Sustainable Agriculture: Biofertilizers withstanding Environmental Stress
Article Information
Sunita Kumari Singh*
PG Department of Botany, Jai Prakash University, Chapra, Bihar, India
*Corresponding Author: Sunita Kumari Singh, PG Department of Botany, Jai Prakash University, Chapra, Bihar, India
Received: 01 November 2020; Accepted: 14 November 2020; Published: 25 November 2020
Citation: Sunita Kumari Singh. Sustainable Agriculture: Biofertilizers withstanding Environmental Stress. International Journal of Plant, Animal and Environmental Sciences 10 (2020): 158-178.
View / Download Pdf Share at FacebookAbstract
World’s mounting population food demand is totally dependent on the chemical fertilizers which destroy the environment and negatively influencing the health of humans. The useful microorganisms inhabit the plant systems and plays substantial role in nutrient uptake from the ecosystems of plant. Biofertilizers augment the plant development by various direct and indirect plant growth promoting mechanisms such as biological nitrogen fixation, production of several plant growth hormones, siderophores, innumerable hydrolytic enzymes and solubilization and mobilization of potassium, zinc, and phosphorus, therefore, they are the finest substitute of chemical fertilizers as they are eco-friendly for plant growth and soil fertility. Despite these utilities, biofertilizers itself are subjected to the extreme environmental conditions and they developed different mechanisms to cope up with them. Extensive work on the biofertilizers has been done, which divulges that these microorganisms have the potential of providing the vital nutrients to the crops in optimum amount for the amelioration of harvest without distressing the ecosystem.
Keywords
Biofertilizers, Biological nitrogen fixation, Plant growth hormones, Hydrolytic enzymes, Soil fertility
Article Details
1. Introduction
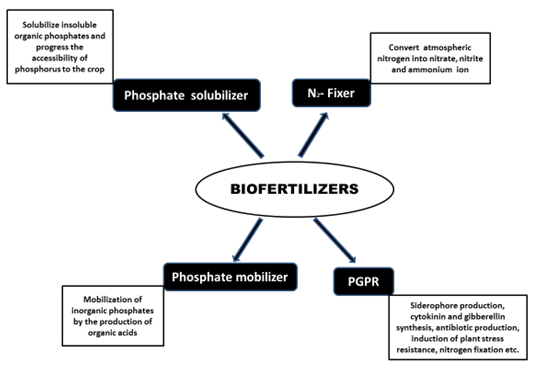
Nowadays increasing population lead to increasing demand of food supply consequently depletion of soil, which is replenished with chemical fertilizers leads to the biomagnifications of chemicals in the food chain, although, biofertilizers retain the soil fertility without any harmful effects. Biofertilizers are microorganisms that accelerate certain microbial processes in the soil which augment the extent of availability of nutrients in a form easily assimilated by plants and help plants uptake of nutrients by their interactions in the rhizosphere when applied through seed or soil. In other words, biofertilizers are living microorganisms which when applied to seed, plant surfaces, or soil colonizes the rhizosphere or the interior of the plant and promotes growth by escalating the accessibility of primary nutrients to the host plant [1]. According to Mishra et al. [2], biofertilizer is a mixture of live or latent cells encouraging nitrogen fixing, phosphate solubilizing etc. inoculated to soil, seed, roots, or composting areas with the purpose of increasing the quantity of mutualistic beneficial microorganisms and accelerating their microbial processes, which enhance the availability of nutrients that can then be easily assimilated and absorbed by the plants. They include nitrogen fixers, potassium and phosphorus solubilizers, growth promoting rhizobacteria (PGPRs), endo and ecto mycorrhizal fungi, cyanobacteria and other useful microorganisms [3, 4] and establish a vivacious module of sustainable agriculture, playing crucial role in sustaining the well-being of the plants against pathogens as well as supporting the growth by making the accessibility of several nutrients, phytohormones and remain viable simultaneously enhancing the productivity as well as the fertility of the soil [5] (Table 1). Biofertilizers can be applied to crops by seed treatment, seedling root dip, soil treatment etc. Rhizobium with phosphotika as seed treatment are recommended for pulses such as pigeon pea, green gram, black gram, cowpea etc. groundnut and soybean, similarly, Azotobacter with phosphotika as seed treatment are useful for wheat, sorghum, maize, cotton, mustard etc. and for transplanted rice, the recommendation is to dip the roots of seedlings in a solution of Azospirillum with phosphotika. Cortivo et al. [6] suggested that seed-applied biofertilizers may be effectually exploited in sustainable wheat cultivation without altering the biodiversity of the inhabitant microbiome. They not only fix atmospheric nitrogen in the soil and make it available to the plant but also solubilize the insoluble forms of phosphates like tricalcium, iron and aluminium phosphates into available forms, scavenge phosphate from soil layers, produce hormones and anti-metabolites which promote root growth, decompose organic matter and help in mineralization in soil and increase the availability of nutrients and improve the yield by 10 to 25% without unfavorably affecting the soil environment. In tropical countries, rice production mainly depends on biofertilizers [7]. Latent cells of competent strains of N2-fixing, phosphate solubilizing or mobilizing microorganisms are used for application to seed or soil with the intent to increase the number of such micro-organisms and hasten those microbial processes which enhance the accessibility of nutrients that can be assimilated by plants [8]. They improve the soil structure, restores soil nutrient, build soil organic matter, water uptake, plant growth and plant tolerance to abiotic and biotic factors [9] (Figure 1). These potential microbes would play a key role in productivity and sustainability of soil and also in protecting the environment as eco-friendly and cost-effective inputs for the farmers [3, 10]. Biopesticides (bacteria that promote plant growth by control of lethal organisms) and bioenhancers (bacteria can augment plant growth by producing phytohormones) should not be included in biofertilizers [11]. Green revolution brought striking increase in food production but with unsatisfactory concern for sustainability. Dependence on chemical fertilizers for forthcoming farming intensification would mean more loss in soil quality, water contamination and unsustainable burden on the economic system [12]. Incorporated use of mineral fertilizers, organic manures, biofertilizers, etc. is the only alternate for improving soil fertility [13]. Organic farming ensures food safety, adds to the biodiversity of soil [14] and additional advantages include longer shelf life causing no adverse effects to ecosystem [15]. Sharma and Upadhyay [16] described the present status of supply demand, marketing strategies, networks and governmental interventions in the pricing policies of biofertilizers in India. The international market for biofertilizers was predictable to be value about five billion USD in 2011 and is doubled in 2017, actively in Latin America, India and China [17-19]. Progress of quality standards of manufacture and a legal framework that guarantees both manufacturers and farmers are needed to maintain such prospective fiscal improvement [20]. Out of total applied fertilizer, only 30-50% of applied nitrogen fertilizers and 10-45% of phosphate fertilizers are taken up by crops [21, 22]. Several biotic, abiotic and anthropogenic factors cause challenges in booming application of commercial biofertilizer [20]. The 50-60% nitrogen requisite is fulfilled through the amalgamation of mineralization of soil organic nitrogen and biological nitrogen fixation by free living and rice plant associated bacteria [23].
|
Roles of biofertilizers |
Plants affected |
Microbes |
References |
|
Increases the photosynthetic rate |
Rice |
Rhizobia |
Mia and Shamsuddin [24] |
|
Basil |
Pseudomonas sp., Bacillus lentus, and Azospirillum brasilense Pseudomonas sp., Bacillus lentus, and Azospirillum brasilense |
Heidari and Golpayegani [25] |
|
|
Potato |
Bacillus sp. |
Gururani et al. [26] |
|
|
Arabidopsis thaliana |
Azospirillum brasilense sp 245 strain |
Cohen et al. [27] |
|
|
Bioremediation |
- |
Achromobacter, Azotobacter, Bacillus, Bradyrhizobium, Brevibacillus, Kluyvera, Mesorhizobium, Ochrobactrum, Pseudomonas, Psycrobacter, Ralstonia, Rhizobium, Sinorhizobium, Variovox, and Xanthomonas |
Shinwari et al. [28] |
|
Pesticides remediation |
- |
Azospirillum, Azotobacter, Bacillus, Enterobacter, Gordonia, Klebsiella, Paenibacillus, Pseudomonas and Serratia, |
Shaheen and Sundari [29] |
|
To overcome abiotic stresses |
- |
Pseudomonas alcaligenes, P. aurantiaca, P. aureofaciens and P. chlororaphis |
Verma et al. [30], Yadav et al. [31] |
|
Platycladus orientalis |
Bacillus subtilis |
Liu et al. [32] |
Table 1: Plausible biotechnological protagonist of biofertilizers [5].
