Recurrent Stroke In 34 Year-Old, Unusual Presentation of Non-Bacterial Endocarditis
Article Information
Zhang Y, Klarich KW, Cicek S, Maleszewski J, Casanegra AI*
Department of Cardiovascular Medicine, Mayo Clinic, 200 FirstStreet SW, Rochester, Minnesota 55905
*Corresponding Author: Dr. Ana I. Casanegra, Department of Cardiovascular Medicine, Mayo Clinic, 200 FirstStreet SW, Rochester, Minnesota 55905, United States
Received: 07 January 2020; Accepted: 15 January 2020; Published: 02 March 2020
Citation: Zhang Y, Klarich KW, Cicek S, Maleszewski J, Casanegra AI. Recurrent Stroke In 34 Year-Old, Unusual Presentation of Non-Bacterial Endocarditis. Archives of Clinical and Medical Case Reports 4 (2020): 192-196.
View / Download Pdf Share at FacebookAbstract
Factor V Leiden (FVL) and prothrombin G20210A (PGM) mutations are the most frequent genetic risk factors involved in deep venous thrombosis in Caucasians. Being heterozygous for both seems to increase the risk of venous events but it is not well established if the association increases the risk of arterial thrombosis or other thrombotic events. We present a case of recurrent stroke caused by non-bacterial thrombotic endocarditis (NBTE) in a 34-year-old Caucasian man heterozygous for both FVL and PGM. His diagnosis of NBTE was confirmed with pathology in the absence of other causes of NBTE.
Keywords
Prothrombin gene G20210A; Factor V Leiden; Cardiac mass; Anticoagulation; Thromboembolism
Prothrombin gene G20210A articles, Factor V Leiden articles, Cardiac mass articles, Anticoagulation articles, Thromboembolism articles
Prothrombin gene G20210A articles Prothrombin gene G20210A Research articles Prothrombin gene G20210A review articles Prothrombin gene G20210A PubMed articles Prothrombin gene G20210A PubMed Central articles Prothrombin gene G20210A 2023 articles Prothrombin gene G20210A 2024 articles Prothrombin gene G20210A Scopus articles Prothrombin gene G20210A impact factor journals Prothrombin gene G20210A Scopus journals Prothrombin gene G20210A PubMed journals Prothrombin gene G20210A medical journals Prothrombin gene G20210A free journals Prothrombin gene G20210A best journals Prothrombin gene G20210A top journals Prothrombin gene G20210A free medical journals Prothrombin gene G20210A famous journals Prothrombin gene G20210A Google Scholar indexed journals gene G20210A articles gene G20210A Research articles gene G20210A review articles gene G20210A PubMed articles gene G20210A PubMed Central articles gene G20210A 2023 articles gene G20210A 2024 articles gene G20210A Scopus articles gene G20210A impact factor journals gene G20210A Scopus journals gene G20210A PubMed journals gene G20210A medical journals gene G20210A free journals gene G20210A best journals gene G20210A top journals gene G20210A free medical journals gene G20210A famous journals gene G20210A Google Scholar indexed journals Factor V Leiden articles Factor V Leiden Research articles Factor V Leiden review articles Factor V Leiden PubMed articles Factor V Leiden PubMed Central articles Factor V Leiden 2023 articles Factor V Leiden 2024 articles Factor V Leiden Scopus articles Factor V Leiden impact factor journals Factor V Leiden Scopus journals Factor V Leiden PubMed journals Factor V Leiden medical journals Factor V Leiden free journals Factor V Leiden best journals Factor V Leiden top journals Factor V Leiden free medical journals Factor V Leiden famous journals Factor V Leiden Google Scholar indexed journals Cardiac mass articles Cardiac mass Research articles Cardiac mass review articles Cardiac mass PubMed articles Cardiac mass PubMed Central articles Cardiac mass 2023 articles Cardiac mass 2024 articles Cardiac mass Scopus articles Cardiac mass impact factor journals Cardiac mass Scopus journals Cardiac mass PubMed journals Cardiac mass medical journals Cardiac mass free journals Cardiac mass best journals Cardiac mass top journals Cardiac mass