Safety and Efficacy of Animal Derived Surfactants in Treating Preterm Infants with Respiratory Distress Syndrome: A Retrospective Cohort Study
Article Information
Xiaoao Dong1, Abdul Haium Abdul Alim2, Seyed Ehsan Saffari3, Mei Chien Chua2, Victor Samuel Rajadurai2, Suresh Chandran2*
1Resident Medical Officer, Department of Neonatology, KK Women’s and Children’s Hospital, Singapore
2Senior Consultant, Department of Neonatology, KK Women’s and Children’s Hospital, Singapore; Duke-NUS Graduate School of Medicine, Singapore; Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Lee Kong Chian School of Medicine, Nanyang Technology University, Singapore
3Center for Qualitative Medicine, Duke-NUS Medical School, Singapore
*Corresponding Author: A/Prof Suresh Chandran, Senior Consultant, Department of Neonatology, KK Women’s and Children’s Hospital, Singapore
Received: 08 May 2021; Accepted: 20 May 2021; Published: 24 May 2021
Citation: Xiaoao Dong, Abdul Haium Abdul Alim, Seyed Ehsan Saffari, Mei Chien Chua, Victor Samuel Rajadurai, Suresh Chandran. Safety and Efficacy of Animal Derived Surfactants in Treating Preterm Infants with Respiratory Distress Syndrome: A Retrospective Cohort Study. Archives of Clinical and Medical Case Reports 5 (2021): 455-465.
View / Download Pdf Share at FacebookAbstract
Background: Animal-derived surfactants contain different chemical compositions with various responses, efficacy and safety profiles in treating preterm infants with respiratory distress syndrome. The study aimed to compare the safety and efficacy between poractant alfa (Curosurf®) and beractant (Survanta®) in preterm infants requiring endotracheal surfactant therapy.
Methods: This is a single-center, retrospective observational cohort study involving preterm infants who required endotracheal surfactant therapy at our hospital from January 2015 to January 2016. Incidence of pneumothorax, chronic lung disease (CLD), mortality, and the composite outcome of CLD and death were compared between the infants who received poractant alfa and beractant.
Results: Overall, 179 preterm infants received endotracheal surfactants. Of these, 70 (31%) and 109 (69%) received poractant alfa and beractant, respectively. The incidence of pneumothorax in infants treated with poractant alfa was significantly lower than those treated with beractant (0.00% vs. 6.42%, P = 0.031). Compared with beractant, poractant alfa significantly reduced the incidence of pneumothorax in very low birth weight (VLBW) infants (0.00% vs. 8.00%, P = 0.035), but not in non-VLBW infants (poractant alfa 0.00% vs. beractant 3.03%, P = 1.00). The rates of CLD, mortality, and composite outcomes of CLD and death were not different between the two groups.
Conclusions: Poractant alfa significantly reduced the rates of pneumothorax in preterm infants with respiratory distress syndrome. This reduction was more significant in VLBW than non-VLBW infants. However, mortality and morbidity were not different between these two groups.
Keywords
Surfactant; Poractant alfa; Beractant; Preterm; Pneumothorax
Surfactant articles; Poractant alfa articles; Beractant articles; Preterm articles; Pneumothorax articles
Surfactant articles Surfactant Research articles Surfactant review articles Surfactant PubMed articles Surfactant PubMed Central articles Surfactant 2023 articles Surfactant 2024 articles Surfactant Scopus articles Surfactant impact factor journals Surfactant Scopus journals Surfactant PubMed journals Surfactant medical journals Surfactant free journals Surfactant best journals Surfactant top journals Surfactant free medical journals Surfactant famous journals Surfactant Google Scholar indexed journals Poractant alfa articles Poractant alfa Research articles Poractant alfa review articles Poractant alfa PubMed articles Poractant alfa PubMed Central articles Poractant alfa 2023 articles Poractant alfa 2024 articles Poractant alfa Scopus articles Poractant alfa impact factor journals Poractant alfa Scopus journals Poractant alfa PubMed journals Poractant alfa medical journals Poractant alfa free journals Poractant alfa best journals Poractant alfa top journals Poractant alfa free medical journals Poractant alfa famous journals Poractant alfa Google Scholar indexed journals Beractant articles Beractant Research articles Beractant review articles Beractant PubMed articles Beractant PubMed Central articles Beractant 2023 articles Beractant 2024 articles Beractant Scopus articles Beractant impact factor journals Beractant Scopus journals Beractant PubMed journals Beractant medical journals Beractant free journals Beractant best journals Beractant