2. Nitrogen fixing biofertilizers
Nitrogen is one of the primary macronutrients which is essential for the formation of base pair for DNA, RNA, phosphate group of protein and hormones such as cytokines, metal uptake, transport in xylem and phloem, as osmoregulater, alkaloids etc. [33], which is absorbed by plants in form of nitrates, ammonium and sometimes urea [34] and is added to the soil through fertilizer, biological nitrogen fixation, rainfall and thunder, and decomposition of organic matter [3]. Symbiotic and free-living eubacteria, including cyanobacteria, are two groups of nitrogen-fixing organisms. Heterocystous cyanobacteria such as Nostoc, Anabaena, Nodularia, Scytonema, Cylindrospermum, Mastigocladus, Calothrix, Anabaenopsis, Aulosira, Tolypothrix, Haplosiphon, Stigonema, Fischerella, Gloeotrichia, Rivularia, Nostochopsis, Westiellopsis, Westiella, Chlorogloea, etc. and non-heterocystous cyanobacteria such as Lyngbya, Oscillatoria, Schizothrix, Plectonema, Trichodesmium etc. are proficient nitrogen fixers [35, 36]. The free-living cyanobacteria fix more than 10 kg of nitrogen per hectare per year; however, dense mats of cyanobacteria fix annually approx. 10-30 kg of nitrogen per hectare [37] as well as fix about 20-30 kg nitrogen per hectare along with organic matter to the paddy fields [38]. It also makes symbiotic associations with different photosynthetic and non-photosynthetic organisms such as algae, fungi, diatoms, bryophytes, hornworts, liverworts, mosses, pteridophytes, gymnosperms, and angiosperms [39, 40]. Successful growth and survival of cyanobacteria in the nitrogen deprived habitats due to its nitrogen fixing ability makes them agronomically and economically significant as biofertilizers [7, 41]. According to Dubey and Rai [42] Anabaena fertilissima and A. doliolum quantitatively and qualitatively increases the yield of rice without chemical fertilizer. Wheat crops are also benefitted by cyanobacterial biofertilizers [43, 44]. It fixes approx. 200 Mt of nitrogen annually [45]. Abdel-Raouf et al. [46] suggested that the humus content generated after death and decay of cyanobacteria, improves the soil structure and fertility.
Arbuscular mycorrhiza (AM) can take up nitrogen both as inorganic (either ammonium or nitrate) and organic form [47], which is important under arid and semi-arid environment, where water availability confines uptake of inorganic nitrogen [48]. Strains of Azotobacter, Azospirillum, Phosphobacter and Rhizobacter, add significant amount of nitrogen to Helianthus annus and also increases the plant height, number of leaves, stem diameter percentage of seed filling and seed dry weight [49], likewise, these microorganisms promote the physiology and improve the root morphology of rice plants [50]. Azolla anabaenae is also one of the important biofertilizer in rice fields as Anabaena lives in symbiosis with Azolla, a small free-floating fresh water fern, fixes nitrogen in rice field in the range of 30-40 up to 70-110 kg N ha−1 [51]. The important factor in using Azolla as biofertilizers for paddy crop is its rapid decomposition in the soil and efficient accessibility of its nitrogen to rice plants. It has symbiotic relation with Anabaena and can help rice or other crops through dual cropping or green manuring of soil [52]. Rhizobium is one of the important symbiotic nitrogen fixing bacteria while Azospirillum, Azotobacter, Clostridium, Frankia etc. are asymbiotic nitrogen fixing bacteria. Rhizobia play a very important role in agriculture by inducing nitrogen fixings nodules on the root of legumes such as peas, beans, clove and alfalfa. They fix up to 90% of the nitrogen requirements of the host [53], but they can also act as plant growth promoting rhizobacteria (PGPR) with non-legumes such as maize, wheat, rice, and canola [54, 55]. Rhizobium not only fixes atmospheric nitrogen in symbiotic association with legumes but also with certain non-legumes like Parasponia [56]. Rhizobium sp. is crop specific as R. trifoli, R. melilotti, R. phaseoli, R. japonicum, R. leguminoserum and R. lupine for berseem, leucerne, gram, soyabean, pea and chickpea respectively [12]. In India, approx. 30 million hectares of land is under pulses (chickpea, red-gram, pea, lentil, black gram etc.), oil-seed legumes (soybean and groundnut) and forage legumes (berseem and lucerne) cultivation which fix 50-100 kg/ha nitrogen by the family Rhizobiaceae which colonizes the roots of specific legumes to form root nodules and produces ammonia.
Azotobacter is a non-symbiotic free-living aerobic bacterium which can fix nitrogen approx. 25 kg/ ha under optimum conditions and increase yield up to 50% and they also improve seed germination and plant growth by producing vitamin B, NAA, GA and plant hormones that are inhibitory to certain root pathogens [57]. This biofertilizer have been successfully used in Triticum aestivum, Zea mays, Gossypium arboretum, Pennisetum glaucum and Oryza sativa. It also protects the roots from other pathogens present in the soil. Similarly, Azospirillum (Bacillus polymixa) fixes approx 20-40 kg/ha nitrogen, secretes growth promoters as IAA, gibberellins and cytokinins and vicissitudes root morphology, which eventually augments the growth of plant [58]. Azospirillum could aid in existence of the plants under distressing conditions by persuading changes in pliability of cell wall, and osmotic regulations [59]. Dipping the roots of rice seedlings in 2% suspension of Azospirillum inoculant increased the yield by 100 kg/ ha [60]. Herbspirillum lives in symbiotic association with the roots of sugarcane and fixes atmospheric nitrogen [8]. Acetobacter can fix approx. 15 kg/ha/year nitrogen as they live endophytically in sugarcane ecosystem [12]. Strains of Azospirillum are vended as biofertilizers in different countries as Africa, Argentina, Australia, Belgium, Brazil, Germany, France, India, Italy, Mexico, Pakistan, Uruguay and USA [61].
