriguez-Sosa M. The role of MIF in type 1 and type 2 diabetes mellitus. Journal of Diabetes Research (2014) 2014.
- Veeramani C, Al-Numair KS, Alsaif MA, et al. Protective effect of Cardiospermum halicacabum leaf extract on glycoprotein components on STZ–induced hyperglycemic rats. Asian Pacific Journal of Tropical Medicine 5 (2012): 939-944.
- Carretero A, da Costa DS, Reis RL, et al. Extracellular matrix-inspired assembly of glycosaminoglycan–collagen fibers. Journal of Materials Chemistry B 5 (2017): 3103-3106.
- Gopalakrishnan A, Ram M, Kumawat S, et al. Quercetin accelerated cutaneous wound healing in rats by increasing levels of VEGF and TGF-β1. Indian Journal of Experimental Biology 54 (2016): 187.
- Shetty S, Udupa S, Udupa L. Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of Ocimum sanctum Linn in rats. Evidence-Based Complementary and Alternative Medicine 5 (2008).
- Süntar IP, Akkol EK, Yalçin FN, et al. Wound healing potential of Sambucus ebulus L. leaves and isolation of an active component, quercetin 3-O-glucoside. Journal of Ethnopharmacology 129 (2010): 106-114.
- Ghaisas MM, Kshirsagar SB, Sahane RS. Evaluation of wound healing activity of ferulic acid in diabetic rats. International Wound Journal 11 (2014): 523-532.
- Lee GH, Lee SJ, Jeong SW, et al. Antioxidative and antiinflammatory activities of quercetin-loaded silica nanoparticles. Colloids and Surfaces B: Biointerfaces 143 (2016): 511-517.
- Li Y, Yao J, Han C, et al. Quercetin, inflammation and immunity. Nutrients 8 (2016): 167.
- Peranteau WH, Zhang L, Muvarak N, et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. Journal of Investigative Dermatology 128 (2008): 1852-1860.


 Impact Factor: * 2.5
Impact Factor: * 2.5 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 