top journals Beractant free medical journals Beractant famous journals Beractant Google Scholar indexed journals Preterm articles Preterm Research articles Preterm review articles Preterm PubMed articles Preterm PubMed Central articles Preterm 2023 articles Preterm 2024 articles Preterm Scopus articles Preterm impact factor journals Preterm Scopus journals Preterm PubMed journals Preterm medical journals Preterm free journals Preterm best journals Preterm top journals Preterm free medical journals Preterm famous journals Preterm Google Scholar indexed journals Pneumothorax articles Pneumothorax Research articles Pneumothorax review articles Pneumothorax PubMed articles Pneumothorax PubMed Central articles Pneumothorax 2023 articles Pneumothorax 2024 articles Pneumothorax Scopus articles Pneumothorax impact factor journals Pneumothorax Scopus journals Pneumothorax PubMed journals Pneumothorax medical journals Pneumothorax free journals Pneumothorax best journals Pneumothorax top journals Pneumothorax free medical journals Pneumothorax famous journals Pneumothorax Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Renocardiac syndrome articles Renocardiac syndrome Research articles Renocardiac syndrome review articles Renocardiac syndrome PubMed articles Renocardiac syndrome PubMed Central articles Renocardiac syndrome 2023 articles Renocardiac syndrome 2024 articles Renocardiac syndrome Scopus articles Renocardiac syndrome impact factor journals Renocardiac syndrome Scopus journals Renocardiac syndrome PubMed journals Renocardiac syndrome medical journals Renocardiac syndrome free journals Renocardiac syndrome best journals Renocardiac syndrome top journals Renocardiac syndrome free medical journals Renocardiac syndrome famous journals Renocardiac syndrome Google Scholar indexed journals Abdominal surgery articles Abdominal surgery Research articles Abdominal surgery review articles Abdominal surgery PubMed articles Abdominal surgery PubMed Central articles Abdominal surgery 2023 articles Abdominal surgery 2024 articles Abdominal surgery Scopus articles Abdominal surgery impact factor journals Abdominal surgery Scopus journals Abdominal surgery PubMed journals Abdominal surgery medical journals Abdominal surgery free journals Abdominal surgery best journals Abdominal surgery top journals Abdominal surgery free medical journals Abdominal surgery famous journals Abdominal surgery Google Scholar indexed journals lymphadenopathy articles lymphadenopathy Research articles lymphadenopathy review articles lymphadenopathy PubMed articles lymphadenopathy PubMed Central articles lymphadenopathy 2023 articles lymphadenopathy 2024 articles lymphadenopathy Scopus articles lymphadenopathy impact factor journals lymphadenopathy Scopus journals lymphadenopathy PubMed journals lymphadenopathy medical journals lymphadenopathy free journals lymphadenopathy best journals lymphadenopathy top journals lymphadenopathy free medical journals lymphadenopathy famous journals lymphadenopathy Google Scholar indexed journals
Article Details
1. Introduction
Respiratory distress syndrome (RDS) is a major cause of morbidity and mortality in preterm infants. In 1980, Fujiwara et al. first reported successful administration of exogenous surfactants in newborns with RDS [1]. Since then, the clinical use of surfactants has been rapidly evolving. Earlier synthetic surfactants contained phospholipids only. The discovery of surfactant proteins led to the development of new protein-containing preparations [2]. Subsequent studies soon established that protein-containing surfactant preparations are more superior than non-protein containing synthetic surfactants [3]. However, the best therapeutic choice and regimen of protein-containing surfactant remain to be established.
Poractant alfa (CurosurfÒ, Chiesi Farmaceutici, Parma, Italy) and beractant (SurvantaÒ, Abbott Laboratories, Abbott Park, IL, USA) are animal-derived, protein-containing surfactants that are commonly used to treat RDS. Poractant alfa is extracted from minced porcine lung tissues, while beractant contains minced bovine lung extract with added synthetic lipids [4]. These surfactants differ in their chemical compositions. Although studies have compared the clinical outcomes of these animal-derived surfactants, their results remain equivocal. A retrospective cohort study by Ramanathan et al. demonstrated that a significant reduction of mortality was associated with poractant alfa usage [5]. However, this was challenged by a subsequent study by Trembath et al., where they reported a similar impact on mortality from the two surfactants [6].