3. Phosphate solubilizing-mobilizing biofertilizer
Phosphorus is needed in relatively large amounts, but in lower quantity as compared to nitrogen and potassium. It promotes legume growth, yield, nodule number and nodule mass. It is important for phospholipids in membrane, phosphor proteins for life functions, improvement of crop yield and quality. Analysis has shown that it forms a substantial component of seeds and fruits [3]. Phosphate either in native or in inorganic form becomes mostly unavailable to crops because of its low levels of mobility and solubility and its propensity to become fixed in soil. The key mechanism for phosphorus solubilization is the lowering of soil pH due to organic acids production by microbes [62-68] and mineralisation by the production of phytase by fungi (Aspergillus candidus, A. fumigatus, A. niger, A. parasiticus, A. rugulosus, A. terreus, Penicillium rubrum, P. simplicissimum, Pseudeurotium zonatum, Trichoderma harzianum, and T. viride) [62, 63, 67, 69-73]. PSBs enhance plant development by producing phytohormones, such as auxins, gibberellins, cytokinins, or polyamides [68, 73-75]. PSB includes bacteria (Bacillus megaterium, B. circulans, Pseudomonas striata, Rhizobium, Burkholderia, Achromobacter, Agrobacterium, Microccocus, Aereobacter, Flavobacterium, Erwinia etc), fungi (Penicillium sp. Aspergillus awamori, Trichoderma viridae etc) and mycorrhiza which solubilize insoluble inorganic phosphate compounds, such as tri-calcium phosphate, dicalcium phosphate, hydroxyapatite, and rock phosphate. In addition to bacteria and fungi, some cyanobacteria also solubilize the insoluble organic phosphates and progress the accessibility of phosphorus to the crop [76, 77]. Aspergillus fumigates and A. niger convert cassava wastes to phosphate biofertilizers [78]. Burkholderia vietnamiensis, a stress tolerant bacterium, secretes gluconic and 2-ketogluconic acids, which solubilises phosphate [79]. Arbuscular mycorrhiza enhances the plant phosphorus uptake [80], solubilization of inorganic phosphorus [81] and hydrolization of organic phosphorus [82]. Begum et al. [83] suggested that nurturing arbuscular mycorrhizal fungi (AMF) plant symbiosis, can significantly improve nutrients accretion, plant physiology and biomass accrual, root growth promotion and abiotic stress resistance. Several PGPR are also very effective in solubilizing phosphorus from the extremely insoluble phosphate forms as tricalcium phosphate, hydroxyl apatite and rock phosphate [84, 85]. Sub-culturing of most of the phosphorus-solubilizing bacteria results in the loss of the phosphate solubilizing activity [86] while fungi retain their activity to leach phosphorus-containing rocks even after prolonged culturing [87]. Rhizobial strains which solubilize phosphorus promote Daucus carota and Lactuca sativa development and a Phyllobacterium strain improves the quality of strawberries [88, 89]. Rhizobium leguminosarum strain PETP01 and R. leguminosarum strain TPV08 solubilize phosphate and are PGPR for pepper and tomato plants [90]. PSB also act as a biocontrol against plant pathogens by production of antibiotics, hydrogen cyanate, and antifungal metabolites, therefore, PSBs embody potential alternatives for inorganic phosphate fertilizers to encounter the phosphorus demands of plants, improving yield in sustainable agriculture. Their application is an ecologically and economically comprehensive approach [91].
The insoluble phosphate compounds are mobilized by the production of organic acids, accompanied by acidification of the medium [12]. Phosphate mobilizing biofertilizers are mycorrhiza which includes both ecto- and endo-mycorrhiza such as Glomus sp., Gigaspora sp., Acaulospora sp., Scutellospora sp., Sclerocystis sp., Rhizoctonia solani, Amanita sp., Boletus sp., Laccaria sp. etc. Not only phosphorus, mycorrhiza also mobilizes zinc, boron and other trace elements. They are broad spectrum biofertilizers. Vesicular arbuscular mycorrhiza facilitates the phosphorus nutrition by not only increasing its availability, but also increasing its mobility. Cyanobacteria contribute to mobilization of inorganic phosphates through excretion of organic acids and extracellular phosphatases [92, 93].
4. Plant growth promoting rhizobacteria (PGPR)
For sustainable agricultural development, crops produced should be disease resistance, salt tolerance, drought tolerance, heavy metal stress tolerance and better nutritional value. To accomplish the above desired crop properties, soil microorganisms (bacteria, fungi, algae, etc.) are used, which increase the nutrient uptake capacity and water use efficiency [94]. Among these soil microorganisms, bacteria known as plant growth promoting rhizobacteria (PGPR) are the most promising. They may be used to augment plant health and promote plant growth rate without environmental contamination [95]. It has been recommended that endophytic N2-fixing bacteria may be more imperative than rhizospheric bacteria in promoting plant growth because they escape competition with rhizosphere microorganisms and achieve close contact with the plant tissues [96, 97]. Azotobacter sp., Azospirillum sp., Pseudomonas florescens, Bacillus, Burkholderia, Enterobacter, Klebsiella etc. colonize the plant rhizosphere and enhance the plant productivity by nitrogen-fixation, solubilization of phosphorus and other nutrients, production of phytohormones, antagonism against pathogens and degradation of phytotoxins [98]. Gou et al. [99] demonstrated that WM13-24 biofertilizer containing Bacillus sp. and the integrated biofertilizer promoted chili plant growth, fruit yield, quality, the rhizosphere soil nitrogen content and enzyme activities. Phosphobacterin could increase plant yield by 10-37% and there are recorded savings approx. US $ 0.67 × 109 by the application of Azospirillum biofertilizer to various cereals [100]. Pseudomonas putida controls both potato soft rot and seed decay by siderophore production which chelates iron, consequently iron become unavailable to these pathogens [101-103]. Rhizobia also act as PGPR with non-legumes such as maize, wheat, rice, and canola [54, 55]. Interaction of PGPR and AMF (arbuscular mycorrhizal fungi) was better suited to 70% fertilizer for phosphate uptake [22].
Pseudomonas, Burkholderia, Acidothiobacillus, Bacillus and Paenibacillus are proficient in releasing potassium from minerals such as mica, illite, muscovite, biotite and orthoclases [32, 104], increasing potassium availability up to 15% [105]. Similarly, Bacillus sp. are silicate and zinc solubilizer biofertilizer. Although PGPR are very effective at promoting plant growth and development, but few bacterial species may inhibit growth. However, this negative impact may only occur under certain specific conditions and by some particular traits. Therefore, selection of a particular strain is of the highest importance in obtaining utmost benefits in terms of improved plant growth and development. Table 2 summarizes some PGPR [106]. Gonzalez et al. [107] in their experiment shown that Azospirillum brasilense improves the salt tolerance of the jojoba plant during in vitro rooting, as the bacteria reduces the undesirable effects of saline conditions. PGPR can be classified as biofertilizers once they act as a plant nutrition and amelioration basis that would replenish the nutrient cycle between the soil, plant roots and microorganisms present [106].
|
Function |
Microorganisms |
Benefitted Crops |
|
Nitrogen Fixation |
Burkholderia, Beijerinckia, Frankia, Gluconacetobacter, Herbaspirillum, Rhizobium, Azorhizobium, Azoarcus, Azotobacter |
Rice, Alnus, sugarcane, legumes |
|
Siderophore production |
Chryseobacterium, Bacillus, Phyllobacterium, Pseudomonas, Rhizobium, Streptomyces |
Tomato, strawberries, potato, maize, pepper, lettuce, carrot, Indian lilac |
|
Phosphate solubilization |
Phyllobacterium |
Strawberries |
|
Potassium solubilization |
Paenibacillus, Bacillus |
Black pepper |
|
Chitinase and β-glucanases production |
Pseudomonas, Sinorhizobium |
Several crops |
|
Indole acetic acid synthesis |
Streptomyces, Rhizobium, Paenibacillus |
Lodgepole pine, pepper, lettuce, carrot, tomato, Indian lilac |
|
Plant stress resistance induction |
Mycobacterium, Rhizobia, Bacillus |
Maize, peanuts |
|
ACC deaminase synthesis |
Pseudomonas, Rhizobium |
Mung beans, wheat, pepper, tomato, mung, beans |
Table 2: Plant growth promoting rhizobacteria and their functions [106].
