Surgery Associated with Radiotherapy for Treatment of Giant Squamous Cell Carcinoma in Hand
Article Information
Bruna Mizobutsi Mendes1, Frederico Gustavo Neiva Ellinger MD2, Mariam Patrícia Auada Souto MD, PhD3, Luís Ricardo Martinhão Souto MD, PhD4*
1Academic of the Medical Course at University of Marília (UNIMAR), Marília, São Paulo state, Brazil
2Medical Pathologist at University of Marília (UNIMAR), Marília, São Paulo state, Brazil
3Professor of Dermatology at University of Marília (UNIMAR), Marília, São Paulo state, Brazil
4Professor of Plastic Surgery at University of Marília (UNIMAR), Maríla, São Paulo state, Brazil
*Corresponding Author: Dr. Luís Ricardo Martinhão Souto, Plastic Surgery at University of Marília (UNIMAR), Maríla, Av. Higyno Muzzy Filho, 1001 - Campus Universitário - Jardim Araxá, São Paulo state, Brazil
Received: 06 March 2019; Accepted: 13 March 2019; Published: 28 March 2019
Citation: Bruna Mizobutsi Mendes, Frederico Gustavo Neiva Ellinger MD, Mariam Patrícia Auada Souto MD, PhD, Luís Ricardo Martinhão Souto MD, PhD. Surgery Associated with Radiotherapy for Treatment of Giant Squamous Cell Carcinoma in Hand. Archives of Clinical and Medical Case Reports 3 (2019): 62-71.
View / Download Pdf Share at FacebookAbstract
Squamous cell carcinoma is the most common tumor in the hand, with actinic keratosis being its precursor lesion. It is a common tumor in light-skinned individuals with a history of excessive sun exposure and advanced age. It presents a wide variety of clinical forms, which can generate diagnostic doubt. The combination of clinical and pathological evaluations, together with detailed anamnesis, is the best way to achieve at the final diagnosis. The gold standard treatment for these tumors is surgical excision of the lesion with adequate safety margins; in some cases adjuvant radiotherapy is an excellent option. In this paper, we review the subject addressed and report the case of a patient with giant squamous cell carcinoma in one hand, with eight months of evolution, treated by surgical excision and adjuvant radiotherapy; the treatment was successful and preserved the motor function of the patient's hand.
Keywords
Actinic keratosis, Adjuvant radiotherapy, Squamous cell carcinoma, Surgery, Surgical margins, Ultraviolet rays
Actinic keratosis articles Actinic keratosis Research articles Actinic keratosis review articles Actinic keratosis PubMed articles Actinic keratosis PubMed Central articles Actinic keratosis 2023 articles Actinic keratosis 2024 articles Actinic keratosis Scopus articles Actinic keratosis impact factor journals Actinic keratosis Scopus journals Actinic keratosis PubMed journals Actinic keratosis medical journals Actinic keratosis free journals Actinic keratosis best journals Actinic keratosis top journals Actinic keratosis free medical journals Actinic keratosis famous journals Actinic keratosis Google Scholar indexed journals Adjuvant radiotherapy articles Adjuvant radiotherapy Research articles Adjuvant radiotherapy review articles Adjuvant radiotherapy PubMed articles Adjuvant radiotherapy PubMed Central articles Adjuvant radiotherapy 2023 articles Adjuvant radiotherapy 2024 articles Adjuvant radiotherapy Scopus articles Adjuvant radiotherapy impact factor journals Adjuvant radiotherapy Scopus journals Adjuvant radiotherapy PubMed journals Adjuvant radiotherapy medical journals Adjuvant radiotherapy free journals Adjuvant radiotherapy best journals Adjuvant radiotherapy top journals Adjuvant radiotherapy free medical journals Adjuvant radiotherapy famous journals Adjuvant radiotherapy Google Scholar indexed journals Squamous cell carcinoma articles Squamous cell carcinoma Research articles Squamous cell carcinoma review articles Squamous cell carcinoma PubMed articles Squamous cell carcinoma PubMed Central articles Squamous cell carcinoma 2023 articles Squamous cell carcinoma 2024 articles Squamous cell carcinoma Scopus articles Squamous cell carcinoma impact factor journals Squamous cell carcinoma Scopus journals Squamous cell carcinoma PubMed journals Squamous cell carcinoma medical journals Squamous cell carcinoma free journals Squamous cell carcinoma best journals Squamous cell carcinoma top journals Squamous cell carcinoma free medical journals Squamous cell carcinoma famous journals Squamous cell carcinoma Google Scholar indexed journals health articles health Research articles health review articles health PubMed articles health PubMed Central articles health 2023 articles health 2024 articles health Scopus articles health