Surgical Excision of Perirectal Melanoma in the Horse. 3 Cases
Article Information
Diakakis Nikolaos1, Billi Theodora2*
1Associate Professor, Equine Unit, Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki
2Practitioner, Equine Unit, Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki, Greece
*Corresponding Author: Billi Theodora, Practitioner, Equine Unit, Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki, Greece
Received: 07 May 2021; Accepted: 17 May 2020; Published: 03 June 2021
Citation: Diakakis Nikolaos, Billi Theodora. Surgical Excision of Perirectal Melanoma in the Horse. 3 Cases. Archives of Veterinary Science and Medicine 4 (2021): 43-54.
View / Download Pdf Share at FacebookAbstract
Equine melanoma is one of the most common skin neoplasms encountered in equine practice. Surgical excision of melanocytic tumors is a viable treatment option and is considered to be locally curative. In the literature, information regarding surgical removal and long-term outcome of perirectal melanomas is limited. A total of three cases were included in this report, all presented with large perirectal melanomas, infiltrating the retroperitoneal musculature, leading to extra-luminal obstruction of the rectum. Horses were showing signs of dyschezia and tenesmus, secondary to rectal fecal impaction. Rectal palpation and transrectal ultrasonography aided in assessing the depth and size of the masses, as well as tumor invasion into the rectal wall. Tumor biopsy by fine needle aspiration was used to confirm the diagnosis. All horses underwent surgical excision of the masses to permanently alleviate clinical signs. In one horse, complete incision and reconstruction of the rectal wall was necessary in order to excise the whole tumor. Although surgery was successful in all three horses, one of them had to be euthanized 5 days after surgery, as clinical signs persisted. The aim of this report was to extensively describe the surgical approach, pre-operative considerations and postsurgical complications associated with surgical excision of perirectal melanoma in the horse.
Keywords
Equine perirectal melanoma; Horse; Rectal impaction; Standing; Dyschezia; Blunt dissection
Equine perirectal melanoma articles; Horse articles; Rectal impaction articles; Standing articles; Dyschezia articles; Blunt dissection articles
Article Details
1. Introduction
Equine melanocytic tumors comprise up to 15% of all equine skin neoplasia, with prevalence rates over 80% in older grey horses [1]. Melanomas appear as firm, variably pigmented, single, multiple or coalescing, spherical masses, arising typically at the skin and subcutis of the perineal and parotid region, the ventral surface of the tail, the prepuce, the vulva and the lips [2]. While the vast majority of melanocytic tumors are benign at initial presentation, it has been estimated that two-thirds may progress to malignancy, spreading via lymphatics, blood, local translocation or a combination of these routes [1-6].
Small and solitary dermal nodules usually cause no clinical sings and often remain unchangeable for years. However, with disease progression, melanocytic tumors grow and coalesce. Larger tumors may eventually interfere with poll flexion, compromise breathing or hinder normal eating and drinking. Overgrowing masses can physically occlude the gastrointestinal and urogenital system, causing dychezia and dysuria, whereas internally disseminated lesions may severely impair organ function [6, 7]. Reported treatment options regarding melanocytic tumors include surgical excision, cryosurgery, radiation therapy, immunotherapy, cimetidine, and intratumoral cisplatin [8]. This paper reviews the surgical management of 3 cases involving perirectal melanocytic masses, infiltrating the retroperitoneal musculature, leading to extra-luminal obstruction of the rectum.