5. Potassium solubilizing-mobilizing biofertilizers
Potassium have effects on water uptake, root growth, maintenance of turgor, transpiration and stomatal regulation of plants [108]. It is present in soil generally as silicate minerals which are inaccessible to plants and is absorbed by plants in the form of potassium ions which is insoluble in water. Microbes as Bacillus sp., Aspergillus niger etc. solubilize silicates by producing organic acids which cause the decomposition of silicates and helps in the removal of metal ions. They are broad spectrum bio-fertilizers [3].
6. Environmental stress on biofertilizers
Environmental stresses have substantial influence on microbial physiology. Mutagenic electromagnetic radiations as ionizing radiation (x-rays or γ radiation) carries enough energy to remove electrons from molecules in a cell consequently free radicals are formed which can damage DNA or RNA by oxidizing them and non-ionizing radiation (ultraviolet radiation) exerts its mutagenic effect by exciting electrons in molecules consequently formation of pyrimidine dimer which often change the shape of the DNA in the cell and can cause tribulations during replication. Since ionizing radiation has high frequency, it can penetrate cells and endospores very easily. The high-energetic ultraviolet radiation has great potential for cell damage moreover by direct effects on biologically pertinent molecules as DNA, proteins and lipids and indirectly by the production of reactive oxygen species, resulting in mutagenesis and impairment of essential cellular physiology [109]. UV radiation affects adversely several life processes of cyanobacteria such as growth, survival, photosynthesis, carbon dioxide uptake, pigmentation, motility, phycobiliprotein composition, and nitrogen metabolism etc. [110-113]. Many workers have suggested that the cellular constituents absorbing radiation between 280-315 nm are destroyed by UV-B radiation, which may further affect the cellular membrane permeability and protein damage eventually resulting in the death of the cell [114, 115]. Reactive oxygen species (ROS) is an important component in signal transduction pathway, resulting in the inhibition of gene expression of key proteins involved in photosynthesis during UV-B exposure [116]. Chen et al. [117] observed that Microcoleus vaginatusa after being irradiated with UV- Chen B radiation, showed decreased photosynthetic activity (Fv/Fm), and increased reactive oxygen species (ROS) generation. Kós et al. [118] suggested that upon exposure of the cells to 500 μmol m-2s-1 intensity visible light psbA3 replaces psbA1 as the dominating psbA mRNA species, and psbD2 increases at the expense of psbD1. RuBISCO is the key target involved in UV-induced inhibition [119] might be due to alteration in mRNA level of RuBISCO [120]. Most of the biofertilizers possess an assimilatory enzyme, nitrate reductase. Thapar et al. [121] suggested that the nitrate reductase is bounded to the chlorophyll-containing membrane fractions; inhibition of chlorophyll may directly affect the nitrate reductase activity. Ultraviolet radiation destroys the complex organization within phycobilisomes because their aromatic amino acids absorbs in the range of UV-B. In cyanobacteria, more than 99% of the UV-B is absorbed by chlorophyll-binding proteins and phycobilisomes [110]. Exposure of Synechocystis cells (phycoerythrin absent) to moderate intensity of UV-B (1.8 Wm-2) induces loss of β-phycocyanin, which may be due to the two bilins present in β-phycocyanin whereas the other biliproteins contain single bilin [122]. Synechococcus sp. PCC 7942 phycobilisomes when exposed to UV-B radiation, showed photodestruction of both α- and β-phycocyanin [123]. DNA is one of the most prominent targets of solar UV radiation, in all living organisms [124]. UV-induced lesions may lead to chronic mutations and even death of the cell. In comparison to UV-B, the wavelength of UV-A has poor efficiency in inducing the DNA damage, since they are not absorbed by native DNA. However, UV-A is able to generate singlet oxygen (1O2) or reactive oxygen species (ROS) that can damage DNA via indirect photosensitization reactions [116, 125]. The ability of UV-B radiation to damage a given base is determined by the flexibility of the DNA; the nature of bases play a major role since the distribution of dimeric photoproducts strongly depends on the pyrimidine bases involved. DNA alteration may occur mainly by mispairing of bases during replication, hydrolytic deamination, depurination/depyrimidination, oxidative damage by ionizing radiation (IR) as well as by free radicals or reactive oxygen species (ROS) and by certain alkylating agents [126]. The incidence of UV-B radiation may result in single as well as double DNA strand breaks (DSBs). DSBs may lead to loss of genetic information. The most potent carcinogenic forms of UV-induced DNA lesions are CPDs, 6-4PPs and their Dewar isomers [127] that may impede with normal cellular capability and functional integrity, reduction of RNA synthesis, arrest of cell cycle progression, resulting in mutagenesis, tumorigenesis and apoptosis [124]. It has been found that thymine-thymine (T-T) and thymine-cytosine (T-C) sequences are more photoreactive than C-T and C-C sequences [128]. The yield ratio of CPDs and 6-4PPs mostly depends upon the two adjacent bases involved in the formation of dimer, though, it has been reported that the amount of CPDs and 6-4PPs are about 75 and 25% respectively of the total UV-induced DNA damage product [129]. If unrepaired, a single CPD is sufficient to completely eliminate expression of a transcriptional unit [130]. It seems probable that UV affects the DNA of cyanobacteria and the killing of these microbes might be due to the irreversible damages caused to DNA by the high energy UVR. UV-B-induced formation of thymine dimer has also been reported in Chroococcus sp. and Anabaenopsis sp. However, cyanobacteria exhibit photoreactivation and excision repair [131] mechanisms to overcome the deleterious effects of UV-B radiation.
7. Mitigation strategies against environmental stress by biofertilizers
Biofertilizers have developed a sequence of strategies to defend against environmental stresses (Figure 2). For example, they sustain the integrity and fluidity of cell membranes by modulating their structure and composition and the permeability and activities of transporters are adjusted to control nutrient transport and ion exchange. Certain transcription factors are activated to augment gene expression and specific signal transduction pathways are induced to acclimatize to environmental changes. They also have repair mechanisms that shield their macromolecules against damages inflicted by environmental stresses [132]. Bacteria have successfully colonised every niche on the planet, as the soil-dwelling Gram-positive Bacillus sp. which can survive radiation doses sufficient to kill all other life forms [133]. Cyanobacteria which are exposed to high solar radiation in their habitats have developed a number of mechanisms to cope up with this stress, one of them is avoidance. Wu et al. [134] proposed that decreased helix pitch in the presence of UV-B radiation to obtain a more compact structure of the spirals, which eventually results in self-shading, is an effective protective mechanism against photoinhibition in Arthrospira platensis. The synthesis of extracellular glycan in Nostoc commune was stimulated by UV-B radiation and was proposed to provide UV resistance by increasing the effective path length for the absorption of radiation [135]. An accumulation of active iron superoxide dismutase (FeSOD) in desiccated field cyanobacterium N. commune was found to reverse the effects of oxidative stress imposed by multiple cycles of desiccation and rehydration during the UV-A or UV-B irradiation [136]. The other mechanism to tolerate UV radiation is synthesis of UV-absorbing or photoprotective compounds in the cyanobacteria. Carotenoid pigments are better known for their function as antioxidants that provide protection against detrimental photoproducts [137]. In the cyanobacterium N. commune, changes in the carotenoids pattern in response to UV-B irradiation was reported and myxoxanthophyll and echinenone were suggested to act as outer membrane-bound UV-B photoprotectors [135]. A possible role of pterins as UV-protecting compounds has been suggested for the marine planktonic cyanobacterium Oscillatoria sp., since UV-A radiation has been reported to be very effective in eliciting biosynthesis of a biopterin glucoside compound [138]. Revelations to varied abiotic stresses have levied cyanobacteria towards gradual evolution to sustain the cell viability. Process of photosynthesis have exposed cyanobacteria to the wide range of solar radiations including harmful doses of ultraviolet radiations in their natural habitats which positively forced them to synthesize a number of photon engrossing substances [139]. A number of cyanobacteria synthesize water-soluble colorless, mycosporine-like amino acids (MAA) and the lipid soluble yellow-brown colored sheath pigment, scytonemin [135, 140-142] to provide protection from deleterious effects of UV radiation. MAAs seem well suited for a sunscreen function due to their high molar extinction coefficients and their absorbance spectra peaking within the UV-B and UV-A regions [143]. The presence of other photoprotective compound, scytonemin most probably helped cyanobacteria to survive from lethal effects of UV radiation when there was no stratospheric ozone layer. This assumption is supported by the fact that scytonemin has an in vivo absorption maximum at 370 nm whereas purified scytonemin shows maximum absorption at 386 nm, but it also absorbs significantly at 252, 278 and 300 nm.