On the other hand, a meta-analysis examining randomized controlled trials (RCT) on porcine and bovine surfactants showed a significant reduction in all-cause mortality in infants treated with poractant alfa [7]. To date, there is no conclusive evidence that supports the clinical superiority of one surfactant over the other. Nonetheless, the use of poractant alfa has been steadily increasing in the United States over the last two decades [8].
Our unit has used beractant for RDS treatment since 1991. In 2015, we introduced poractant alfa as an initiative to improve clinical outcomes in preterm infants. Here, we conducted a single-center retrospective cohort study to compare the safety and efficacy of poractant alfa and beractant in preterm infants requiring surfactant therapy for RDS.
2. Materials and Methods
2.1 Study design and population
This is a single-center retrospective cohort study using the database from infants admitted to our tertiary hospital's neonatal intensive care unit from January 2015 to January 2016. The database was generated from de-identified information extracted from hospital electronic and paper records. This retrospective audit was approved by SingHealth Centralized Institutional Review Board (CIRB: 2016/2327) with an exemption for individual consent. We identified infants born with gestational age (GA) < 37 weeks who received either poractant alfa or beractant for management of RDS. Infants who received the first dose of surfactant in other units and subsequently transferred to our unit for further management were excluded from the study. Infants with major congenital anomalies, such as congenital diaphragmatic hernia or cyanotic heart disease, were also excluded.
All infants received endotracheal surfactant as a therapeutic intervention for RDS. Infants who required intubation during initial resuscitation received endotracheal surfactant within 30 min of life. For infants initially stabilized on continuous positive airway pressure (CPAP) at birth, intubation and surfactant administration were offered if they had FiO2 requirement > 40%, PaCO2 > 65 mmHg, or recurrent apnea consistent with progressive RDS. We used a higher dose regimen for poractant alfa, where it was initially administered at 200 mg/kg/dose for the first dose and repeated at 100 mg/kg/dose 12 hours apart, up to but not exceeding a total dose of 400 mg/kg [9, 10]. Beractant was administered at 100 mg/kg/dose for up to three doses at six-hour intervals [11]. Both surfactants were given endotracheally in two aliquots. After surfactant administration, a unified ventilation strategy to optimize ventilation settings for early weaning with a target tidal volume of four to six ml/kg whenever feasible. Infants were classified to have pneumothorax if there was radiographic evidence of air collection in pleural space at any time following initial surfactant therapy. Infants born at < 32 and ≥ 32 weeks gestation were classified to have CLD if they still received either supplemental oxygen or ventilatory support at 36 weeks postmenstrual age and 28 days of postnatal age, respectively [12]. Those who died before CLD evaluation were classified as not having CLD.
2.2 Statistical analysis
Descriptive statistics (frequency and percent) was reported for the demographic variables of subjects who received the two surfactants (poractant alfa and beractant). Maternal and infant parameters were compared with Chi square test. Clinical outcomes were compared between the two groups using Mann-Whitney U and Chi square test for continuous and categorical variables, respectively. All statistical analysis was performed using SAS version 9.4 for Windows (SAS Institute Cary, NC, USA). Statistical significance was set at P < 0.05.
3. Results
A total of 187 preterm infants received either poractant alfa or beractant therapies during the study period. Of these, 179 were eligible for the study. Eight infants were excluded from our analysis because they had major congenital malformations or hydrops fetalis or received the first dose of surfactant elsewhere. Overall, 70 (39%) and 109 (61%) infants were treated with poractant alfa and beractant, respectively (Figure 1).
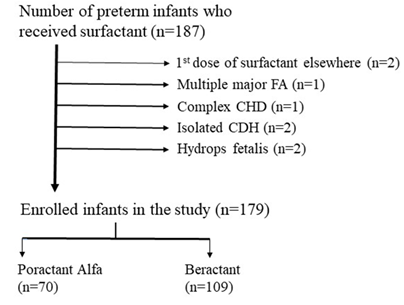
Figure 1: Flow diagram of study eligibility. FA, Fetal anomaly; CHD, Congenital heart disease; CDH, Congenital diaphragmatic hernia.
