Simvastatin Modulates Extracellular Matrix Assembly by Displaying an Antifibrotic Activity in Vitro
Article Information
Annele Sainio1, Asta Laiho2, Hannu Järveläinen1,3
1Institute of Biomedicine, University of Turku, Turku, Finland
2Turku Bioscience/Bioinformatics, University of Turku, Turku, Finland
3Department of Internal Medicine, Satakunta Central Hospital, Pori, Finland
*Corresponding Author: Annele Sainio, Institute of Biomedicine, University of Turku, Kiinamyllynkatu 10, FI-20520 Turku, Finland
Received: 07 August 2019; Accepted: 29 August 2019; Published: 04 September 2019
Citation:
Annele Sainio, Asta Laiho, Hannu Järveläinen. Simvastatin Modulates Extracellular Matrix Assembly by Displaying an Antifibrotic Activity in Vitro. J Pharm Pharmacol Res 3 (2019): 059-073.
View / Download Pdf Share at FacebookAbstract
Statins, competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase, are known to possess properties beyond their cholesterol-lowering effect including anti-inflammatory, antiproliferative and anti-immunomodulatory effects. We examined the effect of simvastatin on extracellular matrix (ECM) assembly by human skin fibroblasts (HSFs) in vitro. Using collagen gel contraction (CGC) assay we showed that simvastatin inhibits contraction of type I collagen-rich gels in a dose-dependent manner. This effect of simvastatin could be overcome by co-incubating the cells with mevalonate. Actin staining revealed that inhibition of CGC by simvastatin is associated with diminished ability of the cells to form aggregates. Using whole human genome Illumina microarray we sought to search for new candidate genes whose expression is regulated by simvastatin during CGC and focused specifically on the genes related to ECM synthesis and remodeling. We found that simvastatin profoundly downregulated gene expression of 27 ECM molecules including proteoglycans decorin and versican, both of which are known to be essential constituents of proper ECM. Expression of these two molecules was further verified by Northern blot analysis. Finally, when simvastatin treated HSFs were activated with TGF-β1, the cell-mediated contraction of collagen gel was restored. Our results indicate that simvastatin markedly alters ECM assembly in vitro possessing an antifibrotic activity.
Keywords
Simvastatin, Collagen gel contraction, Human skin fibroblasts, Extracellular matrix molecules
Article Details
Abbreviations:
CGC-collagen gel contraction; ECM-extracellular matrix; HSF(s)-human skin fibroblast(s); DMEM-Dulbecco’s modified Eagle’s medium; FCS-fetal calf serum; TGF-β1-transforming growth factor-β11. Introduction
Statins, the 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, are one of the most commonly prescribed class of drugs in Western world due to their well-established beneficial action in primary [1] and secondary [2] prevention of cardiovascular diseases. The cardioprotective action of statins has primarily been attributed to their cholesterol-lowering effect [3]. However, it has become apparent that statins also possess properties beyond their influence on cholesterol metabolism [4]. These properties, called pleiotrophic effects, include anti-inflammatory, antiproliferative, anticoagulative and immunomodulatory activities [5]. Lately, attention has been paid to the possibility that statins also modulate fibrotic processes both in extravascular [6] and vascular tissues [7]. Clinically the possible influence of statins on fibrotic processes is intriguing, because it provides an additional mechanism whereby certain beneficial (e.g. risk reduction of acute coronary syndrome) [8] as well as harmful (e.g. tendinopathy) [9] phenomenona associated with statin use could be partially explained.
In order to gain further insight into the effect of statins on extracellular matrix (ECM) remodeling, we performed human skin fibroblast (HSF)-populated collagen gel contraction (CGC) assays with different configurations. Subsequently, candidate molecules involved in the effect of simvastatin on matrix remodeling were search with Illumina microarray and expression of the chosen molecules, namely proteoglycans decorin and versican, was confirmed by Northern blot analyses. Previously both of the above proteoglycans have been shown to be able to drastically modulate ECM assembly [10, 11]. We sought to especially examine whether simvastatin is capable of modulating cell-mediated contraction of type I collagen-rich gel and what molecules are likely to be involved in this action of simvastatin. Finally, attention was paid to the possible underlying mechanism whereby simvastatin might contribute to modulating collagen gel contraction. The results showed that simvastatin markedly alters ECM assembly and likely possesses an antifibrotic activity.
2. Materials and Methods
2.1 Cell culture
Human skin fibroblasts (HSFs) were derived from forearm skin biopsies of three healthy adults. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Paisley, Scotland) containing 10% (v/v) fetal calf serum (FCS; Biochrom AG, Berlin, Germany), 100 IU/ml penicillin and 100 μg/ml streptomycin (Sigma, Saint Louis, Missouri, USA). In collagen gel contraction experiments, DMEM without antibiotics was used in order to avoid the possible effects of antibiotics on the results.