8. Conclusion
Dependence on chemical fertilizers lead to the overutilization of the soil ecosystem and environment. The rising global usage of N fertilizers enhance N2O conc. in the atmosphere 300 times more effective than CO2 that ruins in the atmosphere 100 years or more [144]. Growing global population food requirements can be unravelled by the use of biofertilizers only. Worthy impact of biofertilizers in biocontrol and bioremediation impose a positive impact on crop yield and ecosystem. Hence, reassurance should be specified to its execution in agronomy. Nanotechnology is required to improve the current biofertilizers to sustenance and elevate agricultural sustainability worldwide.
Conflicts of Interest
The author confirms that this article content has no conflict of interest.
References
- Mazid M, Khan TA, Mohammad F. Potential of NO and H2O2 as signaling molecules in tolerance to abiotic stress in plants. Journal of Industrial Research and Technology 1 (2011a): 56-68.
- Mishra D, Rajvir S, Mishra U, et al. Role of bio-fertilizer in organic agriculture: A review. Research Journal of Recent Sciences 2 (2013): 39-41.
- Itelima JU, Bang WJ, Onyimba IA, et al. Bio-fertilizers as key player in enhancing soil fertility and crop productivity: A Review. Direct Research Journal of Agriculture and Food Science 6 (2018): 73-83.
- Ramasamy M, Geetha T, Yuvaraj M. Role of biofertilizers in plant growth and soil health. In Ed.: Nitrogen fixation (2020).
- Kour D, Rana KL, Yadav AN, et al. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatalysis and Agricultural Biotechnology 23 (2020): 101487.
- Cortivo CD, Ferrarri M, Visioli G, et al. Effects of seed-applied biofertilizers on rhizosphere biodiversity and growth of common wheat (Triticum aestivum L.) in the Field. Frontiers in Plant Science 11 (2020): 72.
- Vaishampayan A, Sinha RP, Häder D-P, et al. Cyanobacterial biofertilizers in rice agriculture. Botanical Review 67 (2001): 453-516.
- Khan TA, Mazid M, Mohammad F. Ascorbic acid: an enigmatic molecule to developmental and environmental stress in plant. International Journal of Applied Biology and Pharmaceutical Technology 2 (2011): 468-483.
- Akram MS, Cheema MA, Waqas M, et al. Role of bio-fertilizers in sustainable agriculture. Preprints (2020).
- Bardi L, Malusà E. Drought and nutritional stresses in plant: alleviating role of rhizospheric microorganisms. In: Haryana N, Punj S (Eds) Abiotic stress: new research. Nova Science Publishers Inc, Hauppauge (2012): 1-57.
- Mazid M, Khan TA, Mohammad F. Role of Nitric oxide in regulation of H2O2 mediating tolerance of plants to abiotic stress: A synergistic signaling approach. Journal of Stress Physiology and Biochemistry 7 (2011b): 34-74.
- Mazid M, Taqi Ahmed Khan TA. Future of Bio-fertilizers in Indian Agriculture: An Overview International Journal of Agricultural and Food Research 3 (2014): 10-23.
- Singh S, Singh BK, Yadav SM, et al. Potential of Biofertilizers in Crop Production in Indian Agriculture. American Journal of Plant Nutrition and Fertilization Technology 4 (2014): 33-40.
- Raja N. Biopesticides and biofertilizers: ecofriendly sources for sustainable agriculture. Journal of Biofertilizer Biopesticide. 3 (2013): 112-115.
- Sahoo RK, Ansari MW, Pradhan M, et al. Phenotypic and molecular characterization of efficient native Azospirillum strains from rice fields for crop improvement. Protoplasma 251 (2014): 943-953.
- Sharma A, Upadhyay BK. Marketing Promotion Policies in Agriculture (Special Reference to National Fertilizer Limited). Marketing Promotion Policies in Agriculcure in India 152 (2007): 8-15.
- Fuentes-Ramirez LE, Caballero-Mellado J. Bacterial biofertilizers. In Ed.: Siddiqui ZA. PGPR: biocontrol and biofertilization. Springer, Dordrecht (2005): 143-172.
- Bashan Y, de-Bashan LE. Microbial populations of arid lands and their potential for restoration of deserts. In: Dion P (Ed) Soil biology and agriculture in the tropics, Soil biology series. Springer, Berlin (2010): 109-137.
- Bashan Y, de-Bashan LE, Prabhu SR, et al. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998-2013). Plant Soil 378 (2014): 1-33.
- Malusà E, Pinzari F, Canfora L. Efficacy of Biofertilizers: Challenges to Improve Crop Production D.P. Singh et al. (Eds.), Microbial Inoculants in Sustainable Agricultural Productivity 2 (2016): 17-40.
- Adesemoye AO, Torbert HA, Kloepper JW. Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Canadian Journal of Microbiology 54 (2008): 876-886.
- Adesemoye AO, Torbert HA, Kloepper JW. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microbial Ecology 58 (2009): 921-929.
- Rahman MM, Amano T, Shiraiwa T. Nitrogen use efficiency and recovery from N fertilizer under rice-based cropping systems. Australian Journal of Crop Science Southern Cross Journals 3 (2009): 336-351.
- Mia MB, Shamsuddin Z. Nitrogen fixation and transportation by rhizobacteria: a scenario of rice and banana. International Journal of Botany 6 (2010): 235-242.
- Heidari M, Golpayegani A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). Journal of the Saudi Society of Agricultural Sciences 11 (2012): 57-61.
- Gururani MA, Upadhyaya CP, Baskar V, et al. Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. Journal of Plant Growth Regulation 32 (2013): 245-258.
- Cohen AC, Bottini R, Pontin M, et al. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiologia Plantarum 153 (2015): 79-90.
- Shinwari KI, Shah AU, Afridi MI, et al. Application of plant growth promoting rhizobacteria in bioremediation of heavy metal polluted soil. Asian J ournal of Multidisciplinary Studies 3 (2015): 179-185.
- Shaheen S, Sundari K. Exploring the applicability of PGPR to remediate residual organophosphate and carbamate pesticides used in agriculture fields. International Journal of Agriculture Food Science and Technology 4 (2013): 947-954.