The study population’s baseline characteristics are shown in Table 1. Both groups have similar baseline characteristics, including maternal and infant parameters. There was a preference to use protectant alfa in smaller infants (median birth weight 1075g (IQR 754-1489)) over beractant (median birth weight 1116g (733-1623)). However, this difference did not reach statistical significance (P = 0.225).
|
Parameters
|
Poractant Alfa (CurosurfÒ) |
Beractant (SurvantaÒ) |
p value1
|
|
n (%) |
n (%) |
||
|
Maternal |
|||
|
Hypertension |
11 (15.7) |
22 (20.6) |
0.418 |
|
Diabetes Mellitus |
4 (5.71) |
10 (9.35) |
0.381 |
|
Chorioamnionitis |
23 (33.3) |
34 (31.2) |
0.766 |
|
Antenatal Steroid Complete No or incomplete |
44 (62.9) 26 (31.7) |
64 (58.7) 45 (41.3) |
0.580 |
|
Infant |
|||
|
Gender Male |
45 (64.3) |
65 (59.6) |
0.533 |
|
Gestational Age, week <30 ≥30 |
46 (65.7) 24 (34.3) |
69 (63.3) 40 (36.7) |
0.743 |
|
Birth Weight, gram < 1500 ≥ 1500 |
54 (77.1) 16 (22.86) |
75 (68.8) 34 (31.1) |
0.225 |
|
Apgar at 1 min2 ≤5 6 and above |
42 (60.0) 27 (38.6) |
65 (59.6) 43 (39.4) |
0.947 |
|
1 Chi-square test for analysis. 2 One infant from poractant alfa and one infant from beractant group had no APGAR Score at 1 min of life as both were born before arrival (BBA) |
|||
Table 1: Demographic characteristics of treated infants.
The mean time of administration for the first dose of surfactant was 2.22 hours of life (range 0.5 – 30) for poractant alfa and 2.69 hours of life (range 0.5 to 43) for beractant. More infants required repeated doses of surfactant in the beractant group, but the difference was not statistically significant (beractant 28/109 (25.7%) vs. poractant alfa 14/70 (20%), P = 0.381). Pneumothorax was observed in seven (6.42%) infants treated with beractant, while none was observed in those treated with poractant alfa (P = 0.031) (Table 2). The mean time of pneumothorax occurrence was 39 hours following surfactant administration (range 6 – 165). Further subgroup analyses based on infant’s birth weight showed that the incidence of pneumothorax was significantly higher in very low birth weight (VLBW) infants treated with beractant (poractant alfa 0.00% vs. beractant 8.00%, P = 0.035). No difference was observed in non-VLBW infants (poractant alfa 0.00% vs beractant 3.03%, P = 1.00) (Table 3).
|
Parameter |
Poractant Alfa (CurosurfÒ) |
Beractant (SurvantaÒ) |
p value3 |
|
Pneumothorax |
0 (0.00) 2 |
7 (6.42) |
0.031 |
|
Mortality before discharge |
10 (14.3) |
12 (11.0) |
0.515 |
|
CLD1 |
18 (25.7) |
30 (27.5) |
0.790 |
|
Combined mortality and CLD |
27 (38.6) |
40 (36.7) |
0.800 |
|
Day to first extubation |
1 (1-2) |
1 (1-3) |
0.331 |
|
IPPV1 days |
1 (1-14.5) |
2 (1-6) |
0.852 |
|
NIPPV1 days |
0 (0-3) |
0 (0-4.5) |
0.906 |
|
CPAP1 days |
8.5 (1-33) |
10 (1-29.5) |
0.507 |
|
AV1 days |
11.5 (3-50) |
21 (4-46) |
0.744 |
|
1 CLD, chronic lung disease; IPPV, intermittent positive pressure ventilation; NIPPV, nasal intermittent positive pressure ventilation; CPAP, continuous positive airway pressure; AV, assisted ventilation. 2 Frequency (percent) for categorical variables; Median (First-third quartile) for continuous variables. 3 Chi-square and Mann-Whitney U test for categorical and continuous variables respectively. |
|||
Table 2: Patient outcome comparisons between poractant alfa and beractant therapies.
|
Category |
Poractant Alfa (CurosurfÒ) n (%) |
Beractant (SurvantaÒ) |
p value2
|
|
All (n=179) |
0 (0.00%) |
7 (6.42%) |
0.031 |
|
VLBW1 (n= 128) |
0 (0.00%) |
6 (8.00%) |
0.035 |
|
Non-VLBW1 (n=51) |
0 (0.00%) |
1 (3.03%) |
1.000 |
|
1 VLBW, very low birth weight (birth weight < 1500 grams); non-VLBW, birth weight ≥ 1500 grams. 2 Chi-square test for analysis. |
|||
Table 3: Incidence of pneumothorax in VLBW and non-VLBW infants.
