Sustainable Lithium and Cobalt Recovery from Spent Lithium-ion Batteries: Best Practices for the Future. A review
Article Information
Afreh Paul1, Prof. Gao Lizhen2*, Tetteh Recheal1, Sidhoum Ali1
1College of Environmental Science and Engineering, Taiyuan University of Technology, Taiyuan, Shanxi-Province, China
2College of Chemical Engineering, Shanxi University, Taiyuan, Shanxi-Province, China
*Corresponding Author: Professer Gao Lizhen, College of Chemical Engineering, Shanxi University, Taiyuan, Shanxi-Province, China.
Received: 02 May 2024; Accepted: 08 May 2024; Published: 14 June 2024.
Citation: Afreh Paul, Prof. Gao Lizhen, Tetteh Recheal, Sidhoum Ali. Sustainable Lithium and Cobalt Recovery from Spent Lithium-ion Batteries: Best Practices for the Future. A review. Journal of Analytical Techniques and Research 6 (2024): 43-77.
View / Download Pdf Share at FacebookAbstract
Spent lithium-ion batteries (LIBs) are becoming increasingly common due to their widespread use in various energy-related applications. These batteries contain valuable metals such as cobalt (Co) and lithium (Li), which are in high demand and have limited long-term supply. To recover these valuable metals and avoid environmental pollution, the recycling of spent LIBs using different methods, including hydrometallurgy, pyrometallurgy, direct recycling, and biohydrometallurgy (bioleaching), has been widely explored. Each method has advantages and disadvantages in terms of cost-effectiveness and the recovery of Co and Li from spent LIBs. Thus, a comprehensive and critical analysis of recent studies on the performance of different recycling methods for the extraction of Co and Li from spent LIBs is necessary for the development of novel and practical strategies for effective metal extraction. Specifically, this review focuses on the current advancements in the application of existing recycling methods and emerging recycling technologies in terms of sustainability, efficiency, cost-effectiveness, and environmental friendliness for the recovery of Co and Li from spent LIBs. This review also identifies standardization of LIB design, automation of disassembly of SLIBs and involvement of artificial intelligence/machine learning in the recycling process as some of the best practices for the sustainable recovery of valuable metals from SLIBs and the minimization of pollution from SLIBs.


Keywords
Spent lithium-ion batteries, Emerging technologies, Deep eutectic solvents, Hydrometallurgy, Pyrometallurgy.
Spent lithium-ion batteries articles; Emerging technologies articles; Deep eutectic solvents articles; Hydrometallurgy articles; Pyrometallurgy articles.
Article Details
Highlights
- Traditional recovery technologies for Li and Co from SLIBs are summarized and reviewed
- DES is an emerging green technology with high recovery efficiency for Li and Co.
- Standardization of LIB designs; and
- The automation of disassembly is considered the best practice for the future.
1. Introduction
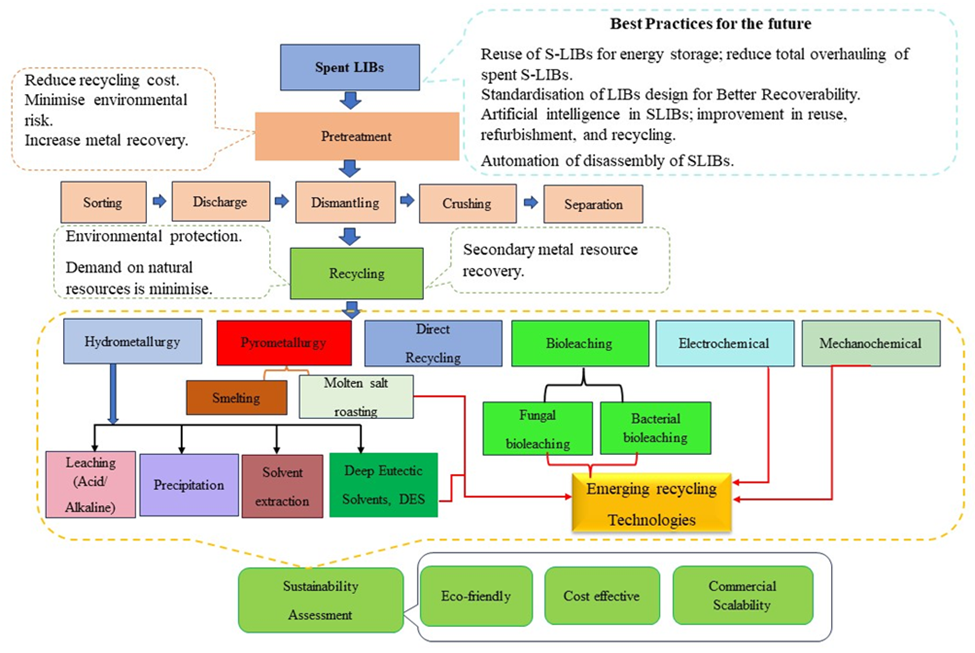
Lithium-ion batteries are widely used in society, powering various technological products such as electric bicycles and mobile phones [1-3]. They are considered the optimal technology for sustainable transportation due to their ability to reduce greenhouse gas emissions [2-4]. Compared to traditional batteries, lithium-ion batteries offer numerous benefits, including high power and energy density, minimal self-discharge, and a long storage life. They can also function at different temperatures, making them ideal for plug-in and hybrid electric cars [5]. The sale of electric battery vehicles is projected to increase significantly (it is anticipated that by 2045, 100 million electric battery vehicles will be sold worldwide) in the coming years [6]. However, the disposal of spent lithium-ion batteries is expected to generate a substantial amount of waste [7]. These batteries contain dangerous components that could harm the environment if not properly managed [8]. Moreover, used lithium-ion batteries are known to ignite instantaneously if not managed properly and are fully discharged, posing risks to the environment, public safety, and human health. The release of toxic elements, such as cobalt, nickel, and lithium, during landfill disposal can also have harmful effects on human health. Increasing the use of these batteries could worsen these negative impacts on human health and the environment [6]. Additionally, extracting natural raw materials for battery production requires more energy than recycling, resulting in a 70% increase in energy usage and CO2 emissions [6]. Lithium, a key component of lithium-ion batteries, is geographically limited to a few areas (approximately 70% of the world's lithium reserves are found in Argentina, Bolivia, and Chile) [4], making the sustainability of supplies uncertain due to market volatility and geopolitical unrest. As the demand for lithium-ion batteries continues to rise, there may be challenges in meeting the demand for lithium [6], and the government needs to implement a strict policy on battery recycling while considering geopolitical and environmental factors. The European Union has already adopted stricter legislation on battery recycling than other countries. The EU Battery Directive 2006/66/EC mandates that by January 1, 2014, the recycling rate of wasted lithium-ion batteries must increase to at least 50% of the weight of lithium batteries and accumulators [3], [8]. From an economic perspective, the active material in a high-energy cell account for 72.2% of the total cost, with the cathode and electrolyte making up 48.8% and 23.4%, respectively. These components contain various metals, such as copper, aluminum, magnesium, nickel, cobalt, and lithium. Certain metals, such as cobalt, nickel, and copper, are recycled due to their economic value. The recovery of lithium becomes economically viable when lithium production reaches one million tons for the lithium-ion battery industry in electric vehicles [4, 7, 9]. Spent lithium-ion batteries typically contain cobalt, nickel, lithium, other metals, organic compounds, and plastics. To extract one ton of lithium, 28 tons of spent batteries are needed, which is equivalent to 250 tons of minerals or 750 tons of brine [10]. The average prices for cobalt and lithium in December 2017 were $72,589 and $22,914 per ton, respectively. Additionally, Zhang et al. (2018) estimated that the value of end-of-life vehicle batteries