free medical journals Cardiac mass famous journals Cardiac mass Google Scholar indexed journals Anticoagulation articles Anticoagulation Research articles Anticoagulation review articles Anticoagulation PubMed articles Anticoagulation PubMed Central articles Anticoagulation 2023 articles Anticoagulation 2024 articles Anticoagulation Scopus articles Anticoagulation impact factor journals Anticoagulation Scopus journals Anticoagulation PubMed journals Anticoagulation medical journals Anticoagulation free journals Anticoagulation best journals Anticoagulation top journals Anticoagulation free medical journals Anticoagulation famous journals Anticoagulation Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals Thromboembolism articles Thromboembolism Research articles Thromboembolism review articles Thromboembolism PubMed articles Thromboembolism PubMed Central articles Thromboembolism 2023 articles Thromboembolism 2024 articles Thromboembolism Scopus articles Thromboembolism impact factor journals Thromboembolism Scopus journals Thromboembolism PubMed journals Thromboembolism medical journals Thromboembolism free journals Thromboembolism best journals Thromboembolism top journals Thromboembolism free medical journals Thromboembolism famous journals Thromboembolism Google Scholar indexed journals NBTE articles NBTE Research articles NBTE review articles NBTE PubMed articles NBTE PubMed Central articles NBTE 2023 articles NBTE 2024 articles NBTE Scopus articles NBTE impact factor journals NBTE Scopus journals NBTE PubMed journals NBTE medical journals NBTE free journals NBTE best journals NBTE top journals NBTE free medical journals NBTE famous journals NBTE Google Scholar indexed journals kidney articles kidney Research articles kidney review articles kidney PubMed articles kidney PubMed Central articles kidney 2023 articles kidney 2024 articles kidney Scopus articles kidney impact factor journals kidney Scopus journals kidney PubMed journals kidney medical journals kidney free journals kidney best journals kidney top journals kidney free medical journals kidney famous journals kidney Google Scholar indexed journals Surgery articles Surgery Research articles Surgery review articles Surgery PubMed articles Surgery PubMed Central articles Surgery 2023 articles Surgery 2024 articles Surgery Scopus articles Surgery impact factor journals Surgery Scopus journals Surgery PubMed journals Surgery medical journals Surgery free journals Surgery best journals Surgery top journals Surgery free medical journals Surgery famous journals Surgery Google Scholar indexed journals
Article Details
1. Case Report
We describe a 34-year-old Caucasian man presenting for evaluation of recurrent stroke of indeterminate etiology.He had history of hypertension, hyperlipidemia, diabetes mellitus type 2, smoking, and obstructive sleep apnea. He was on testosterone replacement therapy since 2015. He denied personal or familial history of thrombotic events. One year prior to our evaluation, he woke up with severe headache, slurred speech, and balance difficulties. An MRI reported several ischemic strokes. He was found to be heterozygous for the Factor V Leiden (FVL) mutation. He was started on Apixaban 5 mg/12 hs for stroke prevention. He cut back on smoking and discontinued the testosterone replacement therapy.
He remained on Apixaban but had recurrent symptoms 6 months after the initial presentation including headache, slurred speech, peripheral vision loss and unsteadiness. A second MRI of the brain indicated 4 new ischemic strokes involving the right median cerebral artery territory, right cerebellar hemisphere, and left occipital lobe, suggesting a central embolic mechanism. MRA of the head and neck was unremarkable and negative for dissection, plaques, or vascular malformations. A transesophageal echocardiogram was reported normal and he was transitioned to Rivaroxaban 20 mg and aspirin 81 mg daily.