The incidence of CLD (poractant alfa 25.7% vs beractant 27.5%, p = 0.790), mortality before discharge (poractant alfa 14.3% vs beractant 11.0%, p = 0.515) and composite outcome of mortality and CLD (poractant alfa 38.6% vs beractant 36.7%, p = 0.800) were not significantly different between the groups. Timing for the first attempt of extubation was comparable between the two groups. Compared to those in the beractant group, infants who received poractant alfa had a shorter duration of assisted ventilation, which included intermittent positive pressure ventilation (IPPV), nasal intermittent positive pressure ventilation (NIPPV), and CPAP (Table 2). However, these differences were not statistically significant.
4. Discussion
Pneumothorax in neonates is associated with significant mortality and morbidity, especially in early preterm infants (gestational age <32 weeks) [13]. With the advent of surfactant therapy and improved ventilation strategies, the incidence of pneumothorax has decreased significantly over the last few decades [14]. Strategies to further reduce its occurrence will help to improve neonatal outcomes further.
We propose that differences in chemical compositions of the two surfactants could have contributed to the differences in the incidence of pneumothorax. When administered at their respective therapeutic doses, poractant alfa contains higher concentrations of both phospholipids and surfactant protein B (SP-B) than beractant [4, 5]. The ability of surfactant to reduce pulmonary surface tension relies on the formation of surface film at the lung air-liquid interface. Phospholipids, especially dipalmitoyldiphosphotidyl choline (DPPC), are the building blocks of this surface film. In contrast, the hydrophobic SP-B interacts with the lipids to promote the formation and adsorption of the surface film to the air-liquid interface [15]. Therefore, a higher concentration of bioactive macromolecules in poractant alfa may facilitate surface film formation and reduce pulmonary surface tension. This biochemical advantage also translates into better in vivo responses. To date, no animal studies have directly compared the in vivo responses between poractant alfa and beractant. Recently, Ricci et al. compared the pulmonary responses of poractant alfa and bovactant (Alveofact®, Lyomark Pharma, Oberhaching, Germany), another bovine-based surfactant, in RDS-induced preterm lambs and adult rabbits. Since bovactant has a lower phospholipid concentration than beractant, it was adjusted to reflect a similar amount of phospholipids of poractant alfa. Despite the dose adjustment, poractant alfa showed a more superior acute pulmonary response, as indicated by significantly higher lung gas volumes, more rapid and sustained recovery [16].
We found 11 randomized controlled trials (RCTs) in the literature that compared the effectiveness of poractant alfa (200 mg/kg) and beractant in neonates [7, 17]. Of these, eight reported pneumothorax as their study outcome (Didzar et al. [18]; Halahakoon et al. [19]; Karadag et al. [20]; Malloy et al. [21]; Ramanathan et al. [22]; Speer et al. [23]; Gharehbaghi et al. [24]; and Mussavi et al. [25]). None of these studies reported a significant difference in preventing pneumothorax occurrence from the two surfactants. However, most of these trials are limited by small sample size and non-vigorous study design [4, 7]. Singh et al. conducted a meta-analysis on these trials and demonstrated a trend favoring poractant alfa in pneumothorax prevention. However, the difference was not statistically significant (beractant vs. poractant alfa: typical risk reduction (RR) 1.24, 95% CI 0.71 to 2.17; typical risk difference (RD) 0.02, 95% CI 0.02 to 0.05) [7]. Trembath et al. analyzed a large cohort with simple logistic regression and random-effects models. They demonstrated a significant reduction of pneumothorax in infants treated with poractant alfa as compared with those treated with beractant (logistic regression: beractant vs. poractant alfa odds ratio (OR) 1.47, 95% CI 1.35 to 1.61; random-effects models: OR 1. 31, 95% CI 1.13 to 1.51) [6]. A recent systematic review by Tridente et al. also demonstrated a significant reduction of pneumothorax incidence associated with poractant alfa (200 mg/kg). However, compared with a pooled bovine surfactant rather than beractant alone (poractant alfa vs. bovine surfactants: RR 0.505, 95% CI 0.308 to 0.827) [17]. Overall, current evidence supports our finding that poractant alfa is more superior to beractant in lowering the incidence of pneumothorax. Various limitations in different RCT settings may have previously overlooked that poractant alfa can potentially prevent pneumothorax occurrence in the neonatal population.