impact factor journals health Scopus journals health PubMed journals health medical journals health free journals health best journals health top journals health free medical journals health famous journals health Google Scholar indexed journals Surgery articles Surgery Research articles Surgery review articles Surgery PubMed articles Surgery PubMed Central articles Surgery 2023 articles Surgery 2024 articles Surgery Scopus articles Surgery impact factor journals Surgery Scopus journals Surgery PubMed journals Surgery medical journals Surgery free journals Surgery best journals Surgery top journals Surgery free medical journals Surgery famous journals Surgery Google Scholar indexed journals Surgical margins articles Surgical margins Research articles Surgical margins review articles Surgical margins PubMed articles Surgical margins PubMed Central articles Surgical margins 2023 articles Surgical margins 2024 articles Surgical margins Scopus articles Surgical margins impact factor journals Surgical margins Scopus journals Surgical margins PubMed journals Surgical margins medical journals Surgical margins free journals Surgical margins best journals Surgical margins top journals Surgical margins free medical journals Surgical margins famous journals Surgical margins Google Scholar indexed journals Ultraviolet rays articles Ultraviolet rays Research articles Ultraviolet rays review articles Ultraviolet rays PubMed articles Ultraviolet rays PubMed Central articles Ultraviolet rays 2023 articles Ultraviolet rays 2024 articles Ultraviolet rays Scopus articles Ultraviolet rays impact factor journals Ultraviolet rays Scopus journals Ultraviolet rays PubMed journals Ultraviolet rays medical journals Ultraviolet rays free journals Ultraviolet rays best journals Ultraviolet rays top journals Ultraviolet rays free medical journals Ultraviolet rays famous journals Ultraviolet rays Google Scholar indexed journals patient articles patient Research articles patient review articles patient PubMed articles patient PubMed Central articles patient 2023 articles patient 2024 articles patient Scopus articles patient impact factor journals patient Scopus journals patient PubMed journals patient medical journals patient free journals patient best journals patient top journals patient free medical journals patient famous journals patient Google Scholar indexed journals medicine articles medicine Research articles medicine review articles medicine PubMed articles medicine PubMed Central articles medicine 2023 articles medicine 2024 articles medicine Scopus articles medicine impact factor journals medicine Scopus journals medicine PubMed journals medicine medical journals medicine free journals medicine best journals medicine top journals medicine free medical journals medicine famous journals medicine Google Scholar indexed journals radiotherapy articles radiotherapy Research articles radiotherapy review articles radiotherapy PubMed articles radiotherapy PubMed Central articles radiotherapy 2023 articles radiotherapy 2024 articles radiotherapy Scopus articles radiotherapy impact factor journals radiotherapy Scopus journals radiotherapy PubMed journals radiotherapy medical journals radiotherapy free journals radiotherapy best journals radiotherapy top journals radiotherapy free medical journals radiotherapy famous journals radiotherapy Google Scholar indexed journals
Article Details
1. Introduction
Approximately 10% to 15% of non-melanoma skin tumors occur on the hand, and the most common of these is the squamous cell carcinoma (SCC) [1]. Actinic keratosis is the precursor lesion of most SCCs, and its presence is an increased risk for skin cancer [2, 3]. Ultraviolet (UV) solar radiation is the primary risk factor for the development of SCC [2, 4]. Clinically, SCC may have a wide variety of presentations with varying degrees of hyperkeratosis [1, 2] Early recognition of these tumors, with rapid surgical intervention, is essential for a good prognosis [3] and for preserving the functionality of the hand [5]. In this paper, in addition to a quick update/review of the subject addressed, we describe an illustrative case of a patient with extensive squamous cell carcinoma and deep invasion of musculature in the back of one hand, with eight months of evolution, submitted to treatment with excision and adjuvant postoperative radiotherapy.
2. Ethical Aspects
This work was approved by the Ethics Committee of Universidade de Marília (UNIMAR), and is in accordance with the Declaration of Helsinki (1964) and its posterior amendments.