is between $1.38 million and $6.76 million by 2035 [5]. Recycling spent lithium-ion batteries is both necessary and potentially profitable. However, the recycling process for these batteries poses challenges and uncertainties. Efficient recycling processes are difficult to design because of the diverse composition of lithium-ion batteries in terms of technology, application, and manufacturing [11]. Additionally, economic benefits and resource demands strongly impact battery recycling [12], with the former relying on the market price of metals and the technologies utilized for cathode materials in lithium-ion batteries. It is important to note that the recycling of batteries can yield lithium carbonate, a significant raw material for lithium-ion battery production [13]. Currently, only 3% of lithium-ion batteries are recycled, with a recovery rate for lithium of less than 1% [13, 14]. The development of new recycling processes is imperative for enhancing efficiency and ensuring environmental safety [9, 11]. Lithium-ion battery recycling may not be able to entirely fulfil the demand for vital metals, but it is an important step towards protecting fundamental resources for future generations. This will help to stabilize the raw material costs and avoid resource shortages [15]. Improving the lithium-ion battery recycling process can help us gain a strategic edge in the future by lowering our dependency on nations possessing mineral deposits [16]. Furthermore, recycling lithium-ion batteries will lessen the environmental risks associated with waste batteries and assist in preventing the buildup of wasted batteries [17]. The scrap quantities will also remain low due to recycling. Recycling can be performed using high-temperature or low-temperature separation techniques. There are three well-known technologies available: pyrometallurgy (high temperature), hydrometallurgy (low temperature) and direct recycling (Fan et al., 2021; Verma et al., 2020; Yun et al., 2018). Hydrometallurgy is considered superior due to its environmental friendliness, low energy requirements, and high efficiency [9]. By employing a hydrometallurgical process, it is possible to recover almost 100% of the lithium, whereas in a pyrometallurgical process, it would be completely lost[9]. The recycling process for spent lithium-ion batteries consists of three stages: pretreatment, secondary treatment, and metal extraction. Pretreatment involves dismantling, crushing, and separating battery constituents to concentrate the metallic portion and eliminate hazardous elements. Secondary treatment aims to dissolve the organic binder to improve separation of the black mass from the current collector [9, 18]. Metal extraction utilizes hydrometallurgical processes to retrieve pure metals and optimize the efficiency of the recycling process [18]. The current methods for recycling spent lithium-ion batteries (LIBs), namely, pyrometallurgy, hydrometallurgy and direct recycling [19-21], are not without negative impacts. Pyrometallurgy generates waste gases and slag at high temperatures [13, 22]. Hydrometallurgy requires complex equipment and poses safety risks due to the use of acids and alkalis [23- 25]. Although strong acids can achieve high metal extraction yields [26, 27, 28], their negative impacts are recognized [29]. More recently, the use of deep eutectic solvents in recycling spent lithium-ion batteries has emerged as a method of hydrometallurgy. The use of natural deep eutectic solvents (DESs) has advantages in terms of cost, toxicity, and biodegradability [27, 30-35]. Direct recycling repairs batteries without destroying the original materials. However, each method has advantages and disadvantages, so there is a need for an environmentally friendly and efficient recycling technology.
Since few of these techniques have been made commercially available, SLIB recycling techniques have achieved modest success in recent years. For lithium-ion batteries, the Umicore, Sony-Sumitomo, and Toxco technologies are the three main industrial recycling procedures. The Recupyl process is a novel plant that has also been commercialized. Although the recovery of lithium is typically not a priority, the primary goal of recycling procedures, both at the industrial and laboratory levels, is to recover cobalt and nickel because they are highly valuable [13]. Using a scientific database (Elsevier-ScienceDirect) (Supplementary Figure 1), this review thoroughly examined the publishing trend over the last 14 years (2010-2024) to determine the current level of knowledge about the recycling of spent LIBs. The methodologies used were adapted and modified from [36]. The two keywords used in the “ScienceDirect” search engine are “spent lithium-ion batteries” and “recycle”, which resulted in 2,286 publications, with 1973 publications between 2010 and 2024 representing 86% of the publications within the selected range of years. The scientific community is becoming increasingly interested in creating innovative and sustainable methods for recycling spent lithium-ion batteries (LIBs) to recover valuable metals from waste and protect the environment. This is indicated by the steady increase in publications about spent LIBs over the past seven years. A major fraction of these publications are related to the recycling of spent LIBs using pyrometallurgy, hydrometallurgy or direct recycling. Previous studies have also reported that the above three methods are commonly used for recycling spent LIBs [18], [37]. Approximately 60% of the articles published worldwide were written by researchers in China, with the United States coming in second with 9% and India third with 6% [36]. Further analysis in the “ScienceDirect” database with the keywords “spent lithium-ion batteries”, “leaching”, “pyrometallurgy”, “direct recycling”, “deep eutectic solvents (DES)” and “bioleaching/biohydrometallurgy” resulted in a total of 1646 publications, 573 publications, 222 publications, 139 publications and 215 publications (research articles), respectively. The recovery rates of the current recycling methods for Li and Co extraction were obtained from the literature, and their average recovery leaching efficiencies were recorded. These average recovery rates are based on general trends observed in the literature and presented in this review and may vary depending on the specific parameters of each study. The flowchart for the review methodology is presented in Supplementary Figure 1.