2. Materials and Methods
Case 1
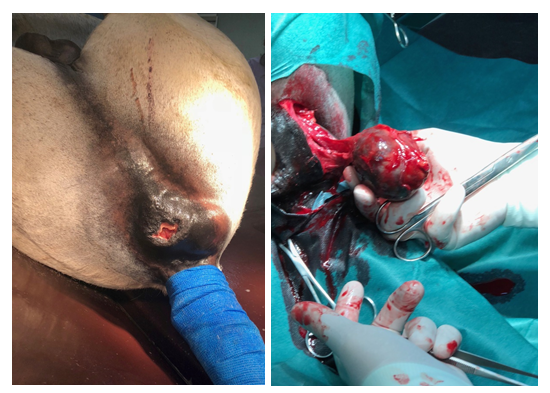
A 23-year-old grey Arabian cross stallion (horse 1) was presented for evaluation and treatment of dyschezia and reduced fecal output. Medical history indicated that horse 1 had been diagnosed with dermal melanomatosis two years ago on presentation, clinical sings were consistent with mild abdominal pain and tenesmus. Small, single melanocytic nodules were noticed at the perineal region in addition to a large, firm mass, dorsal and to the left of the anal orifice (Figure 1a). Upon rectal examination, the tumor could be palpated on the dorsolateral aspect of the rectal wall, extending approximately 6 cm cranially into the pelvic canal, decreasing luminal diameter. Dry, firm feces were evident cranial to the mass. Transrectal ultrasonography revealed a 5.6 × 4.0 cm well-demarcated mass of heterogeneous, hyperechoic echogenicity. There was no clear delineation between the mass and the rectal wall. Abdominal ultrasound was unremarkable. Peritoneal fluid, haematological and biochemical parameters were within normal limits. Percutaneous aspiration of the mass was performed using an 18-gauge, 6-inche spinal needle and the black-pigmented material obtained was pathognomonic for melanoma tissue. Surgical excision of the perirectal tumor was recommended.
Before surgical intervention, treatment centered on dissolving fecal impaction of the rectum and small colon. Mineral oil and water were given three times daily via a nasogastric tube, as well as several rectal enemas consisting of warm soapy water. Abdominal pain was successfully managed by flunixin meglumin (1.1mg/kg i.v. q 24 h). Diet was changed to alfalfa pellets soaked in water, mixed with mineral oil, fed in gradually increasing amounts. Fecal output normalized 72 hours upon admission.
Procaine penicillin G 22.000U/kg bwt i.m. and gentamicin sulfate 6.6mg/kg bwt i.v were administered prior to surgery. The rectum was evacuated and packed caudal to the terminal small colon, with rolled cotton covered by stockinette. Under general anesthesia, with the horse in dorsal recumbensy, a 6 cm skin incision was made centrally over the mass. Blunt dissection was used to expose the tumor, revealing firm, encapsulated, black to grey, homogenous tissue. The medial margin of the mass had infiltrated into the two outer layers of the rectum (Figure 1b), making it impossible to free the tumor without completely incising the rectal wall.
Following mass removal, the rectal wall was sutured through its full thickness using a No.4 Dacron suture material, in a cruciate pattern. The surgical site was closed in a three-layer fashion.
Postoperatively, the stallion received procaine penicillin G 22.000U/kg bwt i.m. q. 24 h, gentamicin sulfate (6.6mg/kg bwt i.v. q. 24 h) and flunixin meglumin (1.1mg/kg bwt i.v. q 24 h) for 5 days. Mineral oil was added twice daily to the horse’s modified diet for the following seven days. Rectal examination of the horse was performed on day 3, to ensure proper healing of the rectal wall. Skin sutures were removed on day 12 and the horse was discharged from the hospital. On follow-up examination, 20 days after surgery, the excision site appeared healthy, without any purulent discharge. The horse was able to defecate normally with no signs of discomfort.
Case 2
A 30-years-old grey Lipizzaner gelding (horse 2) was referred because of a 2-day history of tenesmus and abdominal discomfort, secondary to a perianal mass causing fecal impaction. During the past 6 years, horse 2 had undergone several surgeries associated with perianal melanomas.