- Verma P, Yadav AN, Kumar V, et al. Beneficial plant-microbes interactions: biodiversity of microbes from diverse extreme environments and its impact for crop improvement. In: Plant-Microbe Interactions in Agro-Ecological Perspectives: Microbial Interactions and Agro-Ecological Impacts, Singh DP, Singh HB, Prabha R (Eds), Springer Singapore, Singapore 2 (2017): 543-580.
- Yadav AN, Kumar R, Kumar S, et al. Beneficial microbiomes: biodiversity and potential biotechnological applications for sustainable agriculture and human health. Journal of Applied Biology and Biotechnology 5 (2017): 1-13.
- Liu J, Mehdi S, Topping J, et al. Interaction of PLS and PIN and hormonal crosstalk in Arabidopsis root development. Frontiers in Plant Science 4 (2013): 75.
- Essential elements for plant’s growth. Nature publishers (1999): 1-5.
- Ifokwe NJ. Studies on the production of biological fertilizer from domestic wastes and Azolla pinata (Singh). Unpublished M.Sc. Thesis, Department of Plant Science and Technology, University of Jos (1988): 10- 45.
- Venkataraman GS. Blue-green algae (Cyanobacteria)”. In Eds.: Tata SN, Wadhwani AM, Mehdi MS. Biological Nitrogen Fixation. New Delhi: Indian Council of Agricultural Research (1993): 45-76.
- Pathak J, Rajneesh, Maurya PK, et al. Cyanobacterial Farming for Environment Friendly Sustainable agriculture practices: innovations and perspectives. Frontiers in Environmental Science 6 (2018): 7.
- Watanabe I, Espianas CR, Berja NS, et al. Utilization of the Azolla-Anabaena complex as a nitrogen fertilizer for rice. IRRI Research Paper Series 11 (1997): 1-15.
- Issa AA, Abd-Alla MH, Ohyama T. Nitrogen fixing cyanobacteria: future prospect, In Ed.: Ohyama T. Advances in Biology and Ecology of Nitrogen Fixation. InTech (2014).
- Rai AN, Söderbäck E, Bergman B. Cyanobacterium-plant symbioses. New Phytologist 147 (2000): 449-481.
- Sarma MK, Kaushik S, Goswami P. Cyanobacteria: a metabolic power house for harvesting solar energy to produce bio-electricity and biofuels. Biomass Bioenergy 90 (2016): 187-201.
- Singh JS. Cyanobacteria: a vital bio-agent in eco-restoration of degraded lands and sustainable agriculture. Climate Change and Environmental Sustainability 2 (2014): 133-137.
- Dubey AK, Rai AK. Application of algal biofertilizers (Aulosira fertilissimatenuis and Anabaena doliolum Bhardwaja) for sustained paddy cultivation in Northern India. Israel Journal of Plant Sciences 43 (1995): 41-51.
- Obreht Z, Kerby NW, Gantar M, et al. Effects of root associated N2-fixing cyanobacteria on the growth and nitrogen content of wheat (Triticum vulgare L.) seedlings. Biology and Fertility of Soils 15 (1993): 68-72.
- Karthikeyan N, Prasanna R, Sood A, et al. Physiological characterization and electron microscopic investigations of cyanobacteria associated with wheat rhizosphere. Folia Microbiology 54 (2009): 43-51.
- Guerrero MG, Vega JM, Losada M. The assimilatory nitrate reducing system and its regulation. Annual Review of Plant Physiology 32 (1981): 168-204.
- Abdel-Raouf N, Al-Homaidan AA, Ibraheem IB. Agricultural importance of algae. African Journal of Biotechnology 11 (2012): 11648-11658.
- Hawkins HJ, Johansen A, George E. Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant Soil 226 (2000): 275-285.
- Subramanian K, Charest C. Acquisition of N by external hyphae of an arbuscular mycorrhizal fungus and its impact on physiological responses in maize under drought-stressed and well-watered conditions. Mycorrhiza 9 (1999): 69-75.
- Dhanasekar R, Dhandapani R. Effect of biofertilizers on the growth of Helianthus annuus. International Journal of Plant, Animal and Environmental Sciences 2 (2012): 143-147.
- Choudhury MA, Kennedy IR. Prospects and potentials for system of biological nitrogen fixation in sustainable rice production. Biology and Fertility of Soils 39 (2004): 219-227.
- Wagner GM. Azolla. A review of its biology and utilisation. Botanical Review 63 (1997): 1-26.
- Ghosh N. Promoting biofertilizers in Indian agriculture. Economic and Political Weekly 5 (2004): 5617-5625.
- Franche C, Lindström K, Elmerich C. Nitrogen fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 321 (2009): 35-59.
- Yanni YG, Rizk RY, Abd El-Fattah FK, et al. The beneficial plant growth promoting association of Rhizobium leguminosarum bv. trifolii with rice roots. Australian Journal of Plant Physiology 28 (2001): 845-870.
- Hayat R, Ali S, Amara U, et al. Soil beneficial bacteria and their role in plant growth promotion:a review. Annals of Microbiology 60 (2010): 579-598.
- Saikia SP, Jain V. Biological nitrogen fixation with non-legumes: An achievable target or dogma? Current Science 92 (2007): 317-322.
- Mazid M, Khan TA, Mohammad F. Cytokinins, A classical multifaceted hormone in plant system. Journal of Stress Physiology and Biochemistry 7 (2011c): 347-368.
- Fibach-Paldi S, Burdman S, Okon Y. Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum brasilense. FEMS Microbiology Letters 326 (2011): 99-108.
- Groppa MD, Benavides MP, Zawoznik MS. Root hydraulic conductance, aquaporins and plant growth promoting microorganisms: a revision. Applied Soil Ecology 61 (2012): 247-254.
- Kennedy IR, Choudhury ATMA, Kecskés ML. Non-symbiotic bacterial diazotrophs in crop-farming systems: can their potential for plant growth promotion be better exploited? Soil Biology and Biochemistry 36 (2004): 1229-1244.
- Mehnaz S. Azospirillum: a biofertilizer for every crop. In Ed.: Arora NK. Plant Microbes Symbiosis: Applied Facets. Springer India, New Delhi (2015): 297-314.
- Kumar A, Kumar A, Patel H. Role of microbes in phosphorus availability and acquisition by plants. International Journal of Current Microbiology and Applied Sciences 7 (2018): 1344-1347.
- Satyaprakash M, Nikitha T, Reddi EUB, et al. A review on phosphorous and phosphate solubilising bacteria and their role in plant nutrition. International Journal of Current Microbiology and Applied Sciences 6 (2017): 2133-2144.
- Walpola BC, Yoon M. Prospectus of phosphate solubilizing microorganisms and phosphorus availability in agricultural soils: a review. African Journal of Microbiology Research 6 (2012): 6600-6605.
- Pradhan N, Sukla LB. Solubilization of inorganic phosphate by fungi isolated from agriculture soil. African Journal of Biotechnology 5 (2005): 850-854.
- Son H-J, Park G-T, Cha MS, et al. Solubilization of insoluble inorganic phosphates by a novel salt- and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere. Bioresource Technology 97 (2006): 204-210.
- Selvi KB, Paul JJA, Vijaya V, et al. Analyzing the efficacy of phosphate solubilizing microorganisms by enrichment culture techniques. Biochemistry and Molecular Biology Journal 3 (2017): 1.
- Yousefi A, Khavazi K, Moezi A, et al. Phosphate solubilizing bacteria and arbuscular mycorrhizal fungi impacts on inorganic phosphorus fractions and wheat growth. World Applied Sciences Journal 15 (2011): 1310-1318.