2.2 Collagen gel contraction (CGC) assay
Simvastatin was purchased from Sigma-Aldrich (Schnelldorf, Germany) and converted to open acid form before using, as previously described [12]. Briefly, 20 mg of simvastatin was suspended in 0.4 ml of ethanol, and 2.4 ml of 0.1 M NaOH was added. The solution was heated at 50°C for 2 h, whereafter 3.6 ml of aqueous solution containing 81 mM Na2HPO4 and 15 mM NaH2PO4 was added. The solution was heated at 40°C for 30 min, and the pH was adjusted to 7.3 with concentrated HCl. The mevalonate used in this study was also purchased from Sigma-Aldrich. The collagen solutions for contraction assays were prepared using rat tail type I collagen (BD Biosciences, Bedford, USA). In addition to collagen, these solutions contained the following components: 1 × DMEM (Gibco), FCS (Biochrom AG), simvastatin (Sigma-Aldrich), and in selected experiments also mevalonate (Sigma-Aldrich). The solutions were always prepared for four simultaneous contraction assays, and the assays were conducted in the wells of 24-well culture plates (Nunc, Roskilde, Denmark).
The collagen lattices for contraction assays were prepared in three phases. In phases one and two, the solutions contained type I collagen at the final concentration of 0.5 mg/ml, 10% FCS and simvastatin at concentrations of 50, 100 or 150 mg/ml, or alternatively simvastatin 50 mg/ml plus mevalonate 50 mg/ml. The HSFs (12.5 x 103 HSFs per gel) were added to the second solution. Both of these solutions (the bottom and the middle solutions) were subsequently left to gell at 37°C for one hour before adding the third solution (i.e. culture medium) containing the same ingredients as mentioned above excluding collagen and the cells.
To examine the possible underlying mechanism behind the effect of simvastatin on CGC, TGF-β1 (Santa Cruz Biotechnology, Santa Cruz, CA) at a dose of 5 ng/ml [13] was added together with simvastatin and assayed for CGC. As a control, identical cultures without simvastatin or other additional factors were performed. At the begin of the contraction assays, the gels were gently detached from the wall of the wells to initiate collagen contraction and the cultures were maintained for 24 hours at 37°C in 95% air / 5% CO2. Thereafter, the gels were fixed with 1% neutral-buffered formalin at room temperature. The degree of contraction was assessed by measuring the area of the gels with an imaging densitometer (MCID Image Analyzer). All experiments were performed at least three times, and similar results were always obtained.
2.3 Actin staining of HSFs within collagen lattices
In selected experiments, actin cytoskeletons of control and simvastatin treated HSFs in contracted collagen gels were labeled with phalloidin conjugated to Alexa-Fluor-phalloidin 488 (Molecular Probes, Eugene, Oregon, USA). Briefly, the contracted gels containing the cells were fixed with 1% neutral-buffered formalin, whereafter they were washed with PBS twice for 15 min/time. Next, the gels were permeabilized with acetone for 20 min at -20ºC, and blocked with 1% bovine serum albumin (BSA) in PBS following incubation with Alexa-Fluor-phalloidin (Molecular Probes, 4 units/gel) in PBS. After labeling, the gels were washed with PBS three times at room temperature for 30 min/time and viewed by epifluorescence illumination with a Leica DMRB microscope.
2.4 Cell viability determination
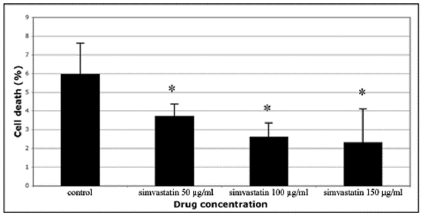
The viability of the cells in the control and simvastatin treated cultures was measured using trypan blue exclusion test as described previously [14]. Briefly, the cells were grown on plastic for 24 hours in the absence or presence of simvastatin (concentrations 25 μg/ml, 50 μg/ml, 100 μg/ml and 150 μg/ml) and viable and death cells were counted using a haemocytometer.
2.5 RNA extraction and microarray analysis
Total RNA was isolated from cell cultures using CsCl-centrifugation and the yield of RNA was determined by measuring the absorbance at 260 and 280 nm. To investigate the effect of simvastatin on total HSF gene expression, Illumina Sentrix® Human-6 Expression BeadChips (Illumina, Inc., San Diego, California, USA) were used. The microarray analysis was performed for biological replicates to ensure data reproducibility. Biotinylated cRNA using the Illumina RNA Amplification kit (Ambion, inc., Austin, Texas, USA) was prepared using ~100 ng total RNA from both control and 150 μg/ml of simvastatin treated HSFs. The biotinylated cRNA was purified using the RNeasy kit (Qiagen, Valencia, California, USA) and hybridized to the chips. The washing and scanning of the chips were performed following standard Illumina protocols. The results were analyzed using Illumina´s BeadStudio software. Microarray results regarding proteoglycans decorin and versican were confirmed by Northern blot analysis as described below.
2.6 Northern blot analysis
Aliquots (10 μg) of total RNA (from control and 150 µg/ml of simvastatin treated HSFs) were size-fractionated by electrophoresis through a denaturing 1% agarose gel and transferred to nitrocellulose membrane as described previously [15] and hybridized with cDNA probes labelled with [α-32P]dNTP (3000 Ci/mmol) (Promega, Madison, WI, USA) by random priming. The hybridization signals of the specific mRNAs of interest were normalized to those of 28S to correct the differences in loading or transfer [16].
Nick translation instead of random priming was used for labelling of the 28S ribosomal RNA cDNA probe. The following cDNA probes were used: a decorin cDNA for the full-length human decorin core protein [17], a versican cDNA for the full-length human versican core protein [18] and a cDNA fragment of 28S ribosomal subunit. Results of the Northern blotting were quantified using TINA (version 2.10f) as previously described [19].