He presented to us for a second opinion regarding cryptogenic strokes. The physical exam was normal, with the exception of a mildly impaired tandem gait and hyperreflexia in the lower extremities. A workup for inherited or acquired thrombophilia resulted in normal levels of protein C, protein S, and antithrombin, no antiphospholipid antibodies or lupus anticoagulant. In addition to his heterozygosity for FVL, he was found to be heterozygous for the mutation in the prothrombin gene G20210A (PGM). He had no evidence of rheumatologic or autoimmune diseases, age appropriate cancer screening was negative.
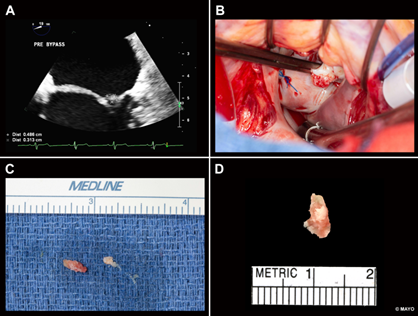
Figure 1: (A )Transesophageal Echocardiogram:Rounded mass measuring 5 mm x 4 mm attached by a broad base to the left atrial side of the lateral P2 scallop of the mitral valve (B)Intraoperative photograph shows a soft, friable mass arising from the mitral valve (C) Resected thrombus growing from the mitral valve (D) The pathology picture of mitral valve vegetation.
A transesophageal echocardiogram demonstrated a rounded mass measuring 5 mm × 4 mm, with small, associated mobile filaments, attached by a broad base to the left atrial side of the lateral P2 scallop of the mitral valve (Figure 1A). There was also a long, linear echodensity, which was originating from the left atrial appendage (LAA), and out past the Coumadin ridge to the left atrium. Blood cultures were negative for bacteria and fungus repeatedly. The differential diagnosis at that point included papillary fibroelastoma, myxoma, thrombus, and sarcoma. The latter was unconvincing considering the characteristics of the image in the MRI, and thrombus was thought to be unlikely due to the high velocities within the left atrial appendage. Given the history of multiple strokes despite anticoagulation, the cardiac mass was removed by open heart surgery (Figure 1B, 1C).
Surgical Pathology confirmed the presence of fibrin-rich thrombus without identifiable microorganisms (Figure 1D). A CT of the chest, abdomen and pelvis was not suggestive of malignancy. The linear echo density from the LAA was an avulsed congenital atrial band which is considered of no clinical consequence and unlikely related to the patients recurrent strokes.
This patient is heterozygous for the prothrombotic FVL and PGM combined, and was diagnosed with pathology confirmed nonbacterial thrombotic endocarditis (NBTE) on the mitral valve. NBTE is usually discovered at postmortem and is found in 1.2% of patients in autopsy series [1]. NBTE vegetations commonly involve the mitral and aortic valves; these lesions can affect both damaged and undamaged cardiac valves, and the chordae tendineae or the endocardium. Compared with the lesions in infective endocarditis, NBTE vegetations are more friable and prone to systemic embolization [2].
FVL and PGM mutations are the most common prothrombotic mutations in the Caucasian population, being present in 5% [3]and 1-3% [4] respectively. Being heterozygous for each of these mutations has shown to have approximately a 5-fold and 3-fold risk increase of venous thromboembolism (VTE) respectively compared to controls [5, 6]. Combined heterozygosity for FVL and PGM is a rare condition present in approximately in 1 in 1000 in the general population and 2.2 per 100 in patients with VTE [7]. Being a compound heterozygous (FVL + PGM) increases the thrombotic risk 20-fold [7].
Whether these mutations also increase the risk of arterial thrombosis is not well established. Although there is not an association between heterozygous FVL and arterial therombosis [8], a retrospective family cohort study showed an association with arterial events in double heterozygous and homozygous carriers [9]. When arterial events occur in a young person, inherited abnormalities of hemostasis seem to play a role, particularly when associated with smoking or estrogen use [7]. We are not aware of other reports of NBTE in patients with FVL and PGM combined in the absence of other causes of NBTE. De Paulis et al. [10] reported chronic thrombotic pannus and later acute thrombosis of a mechanical prosthetic valve while on anticoagulation in a patient with both FVL and PGM mutations, and in the absence of other underlying conditions, besides the acute use of tranexamic acid.