- Tarafdar JC, Bareja M, Panwar J. Efficiency of some phosphatase producing soil-fungi. Indian Journal of Microbiology 43 (2003): 27-32.
- Dodor DE, Tabatabai MA. Effect of cropping systems on phosphatases in soils. Journal of Plant Nutrition and Soil Science 166 (2003): 7-13.
- Khan A, Jilani V, Akhtar MS, et al. Phosphorus solubilizing bacteria: occurrence, mechanisms and their role in crop production. Journal of Agricultural and Biological Science 1 (2009): 48-58.
- Aseri GK, Jain N, Tarafdar JC. Hydrolysis of organic phosphate forms by phosphatases and phytase producing fungi of arid and semi-arid soils of India. American-Eurasian Journal of Agriculture and Environment Science 5 (2009): 564-570.
- Santana EB, Marques ELS, Dias JCT. Effects of phosphate-solubilizing bacteria, native microorganisms and rock dust on Jatropha curcas L. growth. Genetics and Molecular Research, 15 (2016).
- Mittal V, Singh O, Nayyar H, et al. Stimulatory effect of phosphate-solubilizing fungal strains (Aspergillus awamori and Penicillium citrinum) on the yield of chickpea (Cicer arietinum L. cv. GPF2). Soil Biology and Biochemistry 40 (2008): 718-727.
- Vikram A, Hamzehzarghani H. Effect of phosphate solubilizing bacteria on nodulation and growth parameters of green gram (Vigna radiata L. Wilczek). Research Journal of Microbiology 3 (2008): 62-72.
- Dorich RA, Nelson DW, Sommers LE. Estimating algal available phosphorus in suspended sediments by chemical extraction. Journal of Environmental Quality 14 (1985): 400-405.
- Cameron HJ, Julian GR. Utilisation of hydroxyapatite by cyanobacteria as their sole source of phosphate and calcium. Plant Soil 109 (1988): 123-124.
- Ogbo FC. Conversion of cassava wastes for biofertilizer production using phosphate solubilizing fungi. Bioresource Technology 101 (2010): 4120-4124.
- Park J, Bolan N, Megharaj M, et al. Isolation of phosphate-solubilizing bacteria and characterization of their effects on lead immobilization. Pedologist 53 (2010): 67-75.
- Smith SE, Read DJ. Mycorrhizal symbiosis, 3rd Edition, Academic Press, London (2008): 1-637.
- Tawaraya K, Naito M, Wagatsuma T. Solubilization of insoluble inorganic phosphate by hyphal exudates of arbuscular mycorrhizal fungi. Journal of Plant Nutrition 29 (2006): 657-665.
- Richardson AE, Barea JM, McNeill AM, et al. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321 (2009): 305-339.
- Begum N, Qin C, Ahanger MA, et al. Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Frontiers in Plant Science 10 (2019): 1068.
- Rodríguez H, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnology Advances 17 (1999): 319-339.
- Owen D, Williams AP, Griffith GW, et al. Use of commercial bio-inoculants to increase agricultural production through improved phosphorus acquisition. Applied Soil Ecology 86 (2015): 41-54.
- Halder AK, Mishra AK, Chakrabarty PK. Solubilization of phosphate by Aspergillus niger. Scientific Culture 56 (1990): 455-457.
- Kucey RMN. Effect of Penicillium bilaji on the solubility and uptake of phosphorus and micronutrients from soil by wheat. Canadian Journal of Soil Science 68 (1983): 261-270.
- Flores-Felix JD, Menendez E, Rivera LP, et al. Use of Rhizobium leguminosarum as a potential biofertilizer for Lactuca sativa and Daucus carota crops. Journal of Plant Nutrition and Soil Science 176 (2013): 876-882.
- Flores-Felix JD, Silva LR, Rivera LP, et al. Plants probiotics as a tool to produce highly functional fruits: the case of Phyllobacterium and vitamin C in strawberries. PLoS One 10 (2015): e0122281.
- García-Fraile P, Carro L, Robledo M, et al. Rhizobium promotes non-legumes growth and quality in several production steps: towards a biofertilization of edible raw vegetables healthy for humans. PLoS One 7 (2012): 347-368.
- Kalayu G. Phosphate solubilizing microorganisms: promising approach as biofertilizers. International Journal of Agronomy Trends in Plant Science 10 (2019): 22-29.
- Bose P, Nagpal US. Solubilization of tricalcium phosphate by blue-green algae. Current Science 40 (1971): 165-166.
- Rai AK, Sharma NK. Phosphate metabolism in the cyanobacterium Anabaena doliolum under salt stress. Current Microbiology 52 (2006): 6-12.
- Armada E, Portela G, Roldan A, et al. Combined use of beneficial soil microorganism and agrowaste residue to cope with plant water limitation under semiarid conditions. Geoderma 232 (2014): 640-648.
- Calvo P, Nelson LM, Kloepper JW. Agricultural uses of plant biostimulants. Plant Soil 383 (2014): 3-41.
- Assmus B, Hutzler P, Kirchhof G, et al. In situ localization of Azospirillum brasilense in the rhizosphere of wheat with fluorescently labeled, rRNA-targeted oligonucleotide probes and scanning confocal laser microscopy. Applied and Environmental Microbiology 61 (1995): 1013-1019.
- Döbereiner J. History and new perspectives of diazotrophs in association with non-leguminous plants. Symbiosis 13 (1992): 1-13.
- Wani SA, Chand S, Ali T. Potential Use of Azotobacter chroococcum in crop production: an overview. Current Agriculture Research Journal 1 (2013): 35-38.
- Gou J-Y, Suo S-Z, Shao K-Z, et al. Biofertilizers with beneficial rhizobacteria improved plant growth and yield in chili (Capsicum annuum L.). World Journal of Microbiology and Biotechnology 36 (2020): 86.
- Subba Rao NS. Biofertilizers in Agriculture and Forestry, 4th Edn (1986).
- Kloepper JW, Leong J, Teintze M, et al. Enhanced plant growth by siderophores produced by plant-growth-promoting rhizobacteria. Nature London 286 (1980): 885-886.
- Beneduzi A, Ambrosini A, Passaglia M. Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genetics and Molecular Biology 35 (2012): 1044-1051.
- Radzki W, Gutierrez Manero FJ, Algar E. Bacterial siderophores efficiently provide iron to iron-starved tomato plants in hydroponics culture. Antonie Van Leeuwenhoek 104 (2013): 321-330.
- Bennett PC, Choi WJ, Rogera JR. Microbial destruction of feldspars. Mineral Manage 8 (1998): 149-150.
- Supanjani Han HS, Jung SJ, Lee KD. Rock phosphate potassium and rock solubilizing bacteria as alternative sustainable fertilizers. Agronomy for Sustainable Development 26 (2006): 233-240.
- Vejan P, Abdullah R, Khadiran T, et al. Role of plant growth promoting rhizobacteria in agricultural sustainability-a review. Molecules 21 (2016): 573-590.
- Gonzalez AJ, Larraburu EE, Llorente BE. Azospirillum brasilense increased salt tolerance of jojoba during in vitro rooting. Industrial Crops and Products 76 (2015): 41-48.
- Mfilinge A, Mtei K, Ndakidemi. Effect of Rhizobium inoculation and supplementation with phosphorus and potassium on growth leaf chlorophyll content and nitrogen fixation of bush bean varieties. American Journal of Research Communication 2 (2014): 49-87.
- Häder DP, Williamson CE, Wängberg SÅ, et al. Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors. Photochemistry Photobiology Sciences 14 (2015): 108-126.