2.7 Statistical analysis
Data of the CGC assays are shown as a mean ± SD.
Unpaired Student´s t-test was employed for comparing two groups of data. All p-values <0.05 were considered statistically significant. The microarray data was quantile-normalized and the 10-logaritmic signal values were clustered using Pearson´s metric to verify the clustering of controls and treated samples on different branches. The selection criteria concerning microarray data in following analysis were: first, all included genes were either down or upregulated at least 0.9 in a 2-logaritmic scale between the controls and experiments; secondly, the SE (standard error) between the replicates was under 0.5; and thirdly, the mean value of signals from the array had to be over 200.
3. Results
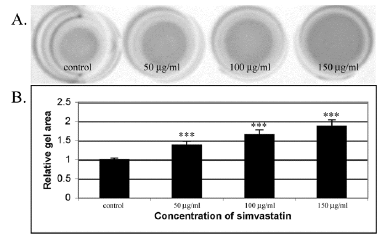
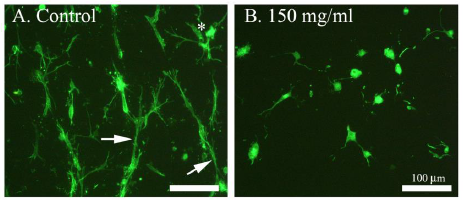
In this study, human skin fibroblasts (HSFs) were used to examine the effect of simvastatin on cell-mediated collagen gel contraction (CGC) in vitro. The cells were seeded into type I collagen-rich matrix and conditioned with different concentrations (50 µg/ml, 100 µg/ml, 150 µg/ml) of simvastatin. As shown in Figure 1, control HSFs contracted collagen gels significantly more than HSFs exposed to simvastatin, and the inhibitory effect of simvastatin on CGC occurred in a dose-dependent manner. The more powerful contraction of collagen gels by control HSFs corresponded to a robust generation of traction by the cells as indicated by the formation of cellular aggregates (Figure 2A). In contrast, HSFs incubated with simvastatin did not aggregate or form fibrillar networks, but on the contrary they were more rounded in shape and had fewer cytoplasmic extensions (Figure 2B). Similar results were obtained with all simvastatin concentrations (data not shown). The possibility that the change in the phenotype of simvastatin treated HSFs was due to a toxic effect of the drug was excluded using trypan blue exclusion test (Figure 3).
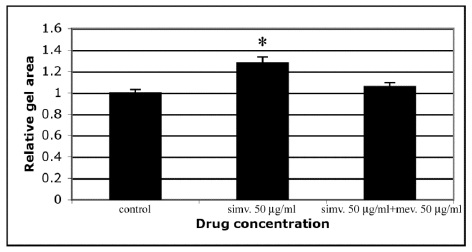
Statins are known to block the mevalonate pathway by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Inhibition of this pathway results not only in decreased cholesterol synthesis, but it also enables the pleiotrophic immunomodulatory, anti-inflammatory and antiproliferative activities of statins [5]. Therefore, we tested whether the inhibitory effect of simvastatin on cell-mediated CGC could be overcome by co-incubating the cells with mevalonate. The results demonstrated that mevalonate abolished the inhibitory action of simvastatin on cell-mediated contraction of type I collagen gels (Figure 4). This indicates that the mevalonate pathway is also of importance in mediating the pleiotrophic effect of statins on ECM assembly.
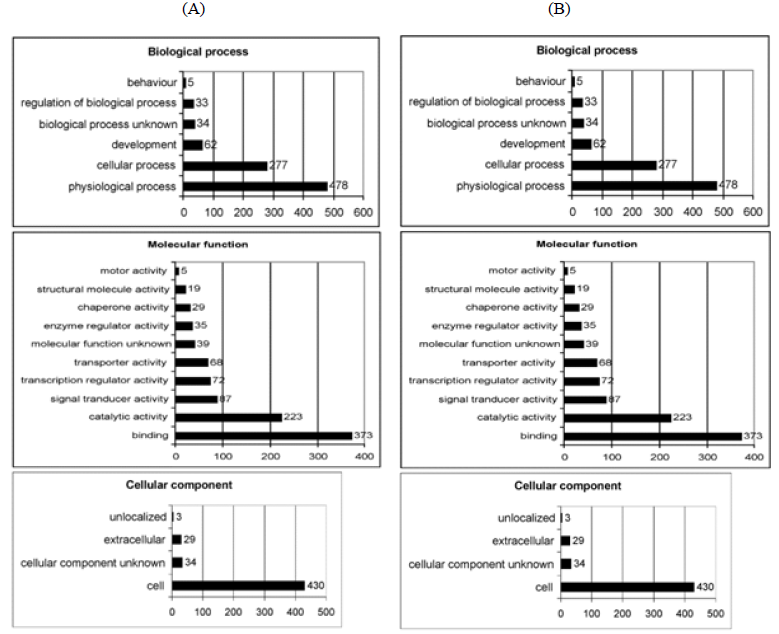
It is well established that cell-mediated CGC is a very complex process requiring the interplay of various cellular and extracellular molecules [20]. To obtain further information about the candidate molecules involved in the inhibitory action of simvastatin on CGC, RNA samples isolated from control and simvastatin conditioned HSFs within collagen gels were used for gene expression profiling using Illumina Sentrix® Human-6 Expression BeadChips. Out of the 26091 RefSeq genes represented on the microarray, 3188 genes showed at least 0.9 time change in expression on the 2-logarithmic scale at least in one of the replicated experiments. Of these regulated genes, 48% showed increased expression in samples derived from simvastatin treated cultures and 52% showed decreased expression. Using DAVID [21], Database for Annotation, Visualization, and Integrated Discovery (http://david.abcc.ncifcrf.gov/home.jsp), simvastatin regulated genes could be assigned to different functional groups: biological process, molecular function and cellular component (Figure 5A and B).