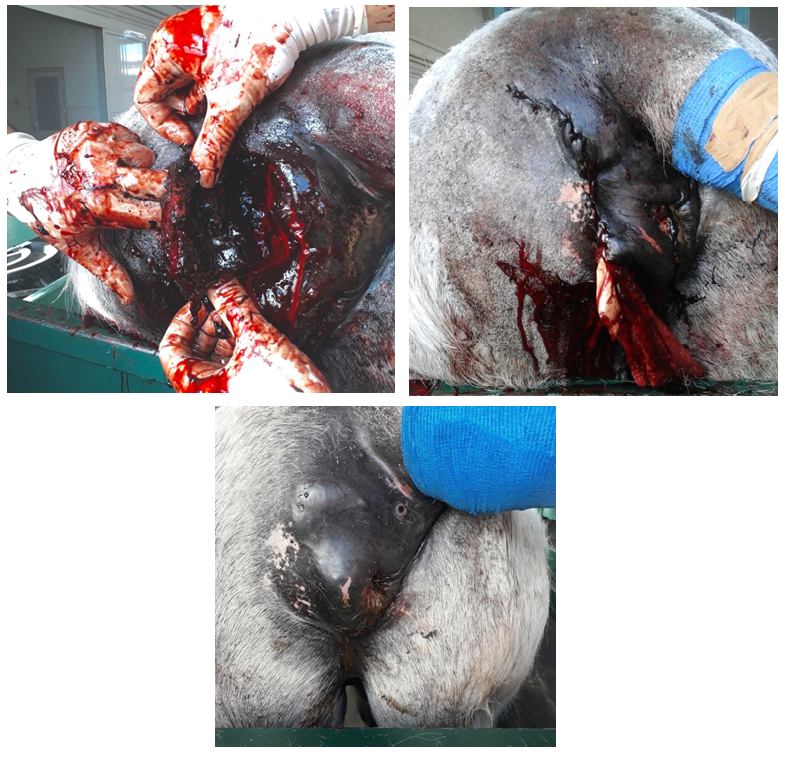
Upon admission, a 7.0 cm firm invasive mass, lying deeper to the skin, was noticed dorsal to the anal orifice and to the left of the midline (Figure 2a). The tumor extended approximately 30 cm cranially into the pelvic canal, along the left dorsolateral rectal wall, showing a multilobulated pattern of varying echogenicity on transrectal ultrasonography. Clear delineation between the rectal serosa and the mass confirmed no tumor invasion into the rectal wall. The mass severely decreased rectal luminal diameter, making defecation almost impossible. The mass was aspirated through the perianal skin and cytological findings were consistent with melanocytic neoplasm. Owing to the extensive nature of the tumor and the poor prognosis, euthanasia was recommended. However, the owner denied and wished for a surgical excision of the perirectal melanoma.
Figure 2a: Pre-operative image of the large perirectal melanoma of horse 2, deforming the anus and interfering with defecation; (b) Intra-operative image of the multilibulated melanoma, extensivly invading the surrounding musculature; (c) Post-operative image of horse 2. Penrose drains were placed in the dead space created by the deep perirectal excision of the tumor.
Horse 2 was given mineral oil and water via a nasogastric tube twice daily in order to resolve impaction and soften feces. Rectal evacuation with soapy enemas was performed several times until fecal output normalized. Flunixin meglumin (1.1mg/kg i.v. q 24 h), procaine penicillin G (22.000U/kg bwt i.m. q. 24 h) and gentamicin sulfate (6.6mg/kg bwt i.v. q. 24 h) were administered to control abdominal pain and prevent possible bacterial infection caused by the repetitive irritation of the rectal mucosa.
Surgery was performed in standing position, following sedation with detomidine 10mg/ml (0.04mg/kg bwt i.v.) in combination with butorphanol 10mg/ml (0.02mg/kg bwt i.v.) and administration of caudal epidural anesthesia with lidocaine hydrochloride 20mg/ml (0.2mg/kg bwt). A 15cm-long skin incision was performed over the tumor mass, from dorso-lateral to vetro-medial, beginning at the level of the tail and ending vetral to the external anal sphincter. Blunt dissection was used to free tumor margins from the surrounding musculature, taking care not to rupture the adjacent rectal wall medial to the mass. Due to the deep invasion of the tumor into the pelvic canal and its mutlilobular structure (Figure 2b), only the caudal two thirds of the mass could be removed. The cranial portion of the tumor was left in place. The dead space, the subcutis and the skin were suture separately in 3-layer fashion after securing penrose drains in the most cranial aspect of the surgical wound (Figure 2c).