Our literature review revealed approximately 677 review papers in the “ScienceDirect” database when the two keywords “spent lithium-ion batteries” and “recycle” were used. The majority of these review papers dealt with spent lithium-ion batteries as a whole and specific component, such as cathodes, anodes, electrolytes and graphite, while others dealt with recycling technologies. However, there is little information on the comparative evaluation of the performance of both traditional and emerging recycling technologies for the recovery of Li and Co, specifically from spent LIBs. This review's primary goal is to conduct a thorough analysis of the most recent advancements in the literature regarding the use of various recycling techniques to recover valuable metals (primarily Co and Li) from spent LIBs, taking into account factors such as cost-effectiveness, efficiency, environmental friendliness, and best practices for spent LIBs.
2. Selection and classification of research methods
There were three stages involved in the selection and classification of research methodologies:
2.1 Selection and classification of the literature
The first step of this research is to conduct literature searches through a comprehensive search of the Elsevier database (ScienceDirect) using the keywords “spent lithium-ion batteries” AND “recycle” AND “leaching” AND “pyrometallurgical” AND “DES” AND “biohydrometallurgy/bioleaching”. Abstracts of the most relevant research papers were assessed by title to check whether they fell within the scope of the study. Additionally, available techniques and technologies were obtained by identifying and using earlier published reviews on the recycling areas of spent lithium-ion batteries. In particular, for the recovery of cobalt (Co) and lithium (Li) from spent lithium-ion batteries, the literature has classified them using a variety of recycling methods.
2.2 Selection and classification of recycling technologies
For traditional recycling technologies such as pyrometallurgy and hydrometallurgy, we selected the technologies commonly used for the recovery of Li and Co and their outstanding improvement in terms of efficiency, cost-effectiveness and environmental protection. In addition, recycling technologies for spent LIBs that have emerged in recent decades were investigated. These technologies are highly important for recycling metals because of their sustainability, environmental friendliness, reusability and recovery efficiency. These include deep eutectic solvents, molten salt roasting, biohydrometallurgy (bioleaching), mechanochemical technology, and electrochemical technologies. The individual recycling technologies are summarized and reviewed.
2.3 Evaluation of technological processes
Both traditional and emerging technologies were analysed for their efficiency in recovering Li and Co from spent LIBs. The efficiency of the different recycling technologies was calculated based on their average recovery rates from the data used in this study and taken from the literature. A filtered literature search was carried out for a specific technical process. Finally, best practices for the future sustainability of spent LIBs to recover valuable metals and minimize pollution are outlined.
3. Lithium and Cobalt
Most of the raw materials used in lithium-ion batteries, which are sourced from ores, are cobalt and lithium due to their dominating market shares in the battery manufacturing sector. [38].
3.1 Cobalt
Cobalt is commonly present in mineral deposits alongside copper or nickel and occasionally contains traces of arsenic and silver. It is typically found in low concentrations and is primarily obtained as a byproduct of other metal extraction processes. Approximately 50% of cobalt production stems from the nickel industry, 44% from copper and other industries, and the remaining 6% from primary cobalt operations. Historically, Africa has been a major source of cobalt, especially the Democratic Republic of the Congo (DRC) and Zambia. Australia, Brazil, Cuba, Russia, Canada, Madagascar, and China are also important contributors. The estimated quantity of refined cobalt as of 2014 was approximately 92,000 tonnes, while the projected reserves were over 13 million tonnes (CDI, 2016)[39]. Due to its expanding applications, the demand for cobalt has intensified despite its limited presence in the market. In terms of production volume, cobalt output exceeds that of lithium, yet its atomic quantity is only a quarter of the latter[40]. Shifts in cobalt demand patterns have been observed, with a notable transition from the United States and Europe to Asia since 2002. This shift has led to a surge in cobalt demand in Asia, particularly driven by chemical applications such as rechargeable batteries and catalysts, which constitute approximately 54% and 46% of the demand, respectively [18]. Generally, cobalt is employed in metallurgical processes, as a constituent of super alloys used in the construction of turbine engines and aircraft, in the chemical industry (powders, adhesives, catalysts, agriculture, medicine, etc.), in the manufacturing of cemented carbides, and in the ceramics and enamels sector [39]. The creation of lithium-ion batteries, which are utilized as a power source for many electronic devices, is nonetheless the most widely utilized purpose. China is the world's major user of cobalt, with almost 80% of this metal coming from the rechargeable battery industry [41].
3.2 Lithium
Currently, more than 32,500 tonnes of lithium are produced annually throughout the world. Much of the world's lithium output is derived from two primary producers: Australia and Chile. The production of lithium carbonate, hydroxide, and chloride has increased in China, which is also becoming a significant producer. In South America, where Chile accounts for approximately 53% and Argentina 14%, the region is believed to have 14 million tonnes of lithium deposits. According to [42], China is thought to hold approximately 23% of global reserves. Lithium production has historically been dominated by subsurface brines due to their low production costs. However, with the current surge in demand for lithium, mineral-sourced lithium has been able to reclaim a significant portion of the market share. In fact, it is estimated that mineral-sourced lithium accounted for half of the world's lithium supply in 2015. Technology corporations in Asia and the United States are now placing a high premium on the security of the lithium supply. To ensure a reliable and diversified supply of lithium for battery suppliers and vehicle manufacturers, cooperative ventures and strategic alliances are being formed between technology companies and exploration companies [42]. Rechargeable batteries, particularly lithium-ion batteries, have shown the most potential for growth in the lithium market. The demand for lithium rechargeable batteries far surpasses that for other types of rechargeable batteries. For instance, a leading electric car manufacturer is in the process of establishing a lithium-ion battery processing plant in the United States capable of producing 500,000 lithium-ion vehicle batteries annually [42].
4. Recycling routes for spent lithium-ion batteries
The process of recycling spent lithium-ion batteries involves three stages: pretreatment, separation of individual components, and recovery of valuable products such as Cu, Al, Fe, Co, Li, Ni, Mn, C, and plastics. The pretreatment stage focuses on removing hazardous sources and separating the components. The second stage involves separating components and dissolving the components. Finally, the last stage is dedicated to recovering valuable materials from spent batteries [18].