3. Case Report
A 69-year-old white male patient, a newspaper delivery man, came to the plastic surgery outpatient clinic with a complaint of "wound" on the back of his left hand for eight months, with rapid growth and with intense local pain limiting movements. Incisional biopsy, performed 6 months before, showed a non-neoplastic lesion and non-specific inflammatory signs. He had an extensive and ulcerated tumor lesion on the left hand, approximately 11 cm in diameter, adhered to deep planes (Figure 1), with hyperkeratosis and seropurulent secretion of strong odor, with intense palpation pain. There was no presence of lymph node enlargement in the left axillary region. The primary clinical diagnostic hypothesis was squamous cell carcinoma.
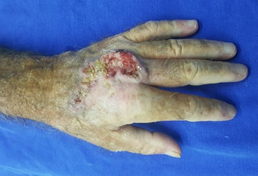
Figure 1: Extensive and ulcerated tumor lesion of left hand dorsum.
Surgical removal of the lesion (Figure 2) was performed with lateral safety margins of 1.0 cm. During excision, tumoral invasion of the superficial muscular fascia and the interosseous musculature was observed at the height of the third metacarpal (Figure 3). Non-removal of the interosseous muscles and closure of the blistering area on the left hand dorsol, with autologous total skin graft (Figure 4) removed from the left inguinal region were used. The surgical specimen was sent for histopathological analysis, which confirmed a well differentiated SCC (Figure 5), with tumor invasion compromising the deep margins of surgical resection (interosseous musculature). After 15 days of surgery, the integration of the skin graft into the recipient bed was almost complete, with a small loss (about 15% of the total graft extension) in the area of ??residual tumor lesion (Figure 6).
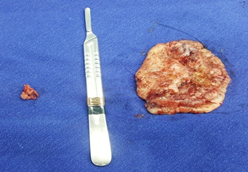
Figure 2: Tumor lesion (anatomical specimen) removed from the left hand dorsum, approximately 11.0 cm in diameter.
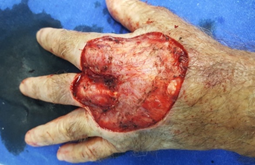
Figure 3: Resection area of ??tumor lesion in left hand dorsum, with clear musculature involvement on 3rd metacarpal.
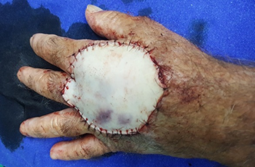
Figure 4: Closure of bloody area in left hand dorsum after resection of tumor lesion.
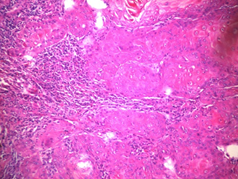
Figure 5: Photomicrography. Hematoxylin-eosin staining. Increased 100 X. Squamous cell carcinoma, showing atypical squamous epithelial cells with pleomorphic nuclei, prominent nucleoli and mitotic figures.
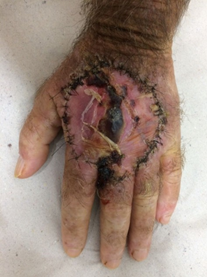
Figure 6: Partial loss (15%) of total skin graft on left hand dorsum 15 days after surgery.
The patient was referred for radiotherapy after surgery, and five weekly sessions of treatment were performed for four weeks. After radiotherapy, there was complete remission of the lesion, and the patient had complete disappearance of pain symptoms, maintaining satisfactory left hand motor function. Patient follow-up was not possible, as he died of gastric bleeding from a peptic ulcer one month after the end of radiotherapy.
4. Discussion
More than 2 million cases of skin cancer are diagnosed each year in the United States [3]. In Brazil, non-melanoma skin cancer is the most frequent, accounting for 30% of all malignancies registered in the country [6]. Squamous cell carcinoma (SCC) is the second most common non-melanoma type of skin cancer, and in some studies its incidence is close to that of basal cell carcinoma (BCC) [7]. SCC is the most common tumor in the hand, ranging from 58% to 90% of cancers at this location. From 11% to 16% of SCCs occur in the hands, and two-thirds of these tumors arise on the back of the hands [1]. SCC may have different clinical presentations, varying the degree of tumor differentiation from small erythematous plaques or nodules to “sizable fungating” and necrotic masses [1]; SCC are endured lesions with potential ulceration and may show spontaneous bleeding [3]. Histopathological subtypes of SCCs with poor prognosis include desmoplastic squamous cell carcinoma, squamous cell carcinoma of adenosquamous cells and squamous cell carcinoma associated with ulceration / healing [7].