To elucidate the relationship between simvastatin and ECM remodeling, the effect of simvastatin on the gene expression of ECM molecules was examined in a more detailed way. We focused our attention on the functional group of extracellular genes within the cellular component resulting in 29 upregulated and 40 downregulated genes in response to simvastatin treatment. Of particular interest were the genes related to ECM synthesis and remodeling. A more exact analysis revealed that the 29 upregulated genes contained only one gene of the ECM, namely type VIII collagen α2-subunit gene. After analyzing the total data on the suppressed genes, the amount of genes was eliminated to 27 genes (Table 1). These included glycoproteins of interstitial connective tissue, e.g. fibrillin 1 and thrombosbondin 1. Different types of collagens representing fibril-forming type V collagen and non-fibril-forming collagens, particularly collagen types IV, VI, VII and XIII were also downregulated. Furthermore, the expression levels of proteoglycans decorin and versican, both of which have previously been shown to be associated with collagen gel contraction, were among the repressed genes. Microarray analyses revealed that, in line with the trypan blue exclusion test, simvastatin did not upregulate expression of any caspase gene.
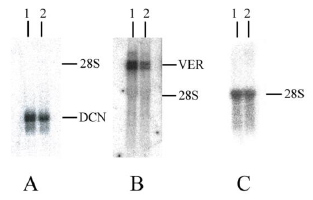
As mentioned above, microarray analysis demonstrated that expression of both decorin and versican decreases when HSF cells are treated with simvastatin. Previously decorin and versican have been shown to be associated with the formation of fibrotic lesions and especially higher levels of decorin together with collagen can lead to a more advanced state of fibrosis [22]. The results of the microarray analysis were further confirmed by Northern blotting in control and HSFs cultures treated with 150 µg/ml of simvastatin (Figure 6). In the presence of simvastatin, the expression of decorin and versican mRNA decreased by 28% and 63%, respectively. These changes were parallel to those observed in the microarray analysis.
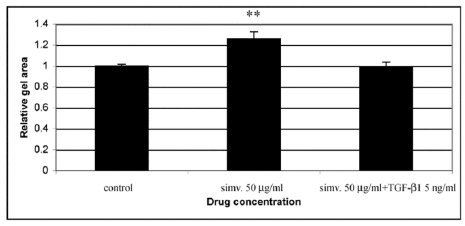
The above results from microarray and Northern blot analyses suggested that simvastatin is likely to possess an antifibrotic activity on HSFs. To examine this possibility more directly, we stimulated the cells with the pro-fibrotic mediator TGF-β1 (5 ng/ml) concomitantly with simvastatin (50 µg/ml), and examined their combined effect on CGC. The results showed that TGF-β1 was able to abolish the inhibitory effect of simvastatin on CGC by HSFs (Figure 7).
Figure 1: Simvastatin inhibits contraction of type I collagen gels by HSFs in a dose-dependent manner. (A) Representative images of contracted collagen gels by control and simvastatin treated HSFs. The gels were prepared as described in Materials and methods. Each gel contained 12.5 x 103 cells/well. The cells were exposed to 50, 100, 150 μg/ml of simvastatin and cultured for 24 hours before assessing gel contraction. Control indicates contraction assay without simvastatin; (B) Quantification of the CGC assays. The degree of contraction was assessed by measuring the area of the gels with an imaging densitometer. The results are expressed as relative changes in the areas of the contracted gels. Capped bars on top of the columns indicate standard deviations (n=12). Statistically significant differences between control and simvastatin treated cultures are indicated by asterisk (***, p<0.001, unpaired Student’s t-test).
Figure 2: Effect of simvastatin on actin cytoskeleton of HSFs. Cells in contracted collagen gels were labelled with Alexa-Fluor-phalloidin as described in Materials and methods. Actin of the cells is seen in green. Control HSFs (not exposed to simvastatin) are shown in (A) and simvastatin (150 µg/ml) treated HSFs in (B). The figures are representative images from three independent experiments. The asterisk in A indicates a typical cellular aggregate and the arrows indicate cytoplasmic extensions between cells or the cellular aggregates. In B, no cellular aggregates can be seen and the cells are more rounded and have fewer cytoplasmic extensions. Scale bar, 100 μm.
Figure 3: Trypan blue exclusion test to examine cell survival after simvastatin treatment. Cells were exposed to simvastatin at concentrations of 50, 100 or 150 μg/ml and cultured on plastic for 24 h, whereafter they were stained with trypan blue. Control indicates cells that were not exposed to simvastatin. Cell death was measured as a percentage of the total cell count. In all, cell death in our experimental setting was low and simvastatin even lowered it in a dose dependent manner. Capped bars on top of the columns indicate standard deviations (n=4). Statistically significant differences between control and simvastatin treated cultures are indicated by asterisk (*, p<0.05, unpaired Student´s t-test).