4.1 Pretreatment process
The recycling of used lithium-ion batteries is a challenging process because accurately determining the remaining power can be difficult. Even after the battery is fully discharged, residual power may remain. Directly treating lithium-ion batteries is ineffective due to their diverse range of elements. [43] After being discharged, they are dismantled and then
separated either manually or mechanically. There are several techniques for separating the active material from discarded batteries. Pretreatments are generally used to separate materials and parts of spent LIBs based on their form, density, conductivity, and magnetic characteristics. Pretreatments such as disassembly, crushing, screening, magnetic separation, washing and thermal pretreatment are examples of physical procedures [44-46].
4.1.1 Discharging: To prevent the potentially dangerous occurrence of self-igniting short circuits and battery rolls during disassembly, it is essential to drain the battery first. This process is known as "discharging" [47]. To recycle lithium-ion batteries (LIBs) based on lithium cobalt oxide (LCO), the batteries can be soaked in a salt solution, typically sodium chloride (NaCl), for the most effective results. However, the optimal discharge level is still uncertain, as full discharge may cause copper to diffuse into the electrolyte, affecting the leaching process. The addition of NaCl to water can accelerate the discharge effect of LIBs. [48-50] reported that a 10% NaCl solution is the best for extracting precious metals while also allowing for the reuse of the leftover liquid. Researchers used a roller presser and then submerged lithium-ion batteries (LIBs) in distilled water to create a short circuit between the cathode and anode [51]. This process was performed for LIBs utilizing LCO and spent LIBs based on NMC [52]. The batteries were fully discharged under 70-minute discharge conditions, causing the residual voltage to drop significantly within 10 minutes.
4.1.2 Dismantling: When disassembling a battery, careful planning and attention to detail are important. There are three methods for removing a battery: manual, semiautomatic, and completely automatic. It is crucial to select the best strategy based on your specific needs, as each approach has advantages and disadvantages. Safety gear such as goggles, gloves, and gas masks is necessary. Manual disassembly involves unscrewing or loosening the battery parts using a pneumatic tool and separating the anode and cathode to remove active components. Semiautomatic and fully automatic dismantling systems are more cost effective and efficient for large-scale operations because they reduce the risk of human error by utilizing machinery [53].
4.1.3 Thermal separation: To obtain valuable cathode materials from dismantled lithium-ion batteries, it is important to remove impurities such as copper, aluminium, iron, and carbon. The most common binder used in lithium-ion batteries is PVDF, which makes it difficult to isolate the cathode materials and aluminum foil for recycling. To eliminate PVDF during pretreatment, thermal treatment is a simple and scalable technique that can be performed at temperatures ranging from 350 to 700 °C. This treatment can also increase the efficiency of lithium leaching by removing carbon, which can adsorb lithium. Thermal pretreatment can be costly due to energy consumption, machinery requirements, and toxic gas byproducts [54]. Vacuum pyrolysis is a process that can decompose cathode material without producing hazardous gases and prevent the oxidation of copper and aluminium. It also allows for the separation of precious metals without the need for scraping or sifting [55]. A recent study by Bi et al. introduced a novel approach using low-temperature heat treatment at 300 °C to effectively recycle LFP batteries [56]. The study revealed that increasing the heat treatment time improved the dissociation of the anode material from the aluminum foil and achieved complete separation without contamination after 120 minutes, although harmful gases were produced during crushing, and the strength of the cathode material was compromised, possibly resulting in the loss of LFP to non-metallic powder. Additionally, in our previous studies [57], high-temperature thermal pretreatment was optimized for maximum enrichment of Li and Co in the black mass of SLIBs produced at 600 °C with a heat treatment time of 35 minutes and a reduction in harmful gases during comminution.
4.1.4 Chemical separation: Lithium-ion battery recycling involves removing the binder from cathode scraps to extract active components. This can be achieved by using solvents such as NMP, DMF, DMSO, and DMAC, with DMAC being the most efficient for extracting aluminum [55]. Citrus fruit juice has also been used as an environmentally friendly solvent. Another approach involves dissolving aluminium in NaOH and burning PVDF residues from cathode materials to avoid hazardous gas release. The resulting powder can be milled for better leaching effectiveness. Overall, organic agents and NaOH are promising methods for lithium-ion battery recycling.
4.2 Mechanical processing
Crushing and shredding batteries is a simpler alternative to disassembling them. The industry combines the discharging and dismantling steps to save costs by shredding or crushing the battery in an inert environment, which is crucial for recycling electrode material comminution [58, 59]. This process benefits the hydrometallurgical recycling process and involves two steps: low-speed rotary milling and high-speed impact mill crushing. The process of crushing or shredding spent lithium-ion batteries is commonly carried out in inert or cryogenic environments to prevent the potentially hazardous reaction of lithium, which occurs when intercalated lithium reacts with water to form lithium hydroxide (LiOH) and hydrogen gas (H2). Overcharged batteries are at risk of developing lithium plating on the surface of the anode. Another rationale for conducting crushing or shredding in an inert atmosphere is that the electrolyte in the batteries contains toxic and flammable compounds, thereby reducing the hazards associated with organics present in the batteries. The interaction of metallic lithium with water or moisture salts leads to highly exothermic reactions [18]. They undergo crushing to produce small size fractions [14]. Following the successful crushing and reduction of lithium-ion batteries into small sizes, the materials are then sorted based on their physical properties. Techniques such as density-based separation are utilized to eliminate plastics, which are lighter than other components. Vibrating tables or flotation can also be utilized for this purpose. Furthermore, the lighter components can be separated from the heavier components based on their differing densities. A magnetic separator can be utilized to extract ferrous components. Mechanical processing can be exclusively employed for the recycling of lithium-ion batteries. This involves grinding post crushing to release the active materials from the spent batteries and separating them from their substrates. Subsequently, the valuable components can be extracted and concentrated through standard mineral processing techniques [60].
4.3 Hydrometallurgy
In the domain of hydrometallurgical processing, this method involves the disintegration of spent lithium-ion batteries, followed by the selective removal of components from the leachate. After that, the leachate was subjected to purification to obtain the essential valuable metals. Typically, crushing and shredding are the preliminary steps in hydrometallurgical processing, facilitating the release of materials. This leads to the integration of mechanical processing with hydrometallurgical techniques in many processing facilities. Studies reveal that the hydrometallurgical extraction of metals from spent lithium-ion batteries is a feasible option because of its numerous advantages, such as high metal recovery rates with purity, low energy consumption, and insignificant gas emissions. However, the process generates liquid waste. Hydrometallurgical processing is effective for extracting lithium from spent lithium-ion batteries [61]. Moreover, hydrometallurgical processes exhibit a high level of selectivity, allowing for the direct separation and retrieval of various materials with great efficiency [16]. The main hydrometallurgical methods used include leaching, precipitation, ion exchange, solvent extraction, and electrochemical separation.