- Sinha RP, Kumar A, Tyagi MB, et al. Ultraviolet-B-induced destruction of phycobiliproteins in cyanobacteria. Physiology and Molecular Biology of Plants 11 (2005): 313-319.
- Xue L, Zhang Y, Zhang T, et al. Effect of enhanced ultraviolet-B radiation on algae and cyanobacteria. Critical Reviews in Microbiology 31 (2005): 79-89.
- Gao K, Yu H, Brown MT. Solar PAR and UV radiation affects the physiology and morphology of the cyanobacterium Anabaena sp. PCC 7120. Journal of Photochemistry and Photobiology B: Biology 89 (2007): 117-124.
- Richa, Sinha RP. Sensitivity of two Nostoc species harbouring diverse habitats to ultraviolet-B radiation. Microbiology 84 (2015): 398-407.
- Vincent WF, Roy S. Solar ultraviolet-B radiation and aquatic primary production: damage, protection and recovery. Environmental Reviews 1 (1993): 1-12.
- Sinha RP, Singh N, Kumar A, et al. Impacts of ultraviolet-B irradiation on nitrogen fixing cyanobacteria of rice paddy field. Journal of Plant Physiology 150 (1997): 188-193.
- Mackerness SAH, Jordan BR, Thomas B. Reactive oxygen species in the regulation of photosynthetic genes by ultraviolet radiation (UV-B: 280-320 nm) in green and etiolated buds of pea (Pisum sativum L.). Journal of Photochemistry and Photobiology B: Biology 48 (1999): 180-188.
- Chen L-Z, Wang G-H, Hong S, et al. UV-B-induced oxidative damage and protective role of exopolysaccharides in desert cyanobacterium Microcoleus vaginatus. Journal of Integrative Plant Biology 51 (2009): 194-200.
- Kós PB, Deák Z, Cheregi O, et al. Differential regulation of psbA and psbD gene expression, and the role of the different D1 protein copies in the cyanobacterium Thermosynechococcus elongatus BP-1. Biochimica et Biophysica Acta 1777 (2008): 74-83.
- Mishra Y, Chaurasia N, Rai LC. Heat pretreatment alleviates UV-B toxicity in the cyanobacterium Anabaena doliolum: A proteomic analysis of cross tolerance. Photochemistry and Photobiology 85 (2009): 824-833.
- Jordon BR. The molecular biology of plants exposed to ultraviolet-B radiation and the interaction with other stresses. In: Interacting stresses on plants in a changing climate, Jackson MB, Black CR (Eds), Springer-Verlag, Berlin (1993): 153-170.
- Thapar R, Srivastava AK, Bhargava P, et al. Impact of different abiotic stresses on growth, photosynthetic electron transport chain, nutrient uptake and enzyme activities of Cu-acclimated Anabaena doliolum. Journal of Plant Physiology 165 (2008): 306-316.
- Zolla L, Bianchetti M, Rinalducci S. Functional studies of the Synechocystis phycobilisomes organization by high performance liquid chromatography on line with a mass spectrometer. European Journal of Biochemistry 269 (2002): 1534-1542.
- Sah JF, Krishna KB, Srivastava M, et al. Effects of ultraviolet-B radiation on phycobilisomes of Synechococcus sp. PCC 7942: alterations in conformation and energy transfer characteristics. Biochemistry and Molecular Biology International 44 (1998): 245-257.
- Jans J, Schul W, Sert Y-G, et al. Powerful skin cancer protection by a CPD-photolyase transgene. Current Biology 15 (2005): 105-115.
- Alscher RG, Danahue JL, Cramer CL. Reactive oxygen species and antioxidants: relationship in green cells. Physiologia Plantarum 100 (1997): 224-233.
- Valko M, Rhodes CJ, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico- Biological Interactions 160 (2006): 1-40.
- De Lima-Bessa KM, Armelini MG, Chigancas V, et al. CPDs and 6-4PPs play different roles in UV-induced cell death in normal and NER-deficient human cells. DNA Repair 7 (2008): 303-312.
- Courdavault S, Baudouin C, Charveron M, et al. Repair of the three main types of bipyrimidine DNA photoproducts in human keratinocytes exposed to UVB and UVA radiations. DNA Repair 4 (2005): 836-844.
- Sinha RP, Dautz M, Häder D-P. A simple and efficient method for the quantitative analysis of thymine dimers in cyanobacteria, phytoplankton and macroalgae. Acta Protozoologica 40 (2001): 187-195.
- Häder D-P, Sinha RP. Solar ultraviolet radiation-induced DNA damage in aquatic organisms: potential environmental impact. Mutation Research 571 (2005): 221-233.
- Kumar A, Tyagi MB, Singh N, et al. Role of white light in reversing UV-B-mediated effects in the N2-fixing cyanobacterium Anabaena BT2. Journal of Photochemistry and Photobiology B: Biology 71 (2003): 35-42.
- Guan N, Li J, Shin H-D, et al. Microbial response to environmental stresses: from fundamental mechanisms to practical applications. Applied Microbiology and Biotechnology 101 (2017): 3991-4008.
- Cox MM, Battista JR. Deinococcus radiodurans the consummate survivor. Nature Reviews Microbiology 3 (2005): 882-892.
- Wu H, Gao K, Villafañe VE, et al. Effects of solar UV radiation and photosynthesis of the filamentous cyanobacterium Arthrospira platensis. Applied and Environmental Microbiology 71 (2005): 5004-5013.
- Ehling-Schulz M, Bilger W, Scherer S. UV-B induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. Journal of Bacteriology 179 (1997): 1940-1945.
- Shirkey B, Kovarcik DP, Wright DJ, et al. Active Fe-containing superoxide dismutase and abundant sodF mRNA in Nostoc commune (cyanobacteria) after years of desiccation. Journal of Bacteriology 182 (2000): 189-197.
- Edge R, Mcgarvey DJ, Truscott TG. The carotenoids as anti-oxidants-a review. Journal of Photochemistry and Photobiology B: Biology 41 (1997): 189-200.
- Matsunaga T, Burgess JG, Yamada N, et al. An ultraviolet (UV-A) absorbing biopterin glucoside from the marine planktonic cyanobacterium Oscillatoria sp. Applied Microbiology and Biotechnology 39 (1993): 250-253.
- Rastogi RP, Madamwar D. Cyanobacteria synthesize their own UV-sunscreens for photoprotection. Bioenergetics 5 (2016): 138-142.
- Sinha RP, Klisch M, Häder D-P. Induction of a mycosporine-like amino acid (MAA) in the rice field cyanobacterium Anabaena sp. by UV irradiation. Journal of Photochemistrty and Photobiology B: Biology 52 (1999): 59-64.
- Sinha RP, Singh SP, Häder D-P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. Journal of Photochemistry and Photobiology B: Biology 89 (2007): 29-35.
- Pattanaik B, Schumann R, Karsten U. Effects of ultraviolet radiation on cyanobacteria and their protective mechanisms. In: Cellular Origin, Life in Extreme Habitats and Astrobiology: Algae and Cyanobacteria in Extreme Environments, (ed. Seckbach, J.) (COLE) Book Series, Springer, Netherlands 11 (2007): 29-45.
- Shick JM Dunlap WC. Mycosporine-like amino acids and related gadusols: biosynthesis, accumulation, and UV-protective function in aquatic organisms. Annual Review of Physiology 64 (2002): 223-262.
- Tian H, Xu R, Yao Y. A comprehensive quantification of global nitrous oxide sources and sinks Nature 586 (2020): 248-256.

 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 