The risk factors for developing SCC are exposure to ultraviolet radiation (solar), areas of chronic inflammation, non-healing ulcers and/or wounds, fair skin (Fitzpatrick phototypes I and II), advanced age, vacations and/or (low latitudes) [8]. Less common factors for the occurrence of these tumors include exposure to arsenic and polyaromatic hydrocarbons [1, 3, 9], as well as immunosuppression [10]. UV (solar) radiation is the primary risk factor for photocarcinogenesis, as well as causing photoaging [4]. Most SCCs grow from actinic keratosis (AK), considered a precursor / pre-malignant lesion [3, 11]. Almost all of the AKs are found on the backs of the hands and upper limbs. These precursor lesions are markers of increased risk for skin cancer and indicate a history of intense exposure to UV radiation. The rate of progression from AK to SCC ranges from 6% to 10% [3].
The recognition of these precursor lesions (AKs) and the prompt and adequate treatment of the disease is of paramount importance, preventing the evolution of the disease from increasing morbidity and mortality [1]. Initially, to determine if a suspected lesion may be a malignant tumor, it is necessary to harvest the detailed history of the injury, including its duration, size and color changes, and other symptoms such as itching and pain. The history of prior and familial skin cancer, sun exposure, benign skin lesions, and areas of chronic inflammation should also be documented [1]. Although SCCs are suspected and/or identified by anamnesis and physical examination and by the clinical course of the lesions, the definitive diagnosis is only obtained after a thorough anatomopathological examination [12].
In 1921, Broders [13] developed a histological classification system for SCC (grades 1 to 4), based on the proportion of histologically differentiated cells versus undifferentiated cells. Grade 1 represents a lesion in which 75% of the cells are well differentiated, grade 2 has 50% well differentiated cells, grade 3 has 25% to 50% well differentiated cells, and grade 4 is less than 25% of well-differentiated cells [13]. In practice, many pathologists use the well-differentiated phrase to indicate that almost all cells are well differentiated, moderate differentiation to indicate areas with no clear keratinization, corneal pearls, and other classic features of SCC, and few differentiation indicates that a keratinocyte lineage is difficult to determine [7]. The presence of poor differentiation indicates a worse prognosis, with a risk of recurrence greater than triple (7% vs. 2%) and approximately twice as many metastatic risk (7% vs. 3%) than well-differentiated SCCs [14].
The latest American Joint Committee on Cancer (AJCC) staging system, eight edition (AJCC-8), published in October 2016, uses tumor diameter ≥ 2 cm as the distinguishing factor between T1 and T2 tumors. High-risk staging of AJCC-8, which results in a staging above (T3), includes tumor diameter ≥ 4 cm, minor bone erosion, invasion of nerves of 0.1 mm caliber or of nerves in subcutis, or even deep invasion (≥ 6 mm or below subcutaneous fat). T4 is reserved for increased bone involvement or invasion of the skull base [7]. SCCs that are AJCC-8 stage T2 or higher are likely to be at high risk with unfavorable clinical outcomes and may warrant further investigation and treatment [7]. The diagnostic hypotheses made by plastic surgeons and dermatologists tend to be more diagnostic accurate compared to anatomopathological examination when compared to the hypotheses formulated by ophthalmologists, general surgeons and general practitioners [15]. The diagnostic accuracy is closely related to the physician's clinical experience to identify such lesions, and specialized referral centers have superior accuracy [16].
Incisional biopsy provides diagnostic information that may aid in the definition of surgical management in relation to non-melanoma skin cancers (BCC and SCC) [17]. There may be disparity between histological results of incisional and excisional biopsies[17]. Surgical variables, tumor evolution, and analysis by the pathologist could explain the variations in histological analysis [17]. Swetter et al. [18] in 2003, proposed that, during the growth phases, tumor cell "nests" could be masked by inflammatory infiltrates [18]. The factors of the sample, such as intensity of the staining or the thickness of the histological cut, may also affect the accuracy of the diagnosis by the pathologist [19].
In a typical setting, the lesion is clinically diagnosed as atypical enough to justify the biopsy, and referred to the pathologist who will give the "gold standard" diagnosis that will guide the behavior. In this sequence, several things can go wrong: the patient may not seek care while the lesion is curable, the physician to whom the patient initially resorts may fail to recognize the lesion as potentially malignant, the pathologist may misdiagnose the lesion, and the surgeon may act wrongly to the pathologist's report [20]. Communication among specialties is essential. Clinical information such as size, age, evolution and reason for excision of the lesion are relevant so that the pathologist can make an accurate diagnosis [21, 22].