4.3.1 Leaching: The process of leaching involves using a solvent to remove a soluble element, typically a metal, from a solid. It can also be selective for particular elements and is used to bring metals into solution. Leaching is an important step in the hydrometallurgy process of recycling lithium and cobalt from used batteries. Different leaching agents are used, including alkaline solutions, inorganic/organic acids, and ammonia-ammonium salt systems. The agent used is contingent on the features of the substances being recycled and the desired outcome. Alkaline solutions are commonly used because they dissolve a broad range of metals, while inorganic acids are useful for extracting cobalt. Ammonia-ammonium salt systems are an eco-friendly alternative to traditional agents. The most common leaching agent or solvent is sulfuric acid, which is also less expensive than other acids, such as hydrochloric or nitric acids, and is easier to obtain. It also poses less of a risk to the environment. The leaching efficiency or selectivity is contingent upon the nature of the leaching media, which can range from basic to acidic, as well as various configurations. By altering the oxidation state of a metal, such as cobalt (CO2+), which is far more water soluble than Co3+, the reduction leaching efficiency increases [16]. Table 1 gives the leaching optimum conditions for Li and Co recovery. Researchers [62] developed a method to recycle cobalt from lithium-ion batteries using a hydrometallurgical process that involves alkali and acid leaching, SX, and chemical precipitation. They used H2SO4 + H2O2 as the leaching agent and reductant, achieving 95% and 96% leaching rates for cobalt and lithium, respectively, under specific conditions. However, the use of strong acids can lead to harmful gas emissions and environmental pollution [63]. [64] used ultrasonic cleaning roasting and an eco-friendly weak organic acid, ascorbic acid, to extract valuable metals (Li and Co) from S-LIBs while minimizing harmful waste. They achieved high leaching rates of 98.5% for Li and 94.8% for Co by using 1.25 M C6H8O6 and a 25 g/L S/L ratio. Ascorbic acid converted insoluble Co3+ in LCO to easily soluble Co2+, while C6H8O6 was oxidized to C6H6O6. This approach is both effective and environmentally sustainable. A new method has been developed by [65] extracting lithium and cobalt from used LiCoO2 The method uses a reductive roasting technique with aluminum foil as a reductant. After 60 minutes at 600 °C, the spent cathode is converted into Li2O, LiAlO2, and CoO. The leaching efficiencies for Li and Al are 93.67% and 95.59%, respectively. This process selectively removes alkaline soluble Li2O and LiAlO2, leaving CoO in the residue. This approach reduces waste, promotes sustainability, and is advantageous compared to traditional thermal reduction techniques.
Table 1: S-LIB leaching optimum conditions
|
Reagent-acid |
Oxidant |
T (°C) |
T (min) |
S/L (g/L) |
Recovery (%) |
Reference |
|
1.25 M citric |
1% H2O2 |
90 |
30 |
20 |
Li: 100%; Co: 90% |
[1] |
|
1.5 M Citric+0.5g/gD-glucose |
— |
80 |
120 |
20 |
Li: 99%; Co: 92%; Ni: 91%; Mn: 94% |
[2] |
|
1.5 M DL-malic |
2% H2O2 |
90 |
40 |
20 |
Li: 100%; Co: 90% |
[3] |
|
1.0 M oxalate |
— |
80 |
120 |
50 |
LiCoO298% |
[4] |
|
1.25 M Ascorbic |
— |
70 |
20 |
25 |
— |
[5] |
|
4 M HCl |
— |
80 |
120 |
Li: 97%; Co: 99% |
[6] |
|
|
4 M HCl (NCA) |
H2O2 |
90 |
1080 |
50 |
Li: 100%; Co: 100% |
[7] |
|
HNO3/H2SO4 |
10% H2O2 |
75 |
120 |
— |
Li: 99%; Co: 99% |
[2] |
|
1 M H2SO4(LCO) |
— |
85 |
— |
— |
Li: 96%; Co: 95% |
[7] |
|
4 M H2SO4 |
CoC2O4↓ |
4.3.2 Precipitation: Leaching is typically followed by precipitation, which is the process of removing components from a solution by forming insoluble compounds. This is accomplished by including substances that react with solvated species to produce precipitate-forming insoluble salts. Filtration or centrifugation can then be used to retrieve the precipitated material. The reuse of the material is an advantage of precipitation, provided that a suitable solidifying agent is used. However, the expected residual solubility of the compound and the possibility of contamination of metal compounds, which would require an additional cleaning step, are disadvantageous [17]. The precipitation method uses varying pH and temperature to separate lithium from other elements in a solution. It is useful for industrial applications where efficient separation is important due to the high demand for lithium [66, 67]. Low-solubility materials, such as transition metal hydroxides, carbonates, or oxalates, can be readily precipitated from the process solution during hydrometallurgical treatment by employing precipitants such as NaOH, Na2CO3, and Na3PO4. These precipitates remove other dissolved metals, leaving behind lithium, which is then extracted in the form of Li2CO3, LiOH, or Li3PO4. This precipitation method is considered the optimal refining process because of its safety, affordability, and efficiency. Table S1 (supplementary material) provides a summary of previous studies supporting this claim [68].
4.3.3 Solvent extraction (SX): Using a two-liquid layer system that takes advantage of ion solubility in polar and nonpolar liquids, the SX technique successfully isolates lithium from leached CAM. Nonpolar extractants are used to remove rare elements such as cobalt, nickel, and manganese from aqueous solutions, whereas lithium is extracted from the solution. To separate Co, Ni, and Mn, several extractants, such as Cyanex 272, PC-88a, Cyphos IL-101, and D2EHPA, can be utilized. Table 2 summarizes previous SX experiments for LiCoO2 CAM, providing better knowledge of the process and its possible applications. [43], [68].
