Schistosomiasis Co-morbid with Posterior Reversible Encephalopathy Syndrome and HIV/AIDS
Article Information
Willem van Aswegen, Dali Magazi*
Department of Neurology, Sefako Makgatho Health Sciences University, South Africa
*Corresponding Author: Dali Magazi, MBBCh, MMed (Neuro), FCP (Neuro)SA, Department of Neurology, Sefako Makgatho Health Sciences University, PO Box 108, Medunsa, 0204, South Africa
Received: 06 January 2021; Accepted: 25 January 2021; Published: 05 February 2021
Citation: Willem van Aswegen, Dali Magazi. Schistosomiasis Co-morbid with Posterior Reversible Encephalopathy Syndrome and HIV/AIDS. Archives of Clinical and Medical Case Reports 5 (2021): 210-215.
View / Download Pdf Share at FacebookAbstract
We describe a young female patient with schistosomiasis, HIV and posterior reversible encephalopathy syndrome (PRES) from a South African township. There are overlapping pathophysiological molecular mechanisms between schistosomiasis and PRES and yet no previous descriptions linking the two conditions. This presentation highlights the need for further investigation of this possible link. It also serves to emphasize the need for an updated geographical distribution map of schistosomiasis in South Africa. The interplay between HIV and schistosomiasis continues to be an important study subject in endemic areas.
Keywords
Schistosomiasis; HIV; PRES; South Africa
Schistosomiasis articles; HIV articles; PRES articles; South Africa articles
Schistosomiasis articles Schistosomiasis Research articles Schistosomiasis review articles Schistosomiasis PubMed articles Schistosomiasis PubMed Central articles Schistosomiasis 2023 articles Schistosomiasis 2024 articles Schistosomiasis Scopus articles Schistosomiasis impact factor journals Schistosomiasis Scopus journals Schistosomiasis PubMed journals Schistosomiasis medical journals Schistosomiasis free journals Schistosomiasis best journals Schistosomiasis top journals Schistosomiasis free medical journals Schistosomiasis famous journals Schistosomiasis Google Scholar indexed journals HIV articles HIV Research articles HIV review articles HIV PubMed articles HIV PubMed Central articles HIV 2023 articles HIV 2024 articles HIV Scopus articles HIV impact factor journals HIV Scopus journals HIV PubMed journals HIV medical journals HIV free journals HIV best journals HIV top journals HIV free medical journals HIV famous journals HIV Google Scholar indexed journals PRES articles PRES Research articles PRES review articles PRES PubMed articles PRES PubMed Central articles PRES 2023 articles PRES 2024 articles PRES Scopus articles PRES impact factor journals PRES Scopus journals PRES PubMed journals PRES medical journals PRES free journals PRES best journals PRES top journals PRES free medical journals PRES famous journals PRES Google Scholar indexed journals Neglected Tropical Diseases articles Neglected Tropical Diseases Research articles Neglected Tropical Diseases review articles Neglected Tropical Diseases PubMed articles Neglected Tropical Diseases PubMed Central articles Neglected Tropical Diseases 2023 articles Neglected Tropical Diseases 2024 articles Neglected Tropical Diseases Scopus articles Neglected Tropical Diseases impact factor journals Neglected Tropical Diseases Scopus journals Neglected Tropical Diseases PubMed journals Neglected Tropical Diseases medical journals Neglected Tropical Diseases free journals Neglected Tropical Diseases best journals Neglected Tropical Diseases top journals Neglected Tropical Diseases free medical journals Neglected Tropical Diseases famous journals Neglected Tropical Diseases Google Scholar indexed journals schistosomiasis articles schistosomiasis Research articles schistosomiasis review articles schistosomiasis PubMed articles schistosomiasis PubMed Central articles schistosomiasis 2023 articles schistosomiasis 2024 articles schistosomiasis Scopus articles schistosomiasis impact factor journals schistosomiasis Scopus journals schistosomiasis PubMed journals schistosomiasis medical journals schistosomiasis free journals schistosomiasis best journals schistosomiasis top journals