Our patient had a pathology proven diagnosis of NBTE, consisting of platelet rich thrombus. The instigating factor causing NBTE is not entirely known but involves endothelial cell injury in the setting of a hypercoagulable state. NBTE is most notably associated with malignancy, antiphospholipid syndrome, and SLE [12-14]. Our patient tested negative for antiphospholipid syndrome, and lupus, additionally there was no malignancy identified by screening, by CT of the chest, abdomen and pelvis, or during follow up. Although platelet-rich thrombus is more commonly associated with arterial events and both FVL and PGM are more commonly associated with venous events, we hypothesize that in the setting of being compound heterozygous in addition with smoking and testosterone supplementation may have created the ideal milieu for this event.
There is no proven therapy to treat NBTE, once diagnosed; treatment usually consists of systemic anticoagulation and treatment of the underlying condition. Addressing the patient’s underlying disease is the most effective management for NBTE. Surgical intervention may be necessary in select cases, especially those presenting with heart failure, acute valve rupture, or recurrent embolism. Our patient underwent surgery because of recurrent emboli despite anticoagulation, and uncertainty of the etiology. Although the NBTE lesions were removed, he will continue on lifelong anticoagulation due to his risk of recurrence of NBTE, stroke and venous thromboembolism[14]in the setting of compound heterozygocity.
Conflict of Interest
None declared.
References
- el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist 12 (2007): 518-23.
- Roldan CA, et al. Libman-Sacks endocarditis and embolic cerebrovascular disease. JACC Cardiovasc Imaging 6 (2013): 973-983.
- Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet 346 (1995): 1133-1134.
- Rosendaal FR, et al. Geographic distribution of the 20210 G to A prothrombin variant. Thromb Haemost 79 (1998): 706-708.
- Middeldorp S, et al. A prospective study of asymptomatic carriers of the factor V Leiden mutation to determine the incidence of venous thromboembolism. Ann Intern Med 135 (2001): 322-327.
- Coppens M, et al. A prospective cohort study on the absolute incidence of venous thromboembolism and arterial cardiovascular disease in asymptomatic carriers of the prothrombin 20210A mutation. Blood 108 (2006): 2604-2607.
- Emmerich J, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism--pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost 86 (2001): 809-816.
- Juul K, et al. Factor V Leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood 100 (2002): 3-10.
- Roach RE, et al. Risk of cardiovascular disease in double heterozygous carriers and homozygous carriers of F5 R506Q (factor V Leiden) and F2 (prothrombin) G20210A: a retrospective family cohort study. Br J Haematol 153 (2011): 134-136.
- De Paulis S, et al. Early postoperative obstructive prosthetic mitral valve thrombosis in a patient double heterozygous for factor V Leiden and prothrombin G20210A mutation. Thromb Haemost 99 (2008): 441-442.
- Roudaut R, Serri K, Lafitte S, et al. Thrombosis of prosthetic heart valves: diagnosis and therapeutic considerations. Heart 93 (2007): 137-142.
- Bhimani AA, Hoit BD. Extensive nonbacterial thrombotic endocarditis isolated to the tricuspid valve in primary antiphospholipid syndrome. J Am Soc Echocardiogr 23 (2010): 107 e5-6.
- Smeglin A, et al. Marantic endocarditis and disseminated intravascular coagulation with systemic emboli in presentation of pancreatic cancer. J Clin Oncol 26 (2008): 1383-1385.
- Middeldorp S, Vlieg AV. Does thrombophilia testing help in the clinical management of patients? British Journal of Haematology 143 (2008): 321-335.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