Table 2: Results of solvent extraction (SX) of various LiCoO2 materials reported in the literature.
|
Electrode material |
Leach reactive and conditions |
SX reagent |
Efficiency |
Purity |
References |
|
LiCoO2 |
600°C/2 h |
PC88a |
Leaching 100% |
>99.5% |
[8] |
|
2.25 M H2SO4(80°C/30min) |
Residue: LiSO4 |
||||
|
Leaching: 100%, purity 99.5% |
|||||
|
LiCoO2 |
0.5M HCl (60°C) |
Cyphos IL-101 Saturated Na2CO3(60\C) |
86.20% |
74.20% |
[9] |
|
Residue: Li2CO3 |
|||||
|
LIB scraps |
4 M H2SO4(100 g L−1) |
glucose [90 °C] |
80% |
>98% |
[10] |
|
D2HEPA, CYANEX 272, saturated Na2CO3 |
|||||
|
LiNi0.3Mn0.3Co0.3O2 |
2 M H2SO4H2O2(33 g L−1) [70 °C] |
Cyanex 272 |
Leaching 94% |
[11] |
|
|
Saturated Na2CO3 |
|||||
|
Li2CO3 |
H2SO4-H2O2mixture |
NaOH, D2EHPA, Cyanex 272, kerosene, Na2CO3[95 °C] |
72% |
99.70% |
[12] |
4.3.4 Selective Adsorption: Selective adsorption is a method that separates lithium ions using a specialized lithium-ion sieve. These inorganic bead-type adsorbents, called spinel-structured Mn-type Li-ion sieves, are very selective for Li ions in a Li-containing solution. This technology involves the extraction of lithium from brines. Li-ion sieves are perfect for Li because they use a vacancy to allow only Li ions to pass through. Among inorganic solvents, Li-Mn oxide spinel is the most selective and stable and has the highest capacity. This material is also commonly used due to its low toxicity [68]. Scientists [69] have developed a new method to recover precious metals from used lithium-ion batteries. The process involves extracting lithium, nickel, and cobalt from pretreated LIB powders using ammonia media and then using manganese-type lithium-ion sieves as adsorbents to separate the lithium from the other metals. The study revealed that the recovery efficiencies of lithium, nickel, and cobalt were 76.19%, 96.23%, and 94.57%, respectively. This process also produces byproducts and allows for the recycling of ammonia. This study explored the separation of Li+ ions through experiments on the adsorption behaviors of lithium-ion sieves under different conditions. An increase in the initial Li+ concentration led to an increase in the Li+ adsorption capacity, potentially because Li+ ions enter the active sites of the sieves faster [70]. This highlights the importance of considering varying lithium concentrations in optimizing the extraction process for valuable metals. The experiment examined the effect of varying concentrations of Li+, Ni2+, Co2+, and NH4+ ions on the adsorption capacity of lithium-ion sieves. The results showed that as the concentration of Li+ decreased, the adsorption capacity of the lithium-ion sieves decreased, while the adsorption capacities of the Ni2+ and Co2+ ions remained relatively stable. Additionally, the experiment revealed that increasing the concentration of NH4+ ions resulted in an increase in the Li+ adsorption capacity but a decrease in the Ni2+ and Co2+ adsorption capacity due to a stronger complexation capacity between NH3 and the Ni2+ and Co2+ The study also revealed that higher pH leads to reduced surface charge in lithium-ion sieves, resulting in greater interactions between lithium ions and hydrogen ions. This discovery can help in the creation of more efficient lithium-ion sieves [71]. Umeno et al. observed that the adsorption capacities of Li+, Ni2+, and Co2+ increased as the temperature increased from 20 to 50 °C while keeping their concentrations constant. This is consistent with previous research and suggests that temperature plays a crucial role in the adsorption process of these ions. The increase in capacity can be attributed to the enhanced mobility of the ions and the increased surface area of the adsorbent [69]. These findings have implications for efficient and cost-effective metal recovery from waste streams.
To ensure the success of the hydrometallurgical flowsheet, it is critical to separate the ionic species in the PLS from each other once they are in solution, which can be accomplished through various methods, such as precipitation, SX, electrochemical processing, and membrane separation. In general, hydrometallurgical processes include dismantling, crushing, leaching, purification, separation and product preparation, among which leaching has been the focus [72]. The implications for hydrometallurgical purification and refining are shown in Table 3. The recovery of materials by hydrometallurgy, which has been intensively researched, is a promising technique. However, because so much acid and base are needed for leaching, this process may result in increased chemical expenses, and disposal costs might be substantial. Before using hydrometallurgy extensively, it is crucial to assess the benefits and drawbacks to make an informed choice.
Table 3: Implications for hydrometallurgical purification and refining (adapted from the work of [13].
|
supplementary material |
Challenges in hydrometallurgy |
Troubleshooting |
|
Electrolyte (EMC, DMC) |
Organochlorine chemicals are produced, harmful gases are released, acid usage increases and prewashing expenditures are necessary. |
Evaporation/decomposition by thermal pretreatment |
|
PVDF (Adhesive; 100% in AM) |
Costs associated with disposal rise due to the filter cake's inability to dissolve in acids. |
Decomposition by thermal pretreatments |
|
LiPF6(conducting electrolyte salt; 100% in AM) |
The HF(g) formation, fast corrosion to the equipment; LiF formation, thus, Li depletion in aqueous and metal-containing phase |
Decomposition by thermal pretreatment |
|
Mn |
Expense on operation surges due to necessary precipitation and disposal, as well as cross-contamination in Fe and Al fraction, a more difficult recycling opportunity |
Slagging Pyrometallurgy |
|
Al and Fe |
operational expenses increase as a result of waste disposal and precipitation. Al decreases filtering effectiveness. |
Slagging Pyrometallurgy |
|
Plastic residues (separator, sleeve, cable covers, etc.) |
Costs for additional filters and incinerator disposal |
Decomposition by thermal pretreatment |
|
Graphite (100% in AM) |
Foam formation with an impact on the plant construction and cost of process additives |
Reducing agent Pyrometallurgy |
|
LiFePO4(100% in AM) |
Hazardous wastewater containing PO4, corrosion due to gas production, the creation of stable Ni and Co PO4s, HF favouritism, issues for fundamental safety, and filtering challenges |
Slagging Pyrometallurgy |
|
Silicon (LiB Gen. Si Anode) |
The high expense of waste disposal and gel formation during filtering |
Slagging Pyrometallurgy |
4.4 Pyrometallurgical Method:
Lithium, cobalt, and nickel are precious metallic components that can be extracted from lithium-ion batteries (LIBs) via pyrometallurgy, a technique that employs high temperatures. The process is extensive and requires a number of phases, such as roasting, smelting, and refining. The purpose of roasting is to remove volatile components and other contaminants by heating the electrode material in the presence of air [73]. The roasted electrode material is melted along with a reducing agent during the smelting process, which eliminates any leftover impurities and leaves the pure metal behind (Figure 6). Finally, the metal is further purified during the refining process using a variety of methods, including electrolysis and distillation. Large amounts of wasted LIBs can be handled by pyrometallurgy, which can also yield excellent metal products and recover metals that are challenging to remove with conventional methods [74]. This method's streamlined extraction and purification operations allow it to produce results that are frequently faster and more affordable than those of alternative techniques. Nevertheless, there are a number of drawbacks to pyrometallurgy, such as the difficulty of recycling the electrolyte and the ease with which lithium can be lost in the slag. Furthermore, during the smelting process, pyrometallurgy discharges pollutants into the atmosphere, including sulfur dioxide and other gases [73]. The Umicore Integrated Smelter and Refinery Plant uses a pyrometallurgical process to recover precious metals from waste electrical and electronic equipment (WEEE), which includes pretreatment, smelting in an Isa Smelt furnace, and electrolytic refining of base metals [75].