schistosomiasis free medical journals schistosomiasis famous journals schistosomiasis Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Pathogenesis articles Pathogenesis Research articles Pathogenesis review articles Pathogenesis PubMed articles Pathogenesis PubMed Central articles Pathogenesis 2023 articles Pathogenesis 2024 articles Pathogenesis Scopus articles Pathogenesis impact factor journals Pathogenesis Scopus journals Pathogenesis PubMed journals Pathogenesis medical journals Pathogenesis free journals Pathogenesis best journals Pathogenesis top journals Pathogenesis free medical journals Pathogenesis famous journals Pathogenesis Google Scholar indexed journals Idiopathic Non-Histaminergic Angioedema articles Idiopathic Non-Histaminergic Angioedema Research articles Idiopathic Non-Histaminergic Angioedema review articles Idiopathic Non-Histaminergic Angioedema PubMed articles Idiopathic Non-Histaminergic Angioedema PubMed Central articles Idiopathic Non-Histaminergic Angioedema 2023 articles Idiopathic Non-Histaminergic Angioedema 2024 articles Idiopathic Non-Histaminergic Angioedema Scopus articles Idiopathic Non-Histaminergic Angioedema impact factor journals Idiopathic Non-Histaminergic Angioedema Scopus journals Idiopathic Non-Histaminergic Angioedema PubMed journals Idiopathic Non-Histaminergic Angioedema medical journals Idiopathic Non-Histaminergic Angioedema free journals Idiopathic Non-Histaminergic Angioedema best journals Idiopathic Non-Histaminergic Angioedema top journals Idiopathic Non-Histaminergic Angioedema free medical journals Idiopathic Non-Histaminergic Angioedema famous journals Idiopathic Non-Histaminergic Angioedema Google Scholar indexed journals
Article Details
Abbreviations:
NTD- Neglected Tropical Diseases; CRP- C-Reactive Protein; HIV- Human immunodeficiency virus; CD4- Cluster of differentiation 4; ICAM 1- Intercellular adhesion molecule 1
1. Introduction
A German physician, Theodor Bilharz described a condition that was to bear his name from autopsy findings of an Egyptian patient in 1851. There are however,earlier descriptions from ancient Egyptian papyri of what is reminiscent of bilharzia, including literature from times of ancient Mesopotamia [1]. In the early 2000’s, the world health organisation (WHO) recognised a group of communicable conditions affecting rural poor communities which were not prioritized on the global agenda, aptly referred to as neglected tropical diseases (NTDs). Schistosomiasis or Bilharzia being amongst the NTDs, affects approximately 230 million people, worldwide, an estimated 85% being in sub-Saharan Africa where the principal species are schistosoma hematobium and mansoni excreted in urine and faeces, respectively. Ambitious goals by the WHO were to have schistosomiasis controlled by 2020 and to have a “world free of schistosomiasis” by 2025 [2].
The parasites causing schistosomiasis are waterborne, found in rivers, ponds and dams with a well described lifecycle from the time the fluke penetrates human skin to the excretion of the eggs in urine or faeces. An estimated 20 – 55% of the eggs are successfully excreted, the rest lodge in various organs including the liver, kidney, brain and even the eyes, resulting in granulomas with fibrotic damage [3]. A cascade of immunological events are triggered by the schistosome in the host cell, including activation of type 2 cytokines like interleukins 4 & 5 and a host of others [4]. Mortality from schistosomiasis continues to be significant, the global estimates being 200 000 per annum [5].
South Africa has approximately 4.5 million people infected with schistosomiasis, the north eastern parts covering a quarter of the country being traditionally endemic [6]. The geographic spread of schistosomiasis in South Africa however, has been shown to be in flux, influenced by various factors, including rural-urban migration and cross border movements from endemic regions [7]. Weather patterns are another identified determinant of schistosomal geographical distribution, meaning that global climate change could possibly have an impact on present day patterns of schistosomiasis [8].