Figure 4: Mevalonate reverses the inhibitory effect of simvastatin on CGC by HSFs. CGC assays were performed as described in Materials and methods. Co-incubation of HSFs with simvastatin (50 µg/ml) and mevalonate (50 µg/ml) was able to abolish the inhibitory effect of simvastatin on CGC. Control indicates contraction assay without simvastatin and mevalonate. Capped bars on top of the columns indicate standard deviations (n=12). Statistically significant differences between control and drug treated cultures are indicated by asterisk (*, p<0.05, unpaired Student´s t-test).
Figure 5: Output of GoCharts. Upregulated (A) and downregulated genes (B) among Gene Ontology (GO) Biological Processes, Molecular Function and Cellular Component in HSFs by simvastatin on the first GO level using DAVID, Database for Annotation, Visualization, and Integrated Discovery (http://david.abcc.ncifcrf.gov/home.jsp). Simvastatin treatment changed the expression of 3188 genes (>0.9 change on 2-fold logarithmic scale), of which 48 % were upregulated and 52 % downregulated. These genes could be assigned to different functional groups: biological process, molecular function and cellular component. X-axis points out the number of simvastatin regulated genes in HSFs in different categories.
|
Gene name |
GenBank |
AvgLogratio |
SE |
|
cartilage oligomeric matrix protein |
1311 |
-1.26 |
0.03 |
|
collagen, type IV, alpha 2 |
1284 |
-1.01 |
0.06 |
|
collagen, type V, alpha 2 |
1290 |
-0.72 |
0.36 |
|
collagen, type VI, alpha 1 |
1291 |
-2.07 |
0.44 |
|
collagen, type VI, alpha 2 |
1292 |
-1.97 |
0.2 |
|
collagen, type VII, alpha 1 |
1294 |
-3.43 |
0 |
|
collagen, type XIII, alpha 1 |
1305 |
-1.24 |
0.01 |
|
collagen, type XVI, alpha 1 |
1307 |
-1.03 |
0.04 |
|
collagen, type XVIII, alpha 1 |
80781 |
-0.86 |
0.1 |
|
decorin |
1634 |
-0.42 |
0.39 |
|
dermatopontin |
1805 |
-1.74 |
0.11 |
|
elastin microfibril interfacer 1 |
11117 |
-1.58 |
0.16 |
|
extracellular matrix protein 1 |
1893 |
-0.72 |
0.13 |
|
fibrillin 1 |
2200 |
-2.45 |
0 |
|
fibrillin 2 |
2201 |
-1.28 |
0.13 |
|
fibulin 1 |
2192 |
-1.38 |
0 |
|
laminin, alpha 2 |
3908 |
-1.35 |
0.48 |
|
laminin, alpha 4 |
3910 |
-1.66 |
0.05 |
|
laminin, beta 2 |
3913 |
-0.87 |
0 |
|
laminin, beta 3 |
3914 |
-0.95 |
0.14 |
|
laminin, gamma 1 |
3915 |
-2.01 |
0.16 |
|
latent transforming growth factor beta binding protein 4 |
8425 |
-0.83 |
0.01 |
|
microfibrillar-associated protein 2 |
4237 |
-1.19 |
0.02 |
|
sparc/osteonectin, cwcv and kazal-like domains proteoglycan 1 |
6695 |
-1.63 |
0.41 |
|
thrombospondin 1 |
7057 |
-1.58 |
0.15 |
|
thrombospondin 2 |
7058 |
-1.92 |
0.07 |
|
versican |
1462 |
-1.35 |
0.16 |
|
Total of 27 genes |
|||
Table 1: List of downregulated ECM genes in response to simvastatin (150 μg/ml) treatment.
Genbank-GenBank geneID; AvgLogratio-average change in 2-logaritmic scale between controls and simvastatin treated cells; SE-standard error of mean.
Figure 6: Expression of decorin and versican by control and 150 µg/ml simvastatin treated human skin fibroblasts (HSFs). Northern blot analyses of 10 µg total RNA from cultures of control HSFs (A, B and C, lane 1) and 150 µg/ml simvastatin treated HSFs (A, B and C, lane 2). The blot was probed with a random primed cDNA for the full-length human decorin (DCN) core protein (A), a random primed cDNA for versican (VER) cDNA (B) and a nick-translated cDNA for 28S (C). Northern blot analyses were performed as described in Materials and methods.
Figure 7: Treatment of HSFs with TGF-β1 (5 ng/ml) concomitantly with simvastatin (50 µg/ml) eliminates the inhibitory effect of simvastatin on gel contraction. CGC assays were performed as described in Materials and methods. Control indicates contraction assay without simvastatin and TGF-β1. Capped bars on top of the columns indicate standard deviations (n=4). Statistically significant difference in CGC between control and simvastatin treated cultures is indicated by asterisk (**, p<0.01, unpaired Student´s t-test).