4.4.1 Smelting: The smelting process in recycling lithium-ion batteries involves heating the electrode material to melt metal oxides, which results in the reduction of metal oxides to a liquid state [74]. This method removes the requirement for pretreatment and enables direct smelting of cells and modules in the smelter [76]. In this process, the electrolyte is heated to a low temperature for evaporation, while the electrode material melts at high temperatures. Carbon and aluminum act as reducing agents, and the addition of appropriate fluxes aids in metal melting by lowering the melting point. An advantage of smelting is its ability to recover various spent LIBs with diverse chemical compositions.
4.4.2 Roasting: Carbonothermal reduction roasting is a crucial method in pyrometallurgy for recovering cathode materials by heating them with a reducing agent such as carbon, promoting the reduction of transition metal oxides and facilitating the leaching of Li and Co [77]. A combination of LCO and graphite was roasted for 30 minutes at 1000 °C in a nitrogen environment by [48]. The findings indicated that 98.93% of the Li, 95.72% of the Co, and 91.05% of the graphite were recovered by magnetic separation from the roasted products, which were made of graphite, Co, and Li2CO3. In contrast to conventional calcination methods, which necessitate heat transfer and result in undue thermal loss and comparatively long heating times, microwave-assisted carbothermal reduction is gaining attention because of its ability to recover metals from spent LIBs by directly heating graphite with microwave energy, improving metal recovery efficiency [78]. A brief overview of the pyrometallurgical recycling process for spent LIBs is shown in Table 4.
Table 4: A concise analysis of pyrometallurgical recycling for LIBs adapted from [14], [15]
|
Spent material |
Reducing agent |
Thermal-treatment condition |
Posttreatment leaching |
Efficiency wt.% |
Ref. |
||
|
Co |
Li |
||||||
|
Polymer LIB |
Al can, pyrolusite, SiO2 |
800°C, 2h |
Conc. H2SO4, H2O |
99.84 |
50.28 |
[16] |
|
|
LiCo0.7Ni0.15Mn0.15O2 |
Activated carbon |
700°C, 1h |
C6H8O7, Na2SO4,1 M, H2O |
>99 |
38.3 |
[15] |
|
|
LiCoO2 |
Graphite, NaOH 10% |
520°C, 3h |
H2O |
- |
93 |
[17] |
|
|
LiCoO2, LiMn2O4, LiNiO2 |
Graphite |
400-700°C, 0.5-1.5h |
- |
- |
- |
[18] |
|
|
LiCoO2, LiMn2O4, LiCoxMnyNizO2 |
Graphite, initial vacuum<1kPa |
700°C, 0.5h |
H2O |
- |
81.9 |
[19] |
|
|
LiMn2O4 |
Carbon, vacuum |
800°C, 45min |
H2O |
- |
91.3 |
[20] |
|
|
LiCoO2 |
Al foil |
600°C, 1h |
NaOH 2.5M |
- |
93.67 |
[21] |
|
|
LiCoO2 |
NaHSO4·H2O |
600°C, 0.5h |
H2O |
72.56 |
0.53 |
[22] |
|
|
Simulated slag xLi2O·yCaO·zAl2O3·nSiO2. |
CaCl2, NaCL, ACl3 |
1000°C, 1.5h |
- |
- |
97.45 LiCl |
[23] |
|
|
LiCoO2 |
HNO3, (70°C, 5h) |
250°C, 1h |
H2O |
<0.1 |
>93 |
[8] |
|
|
LiCoO2 |
NH4Cl |
350°C, 20min |
H2O |
99 |
99 |
[24] |
|
|
Electrode materials |
1700 °C-1750 °C |
>90% with 99% purity |
[25] |
||||
|
LiCoO2+ C |
1000 °C/30 min in O2free |
||||||
|
[3] |
|||||||
|
Cathode materials |
650 °C/3 h |
Water leaching [100 °C/0.5 h] |
99% |
84.70% |
[26] |
||
|
LIB scraps |
Roasting 100-300 °C |
70 °C/5 h HNO3 |
90% with 99.95% purity |
[23] |
|||
|
Electrode materials |
Carbothermal 850 °C/45 min |
61% |
80% with 98% purity |
[27] |
|||
|
Co, Li and graphite |
High temperature, smelting and reduction process |
97.52% |
98.93% |
[3] |
|||
4.5 Emerging sustainable recycling technologies for SLIBs
4.5.1 Deep eutectic solvent: Hydrometallurgy has been the subject of countless investigations in recent decades because of its ability to produce highly pure products and because of the rich chemistry involved, which may enhance overall efficiency [76, 79]. Eco-friendly solvents have replaced traditional acid-based leaching reagents in an attempt to reduce pollution and improve the circular economy throughout the leaching process. The idea of solvometallurgy, a cutting-edge field of study that looks for safer and more efficient substitutes for leaching reagents, was born out of this transition [80]. Among the several solvents being studied for the recycling of lithium-ion batteries (LIBs), deep eutectic solvents (DESs) have garnered much attention due to their special ability to interact with the active components in LIBs. This feature offers the potential for cost-effective and environmentally friendly recycling of LIB cathode materials. DESs are a broad class of solvents that are created when two solid materials are combined, producing a combination that has a significantly lower melting point [81-83].