2. Index Case Presentation
A 31-year old female from Soshanguve, one of the north western townships of Pretoria, South Africa was found unresponsive. She had previously complained of a headache followed by erratic behavior over a period of two weeks. The Glasgow coma scale on hospital arrival was 7/15. She also had bilateral papilloedema with globally absent deep tendon reflexes. A brain CT scan showed extensive hypodense areas in both cerebral hemispheres, a picture suggestive of posterior reversible encephalopathy syndrome (PRES) shown in the figure below. She also had an iron deficiency anemia (hemoglobin of 9.5g/l) with a high C-reactive protein (CRP) of 54 mg/l. She was further diagnosed positive for the human immunodeficiency virus (HIV) with a CD4 count of 13 cells/ µl and a viral load of 284 772 copies/ml. A lumbar puncture was deferred due to clinical and radiological evidence of raised intracranial pressure and was treated empirically for bacterial and cryptococcal meningitis, the latter owing to the profoundly low immunity status. Urine microscopy showed Schistosoma haematobium ova and hematuria for which an antihelminth was commenced.
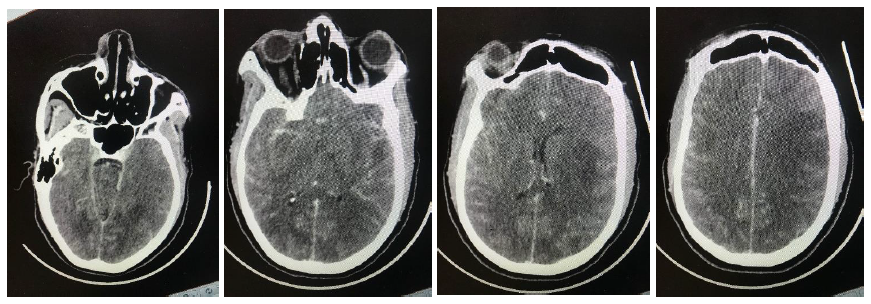
Figure 1: Sequential contrasted CT images illustrating PRES.
3. Discussion
At a molecular level, there exists an overlap in the host response to schistosomal infection and the underlying pathophysiology of PRES. Schistosomiasis results in a demonstrable upregulation of intercellular adhesion molecule1 (ICAM 1), a transmembrane protein found on the surface of leukocytes and endothelium with a resultant increase in vessel permeability [9]. Cytokine activation and an overexpression of ICAM 1 have also been demonstrated and hypothesized to be an important causative contributor for PRES [10]. The latter is a radiologically diagnosed acute condition characterized by bilateral cerebral edema, usually maximal in the occipital lobes with no identifiable solid brain lesions (eg: abscess, granulomas or tumors). Neuroschistosomiasis is a rare manifestation of the helminth infection with a spectrum of clinical manifestations including headaches, confusion and coma [11]. Present day definitions of neuroschistosomiasis are not necessarily hinged on isolation of the helminth in the central nervous system (peripheral isolation and neurological clinical features sufficing), leading to some authors pointing out a gap in diagnostic standards [12]. A literature search on schistosomiasis and PRES did not yield results. HIV has however, been described with PRES and postulated to be largely from endothelial damage in the brain [13]. The index patient could have had endothelial dysfunction as a result of contributory mechanisms from an interplay between HIV and schistosomiasis.
Sub-Saharan Africa, endemic for both HIV and schistosomiasis prompted studies to investigate a potential interaction between that retrovirus and bilharzia. Urogenital ulceration from schistosomiasis has been described to make females particularly vulnerable to contracting HIV during intercourse [14]. The hematuria and iron deficiency anemia seen in our patient was explained by the schistosoma hematobium infection. Upregulation of CD4 receptors which are docking stations for HIV has been demonstrated with schistosomiasis suggesting further interplay between the two conditions at a molecular level. People living with HIV and Schistosomal infection as a co-morbidity have also been associated with higher viral load than those without schistosomiasis [15]. This however, is not universal with some studies finding no such association [16]. The sub-Saharan African region stands to benefit from an in-depth understanding of the interplay between schistosoma and HIV, in the interest of having informed preventative programs.
The index patient serves to highlight that city areas like Pretoria, the capital city of South Africa could be having a silently growing problem of schistosomiasis. Our patient was a rural-urban migrant from an area endemic for schistosomiasis. Informal settlements along the banks of rivers, in townships on the outskirts of cities (eg: Jukskei & Moretele rivers in Alexandra and Mamelodi townships, Johannesburg and Pretoria, respectively) are a possible risk for a surge of schistosomiasis in previously quiescent areas.