In our study, poractant alfa is more superior in protecting VLBW infants against pneumothorax. The incidence of pneumothorax is significantly lower in poractant alfa-treated VLBW infants. However, a reduction in pneumothorax was not statistically significant for non-VLBW infants. Limited studies have compared different surfactant preparations in VLBW cohorts for pneumothorax prevention. Consistent with our findings, a small RCT by Speer et al. showed a reduction in pneumothorax in VLBW infants treated with poractant alfa. Still, it was not statistically significant (adjusted OR 0.49, P = 0.43) [23]. We speculate that biophysical properties of poractant alfa may contribute to this: (1) lower dose volume at 2.5 ml/kg body weight, as compared with 4.0 ml/kg for beractant, and (2) lower viscosity of poractant alfa. These may facilitate endotracheal administration of poractant alfa, especially in smaller infants who are usually intubated with smaller endotracheal tubes. This, in turn, could reduce the cardiorespiratory disturbances during surfactant administration. Moreover, in vitro study has demonstrated that poractant alfa maintains a low viscosity regardless of surface tension, whereas the viscosity of beractant increases rapidly with progressively lower surface tension [26]. Thus, in contrast to beractant, the in vivo distribution of poractant alfa is likely more sustained with progressive reduction of pulmonary surface tension.
Although our study demonstrated a significantly lower incidence of pneumothorax in infants treated with poractant alfa, it did not improve mortality. This contrasts with a study from Singh et al., where beractant was associated with a higher risk of mortality before discharge compared with poractant alfa (RR 1.44, 95% CI 1.04 to 2.00) [7]. Because our study is a retrospective observational cohort study, the subjects are not randomized. Although both VLBW and non-VLBW infants have compatible key baseline characteristics, we may not have accounted for all confounders from our non-randomized study. In addition, more infants received beractant therapy in our center. This unequal distribution is likely because poractant alfa was a relatively new surfactant in our center during the study period. As the sample size becomes unequal, its statistical power also diminishes [27]. Our small number of infants also limits the subgroup analysis in non-VLBW infants. We also observed that infants who received poractant alfa were smaller, reflecting a possible clinician preference towards poractant alfa in smaller infants in our center, possibly due to smaller volume.
5. Conclusions
In summary, our study suggests that poractant alfa is more effective than beractant in preventing pneumothorax, especially in VLBW infants. This is consistent with findings from other observational studies and meta-analysis. It provides additional insights into a potentially significant factor that may influence the choice of surfactant preparations in preterm infants, especially VLBW infants. preparations are more superior than.
Acknowledgments
We thank Mr Eddy Sputra Leman, PhD, Senior Scientific Editor, Duke-NUS Medical School, Singapore for editing the manuscript.
Authors Contribution
The authors’ contributions were as follows: X.D. collected the data and wrote the manuscript; A.A.A.H., M.C.C., V.S.R and S.C. designed, supervised the research project and co-wrote the manuscript; S.E.S. performed the statitical analysis; S.C. had primary responsibility for final content.
Conflict of Interest
The authors have no competing interest to declare.
References
- Fujiwara T, Maeta H, Chida S, et al. Artificial surfactant therapy in hyaline-membrane disease. Lancet 1 (1980): 55-59.
- Mazela J, Merritt T, Gadzinowski J, et al. Evolution of pulmonary surfactants for the treatment of neonatal respiratory distress syndrome and paediatric lung diseases. Acta Paediatr 95 (2006): 1036-1048.
- Soll RF, Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Databbase Syst Rev 2 (2001): CD000144.
- Logan JW, Moya FR. Animal-derived surfactants for the treatment and prevention of neonatal respiratory distress syndrome: Summary of clinical trials. Ther Clin Risk Manag 5 (2009): 251-260.