4. Discussion
Fibroblast-populated gel contraction cultures are considered an excellent in vitro model for examining molecules or factors involved in matrix remodeling and at present, a great number of both cellular and extracellular molecules and factors have been shown to contribute to gel contraction [10, 20, 23]. In the present study we have demonstrated that the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, simvastatin, prevents CGC by HSFs in a dose-dependent manner and that this effect of simvastatin is associated with decreased formation of cellular aggregates. Previously, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors have been shown to inhibit CGC by different other cell types including lung fibroblasts [13], renal myofibroblasts [24] and vascular smooth muscle cells [25]. The above studies collectively indicate that statins´ inhibitory effect on CGC is not a cell specific but a general phenomenon. Thus, it can be suggested that ECM remodeling activity should be included in the list of the pleiotrophic effects of statins, similarly to their anti-inflammatory, antiproliferative, anticoagulative and immunomodulatory activities [5].
In addition to having demonstrated that simvastatin inhibits CGC, we have shown that this effect of simvastatin can be overcome with mevalonate. This suggests that the mevalonate pathway contributes to the effect of simvastatin on the regulation of ECM assembly. Earlier, the mevalonate pathway has been shown to play a crucial role in such pleiotrophic effects of statins as anti-inflammation [26] and antiproliferation [27]. By blocking the mevalonate pathway, statins including simvastatin not only inhibit cholesterol synthesis but they also suppress the synthesis of various isoprenoids derived from this pathway. This in turn can lead to the prevention of RhoA activation [28]. RhoA inactivation has been shown to be critically involved in the organization of actin stress fibres [29]. In our study, incubation of HSFs with simvastatin resulted in a clear decrease in the formation of actin filaments suggesting the possible involvement of RhoA.
Our additional interest in this study was to get an overall view on the candidate molecules involved in the contraction of type I collagen gels by profiling the genome-wide gene expression of HSFs after simvastatin treatment. We sought to focus especially on the expression of different ECM molecules. Adding to previously reported genes including thrombospondin 1 [30], thrombospondin 2 [31] and collagen type IV [32], the mRNA levels of several other ECM genes were affected by simvastatin. Comparing the mRNA expression levels of the control versus simvastatin treated cells we saw inhibition of the extracellular proteoglycans decorin and versican. Decorin, a small chondroitin/dermatan sulphate proteoglycan, has interactions with several other ECM proteins such as collagen type VI [33], thrombospondin 1 [34] and dermatopontin [35]. The mRNA expression levels of the above three molecules were downregulated concomitantly with decorin. Versican, on the other hand, is a large chondroitin sulphate proteoglycan, which interacts among others with fibulin 1 and fibrillin 1 [36]. Interestingly, the gene expression of all these molecules was also markedly downregulated. Both decorin and versican have been associated with the formation of fibrotic lesions [22] and together with glycoproteins and collagens are vitally involved in the organization, stabilization and remodeling of the ECM [37]. Now a question arises whether the overexpression of the above ECM molecules including decorin could antagonize the action of simvastatin on CGC, because recently e.g. decorin overexpression has been shown to enhance the collagen contraction phenomenon [10]. In the present work, we also showed that the results of microarray analysis on the repression of decorin and versican mRNA expression were in parallel with the findings of Northern blot.
The altered expression of ECM molecules due to statin treatment may lead to a less well organized structure of connective tissue, and this can have consequences related to its function. Tendon cells have been reported to establish an internal cytoskeletal tension through interactions with their ECM [38]. Interferences in the ECM structure may lead to alterations in cells’ tensional homeostasis and to weakness in connective tissue, and there have been some indications of tendinopathy caused by statin therapy and their rate of occurrence may be underestimated [9]. Statins have also been indicated with impaired wound healing [39]. Fibroblasts are involved in several steps of wound healing starting from granulation tissue formation to contraction, which brings the margins of an open wound together [23]. According to our results, simvastatin dose-dependently inhibits CGC, which is an in vitro model for wound contraction.
We also demonstrated that the diminished CGC caused by simvastatin can be eliminated with concomitant TGF-β1 stimulation of the cells. TGF-β1 is a major inducer of ECM synthesis and acts as a strong fibrotic factor. Our results together with the observations from microarray and Northern analyses strongly imply that simvastatin is an antifibrotic drug. This provides a possible explanation for the inhibitory effect of simvastatin on CGC.
Fibroblasts are able to synthesize a large number of different ECM components including proteoglycans, the most common of which are decorin, biglycan and versican [40, 41]. The continuous deposition of these and other ECM molecules may ultimately lead to fibrosis, a very complex process which is not yet fully understood [37]. With lung fibroblasts, a fibrotic trait can be identified by higher decorin and collagen production [41]. In systemic sclerosis, the production of proteoglycans is increased and this altered production may affect the organization of matrix fibers and thereby the fibrotic process [22]. Previously, simvastatin has been reported to reduce fibrotic events in normal and systemic sclerosis fibroblasts [42]. Furthermore, in a earlier study another HMG-CoA reductase inhibitor, atorvastatin, has been shown to possess an antifibrotic effect on aortic smooth muscle cells [43]. So far, little has been reported about the effects of statins on proteoglycan regulation. In our study, simvastatin treatment caused a marked change in the expression of two proteoglycans, decorin and versican in healthy human fibroblasts. Our results collectively suggest that statins display an antifibrotic activity.