Post-surgery, horse 2 received procaine penicillin G 22.000U/kg bwt i.m. q. 24 h, gentamicin sulfate 6.6mg/kg bwt i.v. q. 24 h and flunixin meglumin 1.1mg/kg bwt i.v. q 24 h in addition to a fecal softening diet. On day 3, the surgical wound appeared smooth, with clear exudate and no signs of infection. However, the gelding continued to show signs of dyschezia and tenesmus. A new fecal impaction had formed cranial to the portion of the tumor, which had not been surgically removed. On day 5, the owner eventually gave its consent to euthanize the horse.
Case 3
A 21-years-old grey Arabian gelding (horse 3) was admitted for evaluation and treatment of a large perianal mass, partially occluding the rectum. According to the referring veterinarian, eight months prior to admission, a 2 cm firm mass, imbedded in the perianal subcutis, had been noticed in addition to smaller growths present at the ventral aspect of the tail base. Over the past four months, the perianal tumor had doubled in size, impairing normal defecation. During this period, horse 3 had been treated several times for signs of tenesmus and mild abdominal pain.
Upon presentation, the gelding was bright, alert, and responsive, with no signs of abdominal discomfort. Clinical, biochemical, and hematological parameters were within normal limits. A 5.3 cm large mass was presented dorsal and to the left of the anal sphincter, imbedded deep into the surrounding tissue. Transrectal ultrasonography revealed that the mass extended 7 cm into the retroperitoneal space, adjacent but not invading the rectal wall, narrowing the rectal lumen. Transcutaneous aspiration of the mass confirmed the presence of a melanoma tissue. After communicating with the owner, surgical excision of the tumor was chosen as a treatment method.
Two days prior to surgery, a dietary change was initiated. Horse 3 received alfalfa pellets soaked in water, mixed with mineral oil, to soften the feces and to avoid impaction postoperatively. Following administration of procaine penicillin G (22.000U/kg bwt i.m.) and gentamicin sulfate (6.6mg/kg bwt i.v.), the gelding was sedated, and a caudal epidural anesthesia was performed between C1 and C2, using lidocaine hydrochloride 20mg/ml (0.2mg/kg bwt). A 4 cm long skin incision was made over the mass and by finger dissection the tumor was freed from the surrounding tissue. The rectal serosa medial to the mass remained intact. No penrose drain was placed into the dead space, as tissue margins where tightly opposed, using No 0 absorbable polyglactin suture material. The subcutis and skin were sutured separately, using No.0 absorbable polyglactin and No.0 non-absorbable polypropylene suture material, respectively.
Horse 3 remained hospitalized for the following twelve days. Postoperatively, the gelding received procaine penicillin (G 22.000U/kg bwt i.m. q. 24 h), gentamicin sulfate (6.6mg/kg bwt i.v. q. 24 h) and flunixin meglumin (1.1mg/kg bwt i.v. q 24 h) for 5 days. Fecal softeners were continued during the first five days of hospitalization and then gradually removed from the horse’s diet. On day eight, consistency of feces and defecation frequency had normalized. Upon discharge, the surgical wound appeared dry and skin re-epithelization was present. One month after the initial examination the referring veterinarian reported that horse 3 had recovered uneventfully.
3. Results
All horses of the present report were gray, older than 20 years of age and had been diagnosed in the past with dermal melanomatosis. All horses showed signs of dyschezia, tenesmus and abdominal pain, secondary to extraluminal obstruction of the rectum, caused by large perirectal melanomas. Surgical removal of the perirectal tumors was attempted in each horse, with the intention to alleviate clinical sings. Perirectal tumors were larger than 5 cm in diameter and extended at least 6 cm into the retroperitoneal space, with one tumor being approximately 30 cm in length. All were located dorsolateral to the anal orifice and left to the midline, adjacent to the rectum. In one horse the mass had invaded the rectal wall. Removed tumors were firm, black, encapsulated and had a multilobulated structure. None was connected to the skin. Biopsy by percutaneous fine needle aspiration was performed prior to surgery in order to confirm the presence of melanocytic tissue.