The treatment of choice for SCC is surgical excision of the lesion with safety margins, both for low and high-risk lesions [3, 23]. SCCs are said to be high-risk if they have rapid growth (more than 2 cm at presentation), if the biopsy shows invasion equal to or greater than 2 mm deep or the Clark level is at least 4, if they are located in areas with hairs, if there is evidence of perineural invasion in the biopsy, and/or if there is little histological differentiation [3]. For low-risk SCCs, the recommended margin for excision should be at least 4 mm; for high-risk SCCs the recommended margin is at least 6 mm [3]. Although malignancy is limited to skin, excision should be done to subcutaneous fat [3].
A tumor with a diameter greater than 2 cm doubles the risk of recurrence of SCC and triples the risk of metastases when compared to another tumor smaller than 2 cm [10]. The risk factor most associated with recurrence and metastasis is tumor depth; tumors of thickness of Breslow > 2 mm have a 10-fold increased risk of local recurrence, and tumors that extend beyond subcutaneous fat (in deeper layers such as fascia, muscle, perichondrium and periosteum) have an 11-fold increased risk of metastasis compared with more superficial tumors [24]. SCC depth is sometimes described by tissue plane rather than millimeters. In anatomical terms, extension beyond subcutaneous fat is associated with high rates of recurrence (28%) and nodal metastasis (27%) [25]. Perineural invasion of large caliber nerves (nerve measuring ≥ 0.1 mm) is associated with nodal metastases and tumor-specific mortality [26-28]. Tumors with perineural invasion have significant local recurrence and high metastatic risks (47% and 35%, respectively), even after extensive excision [29].
Radiotherapy is reserved for the occurrence of SCCs in older patients and for those patients who do not tolerate surgery, or when it was not possible to obtain clearly adequate surgical margins. Adjuvant radiotherapy is commonly performed after surgical treatment in very extensive or invasive tumors [2]. Many patients with SCC have an excellent prognosis after surgical excision [11]. The rate of healing after excision is around 92% [3]. The recurrence rate for primary tumors on the hand ranges from 5% to 19% [3]. The rate of metastases for SCC in the hand is high, ranging from 6% to 28% [1]. Although most of these tumors are diagnosed early in life (I and II stages), they account for the majority of deaths from non- melanoma skin cancers [11].
Surgical procedures to treat invasive skin cancer on the hand are often complex and limited [5]. Although the excision of a single tumor in the hand should not present difficulties, reconstruction of multiple skin defects represents a challenge due to lack of reserve tissue in this anatomical region [5]. Local reconstruction techniques include the use of grafts and flaps [5]. Successful grafting requires adequate blood flow to the recipient site, immobilization of the skin graft, and prevention of fluid collection formation under the graft [30].
Many experts believe that 80% of skin cancers can be prevented by measures to protect against exposure to solar radiation, such as limiting exposure during certain hours of the day (10:00 a.m. to 4:00 p.m.), wearing clothing that covers most of the body , wear hats to protect the neck, wear sunglasses, apply sunscreen, drink plenty of water, and examine the skin regularly [31]. The regular use of a broad-spectrum sunscreen is effective in preventing UV-induced DNA damage, but irregular or inappropriate use of sunscreen during exposure to UV radiation results in DNA damage that can lead to mutation and subsequent development of skin cancer [4].
The nomenclature adopted in this article - extensive/giant squamous cell carcinoma - is based on a case report on a variation of SCC (Epithelioma Cuniculatum) in the left hand dorsum, where it was suggested that the word "giant" be the most descriptive term with no specific anatomical or histological relationship, and only considers the size of the lesion and the functional impairment of the patient's hand [32].
5. Conclusions
Early diagnosis and surgical treatment of squamous cell carcinoma are fundamental when this type of tumor occurs in any anatomical location, but they are even more important when this type of tumor occurs in the hand, due to the importance of the hands in performing the motor activities daily. Radiotherapy is an excellent adjunct treatment option for cases of extensive and invasive tumor lesions in order to preserve motor function.
Conflicts of Interest
There is no conflict of interest related to this paper.
Funding Statement
There is no funding statement for this work.
References
- Kakar S, Endress R. Skin cancer of the hand: current concepts. J Am Acad Orthop Surg 23 (2015): 307-316.
- Howell JY, Ramsey ML. Cancer, squamous cell, skin (2017).