There is also evidence that simvastatin has beneficial influence on the stability of atherosclerotic plaque [44]. In the present study we have shown that simvastatin strongly downregulates the expression of several types of collagen including collagen type IV, V and VI, which all have been shown to be associated with the formation of atherosclerotic lesions [45]. Because collagens are among others structural providers of mechanical support, changes in their composition may have influence on the formation and stability of atherosclerotic plaques. This may imply that treatment with simvastatin might also have disadvantageous effects on the stability of atherosclerotic plaques, but further studies are still required.
In summary, the results of our present study demonstrated that simvastatin is able to markedly influence ECM assembly and that this effect of simvastatin is mediated through the mevalonate pathway. The results also showed that the effect of simvastatin on CGC can be eliminated with the pro-fibrotic cytokine TGF-β1 suggesting that simvastatin possesses an antifibrotic activity. It remains to be examined whether similar findings are also true in vivo and whether they contribute to physiological and pathological remodeling and fibrotic processes.
Acknowledgements
The authors wish to thank Ms. Marja Nykänen and Ms. Heidi Pakarinen for excellent technical assistance. Financially this study was supported by Finnish Foundation for Cardiovascular Research, Medical Research Fund (EVO) of Turku University Central Hospital, and Turku University Foundation.
Conflict of Interest
The authors state no conflict of interest.
References
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20, 536 high-risk individuals: a randomized placebo-controlled trial. Lancet 360 (2002): 7-22.
- Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344 (1994): 1383-1389.
- Holme I. An analysis of randomized trials evaluating the effect of cholesterol reduction on total mortality and coronary heart disease incidence. Circulation 82 (1990): 1916-1924.
- McFarlane SI, Muniyappa R, Francisco R, Sowers JR. Clinical review 145: Pleiotropic effects of statins: lipid reduction and beyond. J Clin Endocrinol Metab 87 (2002): 1451-1458.
- Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 109 (2004): 39-43.
- Li C, Yang CW, Park JH, Lim SW, Sun BK, Jung JY, et al. Pravastatin treatment attenuates interstitial inflammation and fibrosis in a rat model of chronic cyclosporine-induced nephropathy. Am J Physiol Renal Physiol 286 (2004): 46-57.
- Martin J, Denver R, Bailey M, Krum H. In vitro inhibitory effects of atorvastatin on cardiac fibroblasts: implications for ventricular remodelling. Clin Exp Pharmacol Physiol 32 (2005): 697-701.
- Schwartz GG, Olsson AG, Ezekowiz MD, Ganz P, Oliver MF, Waters D, et al. Myocardial Ischemic Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 285 (2001): 1711-1718.
- Chazerain P, Hayem G, Hamza S, Best C, Ziza JM. Four cases on tendinopathy in patients on statin therapy. Joint Bone Spine 68 (2001): 430-433.
- Jarvelainen H, Vernon RB, Gooden MD, Francki A, Lara S, Johnson PY, et al. Overexpression of decorin by rat arterial smooth muscle cells enhances contraction of type I collagen in vitro. Arterioscler Thromb Vasc Biol 24 (2004): 67-72.
- Wight TN, Merrilees MJ. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res 94 (2004): 1158-1167.
- Liu L, Moesner P, Kovach NL, Bailey R, Hamilton AD, Sebti SM, et al. Integrin-dependent leukocyte adhesion involves geranylgeranylated protein(s). J Biol Chem 274 (1999): 33334-33340.
- Watts KL, Sampson EM, Schultz GS, Spiteri MA. Simvastatin inhibits growth factor expression and modulates profibrogenic markers in lung fibroblasts. Am J Respir Cell Mol Biol 32 (2005): 290-300.
- Jarvelainen H, Pelliniemi TT, Ronnemaa T. The stabilizing effect of glucocorticoids on human endothelial cells in culture. Scand J Clin Lab Invest 45 (1985): 223-228.
- Jarvelainen HT, Kinsella MG, Wight TN, Sandell LJ. Differential expression of small chondroitin/dermatan sulfate proteoglycans, PG-I/biglycan and PG-II/decorin, by vascular smooth muscle and endothelial cells in culture. J Biol Chem 266 (1991): 23274-23281.
- Iruela-Arispe ML, Hasselaar P, Sage H. Differential expression of extracellular proteins is correlated with angiogenesis in vitro. Lab Invest 64 (1991): 174-186.
- Fisher LW, Termine JD, Young MF. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem 264 (1989): 4571-4576.
- Krusius T, Gehlsen KR, Ruoslahti E. A fibroblast chondroitin sulfate proteoglycan core protein contains lectin-like and growth factor-like sequences. J Biol Chem 262 (1987): 13120-13125.
- Nelimarkka L, Kainulainen V, Schonherr E, Moisander S, Jortikka M, Lammi M, et al. Expression of small extracellular chondroitin/dermatan sulfate proteoglycans in differentially regulated in human endothelial cells. J Biol Chem 272 (1997): 12730-12737.
- Ngo P, Ramalingam P, Phillips JA, Furuta GT. Collagen gel contraction assay. Methods Mol Biol 341 (2006): 103-109.
- Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biology 4 (2003): 3.
- Westergren-Thorsson G, Coster L, Akesson A, Wollheim FA. Altered dermatan sulphate proteoglycan synthesis in fibroblast cultures established from skin of patients with systemic sclerosis. J Rheumatol 23 (1996): 1398-1406.
- Guidry C and Grinnell F. Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J Cell Sci 79 (1985): 67-81.