Horse 1 and horse 3 recovered uneventfully. Both horses were re-examined by rectal palpation and transrectal ultrasonography in a six-month interval. Follow-up information of up to three years revealed no evidence of tumor regrowth at the excision site, nor reappearance of initial clinical sings. Pre-existing melanocytic nodules continue to grow on remote sites and horse 1 was subjected to an additional surgery, 2 years later, associated with a melanocytic cluster at the base of the tail. Horse 2 was subjected to euthanasia, five days post-surgery, due to persistent signs of dyschezia.
4. Discussion
Melanocytic tumors are reported to account for up to 34% of all equine neoplasms [2]. According to their clinical and histopathology features, equine melanoma can be classified into four types: melanocytic naevus, dermal melanoma, dermal melanomatosis and anaplastic melanoma, [2, 9]. In this report, all horses were diagnosed with dermal melanomatosis.
All horses were presented with melanocytic masses infiltrating the perirectal musculature. Perirectal melanomas were located dorsal to the rectum and to the left of the midline, at the level where anorectal lymph nodes are positioned within the pelvic canal, adjacent to the rectum [10]. In the literature, it is suggested that dermal melanoma demonstrates a proclivity for local invasion and metastasis. Currently it is not uniformly accepted, whether multiple lesions on a single horse are metastatic or arise spontaneously as multicentric, separate [6]. However, it is most likely that the perirectal tumors of the horses in this report, are secondary metastasis, originating from the melanocytic nodules detected on the perianal skin. Due to the proximity of the masses to the anorectal lymph nodes, it is possible that tumors had spread to the retroperitoneal space, via the lymphatic circulation.
Conditions associated with obstructive diseases of the rectum include perirectal abscessation, anorectal adenopathy, rectal strictures, rectal polyps and neoplasms [11, 12]. The most common neoplasms of the perianal region and anus are squamous cell carcinoma and melanoma, whilst adenocarcinoma and leiomyosarcoma are rare in the horse [12]. In this report, diagnosis of the perirectal melanomas was based on signalment, location and gross appearance of the masses. The presence of black, pigmented tissue obtained by percutaneous fine needle aspiration was pathognomonic [2].
In equine practice, melanomas are often presented in advanced stages, when tumor expansion has already compromised the affected tissue or structure. Large parotid masses may interfere with neck flexion or compress the upper airway, while tumor bulks can physically obstruct the anal sphincter, penis, or prepuce [13]. Overgrowing masses may locally infiltrate musculature [14], soft tissue or hinder blood supply [6]. In this report, perirectal invasion of melanocytic tumors resulted in decreasing rectal luminal diameter of the affected horses, causing fecal impaction in the rectum and small colon.
Clinical signs consistent with extraluminal obstruction of the rectum are mild abdominal pain, reduced fecal output, dyschezia, tenesmus and less frequently dysuria. Dysfunction of the urinary tract may be caused by direct pressure of the underlying urethra or by neuritis secondary to regional inflammation [11]. None of the horses in this report showed signs of dysuria, but all were presented with abdominal discomfort, dyschezia and tenesmus.
Treatment options that have been reported regarding melanocytic tumors, include the use of intratumoral cytotoxic drugs [15], immunotherapy [16, 17], electronic brachytherapy [18], electrochemotherapy [19, 20] and surgical excision [13, 21]. Although localized excision of melanocytic masses does not prevent further progression of the disease, as pre-existing lesions may continue to grow and new tumors may form at remote sites [6], surgical removal of troublesome melanomas does alleviate clinical signs and improves quality of life for the affected horse [13, 2, 21]. Treatment of the horses in this report focused on alleviating clinical sings by surgically removing perirectal melanomas.
Blunt dissection was preferred over sharp dissection as it is considered a more atraumatic surgical method [22]. Perirectal tumors of the treated horses were deeply imbedded within the retroperitoneal musculature and to a close proximity to the rectal wall. By performing finger dissection, the authors were able to free the tumor from the surrounding tissue, without damaging adjacent vital structures. Surgical margins were determined based on gross appearance of the surrounding tissue and not on histological examination. In this report, penrose drains were placed only in one case, where the excised tumor was larger than 10 cm. Placement of penrose drains lies with the individual preference of each surgeon, related to the dead space created after tumor removal.