- Ilyas EN, Leinberry CF, Ilyas AM. Skin cancers of the hand and upper extremity. J Hand Surg Am 37 (2012): 171-178.
- Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection. J Am Acad Dermatol 76 (2017): S100-S109.
- Hessam S, Georgas D, Sand M, Bechara FG. Complete skin resection of the dorsum of the hand: a prophylactic approach using a dermal regeneration template. J Cutan Med Surg 18 (2014): 56-59.
- Ministry of Health of Brazil. Estimate/2016 – Incidence of Cancer in Brazil. (Instituto Nacional do Câncer web site) (2017).
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol 78 (2018): 237-247.
- Milon A, Bulliard JL, Vuilleumier L, et al. Estimating the contribution of occupational solar ultraviolet exposure to skin cancer. Br J Dermatol 170 (2014): 157-164.
- Bonerandi JJ, Beauvillain C, Caquant L, et al. Guidelines for the diagnosis and treatment of cutaneous squamous cell carcinoma and precursor lesions. J Eur Acad Dermatol Venereol 5 (2011): 1-51.
- Xiang F, Lucas R, Hales S, et al. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978-2012: empirical relationships. JAMA Dermatol 150 (2014): 1063-1071.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous Squamous Cell Carcinoma: Review of the Eighth Edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol 24 (2017): 171-194.
- Souto LR, Silva RD. Super giant squamous cell carcinomas. Cutis 91 (2013): 78-80.
- Broders AC. Squamous cell epithelioma of the skin: a study of 256 cases. Ann Surg 73 (1921): 141-160.
- Brantsch KD, Meisner C, Schönfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol 9 (2008): 713-720.
- Mohammad EA, Mansour M, Parichehr K, et al. Assessment of clinical diagnostic accuracy compared with pathological diagnosis of basal cell carcinoma. Indian Dermatol Online J 6 (2015): 258-262.
- Curley RK, Cook MG, Fallowfield ME, et al. Accuracy in clinically evaluating pigmented lesions. BMJ 299 (1989): 16-18.
- Armstrong A, Hart-Pinto A, Sharma N, et al. Discrepancies between biopsy and formal excision histology of non-melanoma skin cancer. J Plast Reconstr Aesthet Surg 69 (2016): 1714-1716.
- Swetter SM, Boldrick JC, Pierre P, et al. Effects of biopsy-induced wound healing on residual basal cell and squamous cell carcinomas: rate of tumor regression in excisional specimens. J Cutan Pathol 30 (2003): 139-146.
- Viray H, Li K, Long TA, et al. A prospective, multi-institutional diagnostic trial to determine pathologist accuracy in estimation of percentage of malignant cells. Arch Pathol Lab Med 137 (2013): 1545-1549.
- Fleming MG. Pigmented lesion pathology: what you should expect from your pathologist, and what your pathologist should expect from you. Clin Plast Surg 37 (2010): 1-20.
- de la Fouchardière A. Clinical practice evolution in dermatology: consequences for the pathologist. Ann Pathol 31 (2011): S117-S120.
- Rademaker M, Thorburn M. Pathology referrals for skin lesions--are we giving the pathologist sufficient clinical information? N Z Med J 123 (2010): 53-58.
- Yun MJ, Park JU, Kwon ST. Surgical options for malignant skin tumors of the hand. Arch Plast Surg 40 (2013): 238-243.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-analysis. JAMA Dermatol 152 (2016): 419-428.
- Karia PS, Jambusaria-Pahlajani A, Harrington DP, et al. Evaluation of American Joint Committeeon Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneoussquamous cell carcinoma. J Clin Oncol 32 (2014): 327-334.
- Carter JB, Johnson MM, Chua TL, et al. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11-year cohort study. JAMA Dermatol 149 (2013): 35-41.
- Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg 35 (2009): 1859-1866.
- Cottel WI. Perineural invasion by squamous-cell carcinoma. J Dermatol Surg Oncol 8 (1982): 589-600.
- Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg 148 (1984): 542-547.
- Goto H, Yoshikawa S, Mori K, et al. Retrospective evaluation of factors influencing successful skin grafting for patients with skin cancer of the foot. J Dermatol 44 (2017): 1043-1045.
- Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet 370 (2007): 528-537.
- McCormack D, Scannell T, Cunningham F. Giant squamous cell carcinoma of the hand: epitheliomacuniculatum-a case report. Ir J Med Sci 163 (1994): 379-380.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