- Kelynack KJ, Hewitson TD, Martic M, McTaggart S, Becker GJ. Lovastatin downregulates renal myofibroblasts function in vitro. Nephron 91 (2002): 701-707.
- Kuzuya M, Cheng XW, Sasaki T, Tamaya-Mori N, Iguchi A. Pitavastatin, a 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, blocks vascular smooth muscle cell populated-collagen lattice contraction. J Cardiovasc Pharmacol 43 (2004): 808-814.
- Zeinstra E, Wilczak N, Chesik D, Glazenburg L, Kroese FGM, Keyser JD. Simvastatin inhibits interferon-g-induced MHC class II up-regulation in cultured astrocytes. J Neuroinflammation 3 (2006): 16.
- Takeda N, Kondo M, Ito S, Ito Y, Shimokata K, Kume H. Role of RhoA inactivation in reduced cell proliferation of human airway smooth muscle by simvastatin. Am J Respir Cell Mol Biol 35 (2006): 722-729.
- Xu H, Zeng L, Peng H, Chen S, Jones J, Chew TL, et al. HMG-CoA reductase inhibitor simvastatin mitigates VEGF-induced “inside-out” signaling to extracellular matrix by preventing RhoA activation. Am J Physiol Rena Physiol 291 (2006): 995-1004.
- Hahn A, Heusinger-Ribeiro J, Lanz T, Zenkel S, Goppelt-Struebe M. Induction of connective tissue growth factor by activation of heptahelical receptors. Modulation by Rho proteins and the actin cytoskeleton. J Biol Chem 275 (2000): 37429-37435.
- Riessen R, Axel DI, Fenchel M, Herzog UU, Rossmann H, Karsch KR. Effect of HMG-CoA reductase inhibitors on extracellular matrix expression in human vascular smooth muscle cells. Basic Res Cardiol 94 (1999): 322-332.
- Siegel-Axel DI, Runge H, Seipel L, Riessen R. Effects of cerivastatin on human arterial smooth muscle cell growth and extracellular matrix exspression at varying glucose and low-density lipoprotein levels. J Cardiovasc Pharmacol 41 (2003): 422-433.
- Nogaki F, Muso E, Yashiro M, Kasuno K, Kamata T, Ono T, et al. Direct inhibitory effects of simvastatin on matrix accumulation in cultured murine mesangial cells. Kidney Int Suppl 71 (1999): 198-201.
- Nareyeck G, Seidler DG, Troyer D, Rauterberg J, Kresse H, Schönherr E. Differential interactions of decorin and decorin mutants with type I and type VI collagens. Eur J Biochem 271 (2004): 3389-3398.
- Winnemöller M, Schon P, Vischer P, Kresse H. Interactions between thrombospondin and the small proteoglycan decorin: interference with cell attachment. Eur J Cell Biol 59 (1992): 47-55.
- Okamoto O, Suzuki Y, Kimura S, Shinkai H. Extracellular matrix 22-kDa protein interacts with decorin core protein and is expressed in cutaneous fibrosis. J Biochem 119 (1996): 106-114.
- Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res 15 (2005): 483-494.
- Eckes B, Kessler D, Aumailley M, Krieg T. Interactions of fibroblasts with the extracellular matrix: implications for the understanding of fibrosis. Springer Semin Immunopathol 21 (1999): 415-429.
- Lavagnino M and Arnoczky SP. In vitro alterations in cytoskeletal tensional homeostasis control gene expression in tendon cells. J Orthop Res 23 (2005): 1211-1218.
- Vincent L, Chen W, Hong L, Mirshahi F, Mishal Z, Mirshahi-Khorassani T, et al. Inhibition of endothelial cell migration by cerivastatin, an HMG-CoA reductase inhibitor: contribution to its anti-angiogenic effect. FEBS Lett 495 (2001): 159-166.
- Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem 67 (1998): 609-652.
- Westergren-Thorsson G, Sime P, Jordana M, Gauldie J, Sanstrand B, Malmstrom A. Lung fibroblast clones from normal and fibrotic subjects differ in hyaluronan and decorin production and rate of proliferation. Int J Biochem Cell Biol 36 (2004): 1573-1584.
- Louneva N, Huaman G, Fertala J, Jiménez SA. Inhibition of systemic sclerosis dermal fibroblasts type I collagen production and gene expression by simvastatin. Arthritis Rheum 54 (2006): 1298-1308.
- Rupérez M, Rodrigues-Díez R, Blanco-Colio LM, Sánchez-López E, Rodríguez-Vita J, Esteban V. HMG-CoA reductase inhibitors decrease angiotensin II-induced vascular fibrosis: Role of RhoA/ROCK and MAPK pathways. Hypertension 50 (2007): 377-383.
- Cipollone F, Fazia M, Iezzi A, Zucchelli M, Pini B, De Cesare D, et al. Suppression of the functionally coupled cyclooxygenase-2/prostaglandin E synthase as a basis of simvastatin-dependent plaque stabilization in humans. Circulation 107 (2003): 1479-1485.
- Katsuda S, Okada Y, Minamoto T, Oda Y, Matsui Y, Nakanishi I. Collagens in human atherosclerosis. Immunohistochemical analysis using collagen type-specific antibodies. Arterioscler Thromb 12 (1992): 494-502.


 Impact Factor: * 2.5
Impact Factor: * 2.5 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 