- Ramanathan R, Bhatia JJ, Sekar K, et al. Mortality in preterm infants with respiratory distress syndrome treated with poractant alfa, calfactant or beractant: a retrospective study. J Periatol 33 (2013): 119-125.
- Trembath A, Hornik CP, Clark R, et al. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr 163 (2013) 955-960.
- Singh N, Halliday HL, Stevens TP, et al. Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev 12 (2015): CD010249.
- Trembath A, Clark R, Bloom BT, et al. Trends in surfactant use in the United States: changes in clinical practice. E-Journal of Neonatology Research 1 (2011): 23-30.
- Halliday HL, Tarnow-Mordi WO, Corcoran JD, et al. Multicentre randomised trial comparing high and low dose surfactant regimens for the treatment of respiratory distress syndrome (the Curosurf 4 trial). Arch Dis Child 69 (1993): 276-280.
- Curosurf product Information, Cornerstones Therapeutics, Illinois (2010).
- Zola EM, Overbach AM, Gunkel JH, et al. Treatment investigational new drug experience with Survanta (beractant). Pediatrics 91 (1993): 546-551.
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163 (2011): 1723-1729.
- Duong HH, Mirea L, Shah PS, et al. Pneumothorax in neonates?: Trends , predictors and outcomes. J Neonatal Perinatal Med 7 (2014): 29-38.
- Shetty S, Greenough A. Neonatal ventilation strategies and long-term respiratory outcomes. Early Hum Dev 90 (2014): 735-739.
- Daniels CB, Orgeig S. Pulmonary surfactant: the key to the evolution of air breathing. News Physiol Sci 18 (2003): 151-157.
- Ricci F, Salomone F, Kuypers E, et al. In Vivo Evaluation of the Acute Pulmonary Response to Poractant Alfa and Bovactant Treatments in Lung-Lavaged Adult Rabbits and in Preterm Lambs with Respiratory Distress Syndrome. Front Pediatr 5 (2017): 186.
- Tridente A, Martino L De, Luca D De. Porcine vs bovine surfactant therapy for preterm neonates with RDS?: systematic review with biological plausibility and pragmatic meta-analysis of respiratory outcomes. Respir Res 20 (2019): 28.
- Dizdar EA, Sari FN, Aydemir C, et al. A randomized, controlled trial of poractant alfa versus beractant in the treatment of preterm infants with respiratory distress syndrome. Am J Perinatol 29 (2012): 95-100.
- Halahakoon WL. A study of cerebral function following surfactant treatment for respiratory distress syndrome. Doctoral Diss Queen’s Univ, Belfast (1999).
- Karadag N, Dilli D, Zenciroglu A, et al. Perfusion index variability in preterm infants treated with two different natural surfactants for respiratory distress syndrome. Am J Perinatol 31 (2014): 1015-1022.
- Mallory CA, Nicoski P, Muraskas JK. A randomized trial comparing beractant and poractant treatment in neonatal respiratory distress syndrome. Acta Paediatr 94 (2005): 779-784.
- Ramanathan R, Rasmussen MR, Gerstmann DR, et al. A randomized, multicenter masked comparison trial of poractant alfa (Curosurf) versus beractant (Survanta) in the treatment of respiratory distress syndrome in preterm infants. Am J Perinatol 21 (2004): 109-119.
- Speer CP, Gefeller O, Groneck P, et al. Randomised clinical trial of two treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed 72 (1995): F8-13.
- Gharehbaghi MM, Sakha SHP, Ghojazadeh M, et al. Complications among premature neonates treated with beractant and poractant alfa. Indian J Pediatr 77 (2017): 751-754.
- Mussavi M, Mirnia K, Asadollahi K. Comparison of the efficacy of three natural surfactants (Curosurf Survanta , and Alveofact) in the treatment of respiratory syndrome among neonates?: a randomized controlled trial. Iran J Pediatr 26 (2016): e5743.
- Rüdiger M, Tölle A, Meier W, et al. Naturally derived commercial surfactants differ in composition of surfactant lipids and in surface viscosity. Am J Physiol Cell Mol Physiol 288 (2004): L379-383.
- Rusticus SA, Lovato CY. Impact of sample size and variability on the power and type I error rates of equivalence tests: A simulation study. Practical Assessment, Research and Evaluation 19 (2014): 11.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