In horse 1, complete incision and reconstruction of the rectal wall was performed in order to excise the whole tumor. In cases where extensive reconstruction of the wall is necessary, fecal diversion techniques, such as the placement of a temporary indwelling rectal liner [23] can be used to prevent fecal contamination of perirectal tissues or the development of septic peritonitis [24]. However, in horse 1, no rectal liner was used, because of the possible complications associated with its placement. In horse 2, only two-thirds of the perirectal tumor could be removed, due to the depth in which the mass had invaded into the pelvic canal.
As expected, knowing the poor prognosis given to this case in first place, partial excision of the tumor did not alleviate clinical signs. The gelding was subjected to euthanasia five days post-surgery. In horse 3, the entire mass was successfully removed, and recovery was uneventful. Cisplatin-impregnated beads were not implanted within the excision sites, due to financial constraints of the owner.
Perirectal melanoma excision can be performed both in a standing or recumbent horse. If the surgery is performed in standing position, deep sedation of the horse in combination with epidural anesthesia is essential for the safety of the surgeon. In the authors’ opinion, having the horse standing is more advantageous, as the surgical site is free of anatomical distortions associated with recumbent position. Preoperatively, horses subjected to perirectal surgery should be fed a laxative diet, to soften fecal consistency and minimize postoperative defecation stress [25]. In this report, horses were fed alfa-alfa pellets soaked in water mixed with mineral oil. Just before surgery, the rectum was manually evacuated and packed with roll cotton to prevent intraoperative contamination of the surgical field.
Complications associated with perirectal melanoma excision include iatrogenic damage to surrounding vital structures, inability to remove the entire tumor and postoperative impaction secondary to tissue edema. Anatomically, the rectum is surrounded by the rectococcygeus muscles, the levator ani, the coccygeus muscle and the internal and external anal sphincter. The pudendal and caudal rectal nerves arising from spinal nerves S2–S4, supply the perineum with motor and sensory innervation, while the internal pudendal artery and the caudal gluteal artery are the main blood suppliers of this anatomical region.
Ventral to the rectum lies the urogenital canal, similar in the male as in the female horse. In the male the urethra can be palpated as it turns around the ischial arch [26]. Depending on the size of the tumor and its depth of invasion, some these structures may be compromised or displaced. Rectal palpation and transrectal ultrasonography prior to surgery, may aid in locating those structures in relation to the mass, while blunt dissection or finger dissection can be used to safely expose tumor margins. Inability to remove the entire tumor may be associated with extreme overgrowth. Lesions imbedded deep within the pelvic canal are often hard to reach, as instrument manipulation may be difficult because of limited surgical access. Postsurgical impactions may be prevented by perioperative dietary modification.
Although it has been suggested that surgical excision of advanced lesions is often unrewarding [6], the authors of this report support that surgical removal of perirectal melanomas alleviates clinical sings, improves quality of life and carries a good prognosis for the affected horse. In literature, there have been limited information regarding perirectal tumor removal [12]. To our knowledge, this is the first case report, describing in detail the surgical approach, pre-operative considerations and postsurgical complications associated with perirectal melanoma excision.
Author’s Declaration of Interests
No conflict of interests has been declared.
Ethical Animal Research
The client-owned animals were treated by routine surgery after informing the client of inherent risks. The client consented to surgery.
Source of Funding
None.
References
- Phillips JC, Lembcke LM. Equine melanocytic tumors. Vet. Clin. North. Am. Equine. Pract 29 (2013): 673-687.
- Knottenbelt DC. Tumours of the skin. In: Clinical Equine Oncology, Eds: Knottenbelt DC, Patterson-Kane JC, Snalune KL, Elsevier Health Sciences, UK (2015): 544-584.
- Knottenbelt DC. Melanocytic neoplasms. In: Clinical Equine Oncology, Eds: Knottenbelt DC, Patterson-Kane JC, Snalune KL, Elsevier Health Sciences, UK (2015): 237-246.
- Scott HC, Major MD, Grant RD, et al. Melanoma as a cause of spinal cord compression in two horses. J. Am. Vet. Med. Assoc 196 (1990): 1820-1822.
- MacGillivray K, Sweeney R, Del Piero F. Metastatic melanoma in horses. J. Vet. Intern. Med 16 (2002): 452-456.