4. Conclusion
The authors realize that a single case report is not enough to prove an association between schistosomiasis and PRES. This is however, a call for further robust studies to interrogate a possible link between the two conditions. An updated national disease burden registry for schistosomiasis would be important for South Africa in order to inform government preventative programs way ahead of possible future epidemics. A better understanding of a possible link between schistosomiasis and HIV is of paramount importance in endemic areas. A complete global elimination of Bilharzia, worldwide as per the WHO goals can only come about from well grounded preventative programs which in turn would be from an informed standpoint.
Acknowledgments
Not applicable
Funding
No special funding was received
Disclosure and Conflict of Interests
None of the authors have any disclosures to make with no conflict of interest
Ethical Approval
Permission was sought and granted by the patient’s family to publish the presentation anonymously.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Di Bella S, Riccardi N, Giacobbe DR, et al. History of schistosomiasis (bilharziasis) in humans: from Egyptian medical papyri to molecular biology on mummies. Pathogens and Global Health 112 (2018): 268-273.
- Deol AK, Fleming FM, Calvo-Urbano B, et al. Schistosomiasis—assessing progress toward the 2020 and 2025 global goals. New England Journal of Medicine 381 (2019): 2519-2528.
- Costain AH, MacDonald AS, Smits HH. Schistosome egg migration: mechanisms, pathogenesis and host immune responses. Frontiers in Immunology 9 (2018): 3042.
- de Oliveira Fraga LA, Torrero MN, Tocheva AS, et al. Induction of type 2 responses by schistosome worms during prepatent infection. The Journal of Infectious Diseases 201 (2010): 464-472.
- Verjee MA. Schistosomiasis: Still a cause of significant morbidity and mortality. Research and reports in Tropical Medicine 10 (2019): 153.
- Magaisa K, Taylor M, Kjetland EF, Naidoo PJ. A review of the control of schistosomiasis in South Africa. South African Journal of Science 111 (2015): 1-6.
- Appleton CC, Ngxongo SM, Braack LE, et al. Schistosoma mansoni in migrants entering South Africa from Moçambique--a threat to public health in north-eastern KwaZulu-Natal?. South African Medical Journal 86 (1996): 350-353.
- Moodley I, Kleinschmidt I, Sharp B, et al. Temperature-suitability maps for schistosomiasis in South Africa. Annals of Tropical Medicine & Parasitology 97 (2003): 617-627.
- Ritter DM, McKerrow JH. Intercellular adhesion molecule 1 is the major adhesion molecule expressed during schistosome granuloma formation. Infection and Immunity 64 (1996): 4706-4713.
- Marra A, Vargas M, Striano P, et al. Posterior reversible encephalopathy syndrome: the endothelial hypotheses. Medical Hypotheses 82 (2014): 619-622.
- Ferrari TC, Moreira PR. Neuroschistosomiasis: clinical symptoms and pathogenesis. The Lancet Neurology 10 (2011): 853-864.
- Härter G, Frickmann H, Zenk S, et al. Diagnosis of neuroschistosomiasis by antibody specificity index and semi-quantitative real-time PCR from cerebrospinal fluid and serum. Journal of Medical Microbiology 63 (2014): 309-312.
- Nightingale S, Wood C, Ainsworth J. The posterior reversible encephalopathy syndrome in HIV infection. Case Reports 2012 (2012): bcr0120125647.
- Wall KM, Kilembe W, Vwalika B, et al. Schistosomiasis is associated with incident HIV transmission and death in Zambia. PLoS Neglected Tropical Diseases 12 (2018): e0006902.
- Walson JL, Herrin BR, John-Stewart G. Deworming helminth co-infected individuals for delaying HIV disease progression. The Cochrane Database of Systematic Reviews 8 (2009): CD006419.
- Bochner AF, Secor WE, Baeten JM, et al. Schistosomiasis was not associated with higher HIV-1 plasma or genital set point viral loads among HIV seroconverters from four cohort studies. PLoS Neglected Tropical Diseases 13 (2019): e0007886.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