- Moore JS, Shaw C, Shaw E, et al. Melanoma in horses: current perspectives. Equine Vet. Educ 25 (2012): 144-151.
- Johnson PJ. Dermatologic tumours (excluding sarcoids). Vet. Clin. North Am. Equine Pract 14 (1998): 643-658.
- Scott DW, Miller WH. Equine Dermatology. Philadelphia: Saunders (2011): 504-508
- Valentine B. Equine melanocytic tumors: a retrospective study of 53 horses. J. Vet. Intern. Med 9 (1995) : 91-297.
- Saar LI, Getty R. Equine lymphatic system. In:The Anatomy of Domestic Animals. 5th ed., W.B. Saunders, Philadelphia (1975): 625-629.
- Magee AA, Ragle CA, Hines MT, et al. Anorectal lymphadenopathy causing colic, perirectal abscesses, or both in five young horses. Jour- nal of the American Veterinary Medical Association 210 (1997): 804-807.
- Freeman DE. Rectum and Anus, In: Auer JA, Stick JA, Kümmerle JM, Prange T. (eds.). Equine Surgery. 5th ed., Saunders WB, Philadelphia (2019): 632-645
- Rowe EL, Sullins KE. Excision as treatment of dermal melanomatosis in horses: 11 cases (1994-2000). J. Am. Vet. Med. Assoc 225 (2004): 94-96.
- Billi T, Karadima V, Tyrnenopoulou P, et al. Surgical excision of a malignant metastatic melanoma located in a skeletal muscle of the lateral thorax of a horse. Vet. Med. Sci (2020): 1-6.
- Theon AP, Magdesian KG, Snyder JR. Long-term outcome associated with intratumoral chemotherapy with cisplatin for cutaneous tumors in equidae: 573 cases (1995-2004). J. Am. Vet. Med. Ass 230 (2007): 1506-1513.
- Heinzerling L, Feige K, Rieder S, et al. Tumor regression induced by intratumoral injection of DNA coding for human interleukin 12 into melanoma metastases in gray horses. J Mol Med 78 (2001): 692-702.
- Müller JMV, Feige K, Wunderlin P, et al. Double-blind placebo-controlled study with interleukin-18 and interleukin-12-encoding plasmid DNA shows antitumor effect in metastatic melanoma in gray horses. J Immunother 34 (2011): 58-64.
- Bradley WM, Schilpp D, Khatibzadeh SM. Electronic brachytherapy used for the successful treatment of three different types of equine tumours. Equine Vet Educ 29 (2017): 293-298.
- Spugnini EP, D’Alterio GL, Dotsinsky I, et al. Electrochemotherapy for the treatment of multiple melanomas in a horse. J Equine Vet Sci 31 (2011): 430-433.
- Scacco L, Bolaffio C, Romano A, et al. Adjuvant electrochemotherapy increases local control in a recurring equine anal melanoma. J Equine Vet Sci 33 (2013): 637-639.
- Groom LM, Sullins KE. Surgical excision of large melanocytic tumours in grey horses: 38 cases (2001–2013). Equine Vet. Educ 30 (2017): 438-443.
- Verwilghen D. Surgical techniques, In: Auer JA, Stick JA, Kümmerle JM, Prange T (eds.). Equine Surgery. 5th ed., Saunders WB, Philadelphia (2019): 198-213
- Watkins JP, Taylor TS, Schumacher J, et al. Rectal tears in the horse: an analysis of 35 cases. Equine Vet. J. 3 (1989): 186-188.
- McMaster M, Caldwell F, Schumacher J, et al. A review of equine rectal tears and current methods of treatment. Equine vet. Educ 27 (2015): 200-208.
- DeBowes RM. Standing rectal and tail surgery. Vet. Clin. North Am. Equine Pract 7 (1991): 649-667.
- Budras KD, Sack WO, Röck S. Pelvis, Inguinal Region, and Urogenital Organs, In: Anatomy of the Horse. 5th ed. Schlutersche, Hannover (2012): 72-87.

 Impact Factor: * 1.1
Impact Factor: * 1.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 