The extraction of different kinds of cathode materials was discovered to be feasible in 2019 with DESs [84], introducing a new phase in the recycling of LIBs via hydrometallurgy. Compared to ionic liquids and conventional solvents, DESs for cathode recycling in LIBs have a number of advantages. They are inexpensive, easily scalable, nontoxic, and eco-friendly [85, 86]. When using DES as a leaching agent, further pollution is prevented, including the release of hazardous waste gases such as SO2, Cl2, and NO2 [25], and additional reducing agents are not needed, in contrast to traditional acid-based leaching agents such as H2NO3, HCl, and H2SO4 [87-89]. As a result, complicated processes and additional reagents are not required during the leaching process. Moreover, DESs can be recycled numerous times while maintaining the same efficiency. The focus on DES recycling has increased recently, as evidenced by the numerous studies conducted to find the best DES system with better leaching efficiency [90], less environmental impact (Lu et al., 2022), and simpler leached element extraction [34]. Many facets of this field have been examined in a number of research papers, such as the physicochemical properties of DESs [91-93], the impact of functionalizing DESs on reaction rates [94], and the ways in which the composition of hydrogen donors and acceptors affects DES properties and the subsequent recovery of metal from LIBs [95]. These studies also weigh the benefits and limitations of DESs in comparison to those of conventional recycling techniques [96], examine the financial and environmental benefits of utilizing DESs to recycle used LIBs [97], and explore the direct extraction of metals from LIBs using acid-based DESs. More studies are necessary to continue growth in this area even though multiple studies have revealed considerable breakthroughs in waste LIB recycling utilizing Dess. Reported DES-based approaches for recycling Co from spent LIBs is provided in Table 5.
Table 5: Reported DES-based approaches for recycling Co from spent LIBs
|
Reaction system |
Reaction condition |
Leaching efficiency |
Ref. |
|
|
DES (ChCl + EG |
LiCoO2 |
195°C, 24 h |
Co: 69.14 |
23[28] |
|
DES (ChCl + urea) |
160°C,2 h |
Co: 60 |
[29] |
|
|
DES (ChCl + CA) |
60°C,4 h |
Co: 99 |
[30] |
|
|
DES (ChCl + Gly) |
200°C, 10 h |
Co: 92.7 |
[31] |
|
|
DES/H2C2O |
LiFePO cathodes |
106°C, S/L ratio of 0.02, 110min |
Li and Fe is 95.3% 85.2%, respectively |
|
|
Choline chloride-citric acid |
60 °C, S/L = 20 g/L, Al: LiCoO2 = 12 wt% ,900 rpm |
Co: 99.6 |
||
|
[32] |
||||
|
Choline chloride-malic acid |
LiCoO2 |
Co:81.2 |
||
|
DES (ChCl + H3PO4) |
Ethanol aqueous/NaOH 100 °C |
Li (100%) and Co (92.8%) |
[33] |
|
|
LiCoO2 |
||||
|
DES (BeCl +Lactic acid) combined DES−ethanol |
Time:2.2 h |
Co: 99.86 |
||
|
S/L: 20(mg/g) |
Li: 99.98 |
|||
|
T:120 °C |
||||
|
LiCoO2 |
Time:0.3 h |
Co:99.7 |
[34] |
|
|
S/L: 20(mg/g) |
Ni:99.3 |
|||
|
T:110 °C |
Mn:99.9 |
|||
|
Li:99.9 |
||||
|
DES (GHC −LA) |
LiCoO2 |
Time:24 h |
Co: 100 |
[35] |
|
S/L: 19.9(mg/g) |
Li: 100 |
|||
|
T:80 °C |
||||
|
ChCl−LA |
LiCoO2 |
Time:24 h |
Co: 100 |
[36] |
|
S/L: |
Li: 100 |
|||
|
T:105 °C |
||||
|
ChCl-urea |
LiCoO2 |
Time:18 h |
Co: 94.7 |
[37] |
|
S/L: 20(mg/g) |
Li: 97.9 |
|||
|
T:180 °C |
||||
|
ChCl−FA |
LiCoO2 |
Time:12 h |
Co: 100 |
[38] |
|
S/L: 20(mg/g) |
Li: - |
|||
|
T:70 °C |
||||
|
PEG −thiourea |
LiCoO2 |
Time:24 h |
Co: 71.5 |
[39] |
|
S/L: 20(mg/g) |
Li: - |
|||
|
T:160 °C |
||||
|
SAD/EG |
LiCoO2 |
Time:6 h |
Co:94.8 |
[40] |
|
S/L: 20(mg/g) |
Ni:99.1 |
|||
|
T:110 °C |
Mn:100 |
|||
|
Li:100 |
||||
|
ChCl−OxA |
LiCoO2 |
Time: |
Co:95.1 |
[41] |
|
S/L: 20(mg/g) |
Ni:95.5 |
|||
|
T:120 °C |
Mn:99.1 |
|||
|
Li: |
||||
|
BeCl−EG |
LiCoO2 |
Time:2.5 h |
Co:99.5 |
[42] |
|
S/L: 25(mg/g) |
Ni:99.6 |
|||
|
T:140 °C |
Mn:99.7 |
|||
|
Li:99.1 |
ChCl: choline chloride, GHC: guanidine hydrochloride, FA: formic acid PEG: polyethylene glycol, SAD: sulfosalicylic acid dihydrate. BeCl: Betaine hydrochloride
4.5.2 Molten salt roasting: Molten salt roasting is a pyrometallurgy process that involves heating spent lithium-ion batteries (LIBs) in the presence of a salt mixture at high temperatures, which facilitates the subsequent recovery of essential metals and speeds up the disintegration of the battery components. (Li et al., 2023). Through techniques including chlorination, sulfation, and nitrification roasting, transition metal oxides are transformed into soluble metal salts through salt-assisted roasting. This process uses salts with certain qualities to lower acid use and enhance metal recovery. Compared to roasting techniques such as chlorination and nitrification, sulfation roasting has been demonstrated to be more environmentally harmless and friendly. This technique is an emerging recycling method aimed at enhancing the efficiency of LIB recycling while attempting to minimize environmental repercussions [98] (Li et al., 2023). By using a vulcanizing agent, metal oxides can be transformed into low-valence sulfates, preventing SOx generation and secondary pollution [99-101]. Adjusting parameters such as temperature and additives in salt-assisted roasting can enhance the metal recovery efficiency and increase the eco-friendliness of the process. By using this technique, the temperature at which valuable metals are recovered is lowered, the cathode powder structure is disassembled at lower temperatures, and the calcination temperature is decreased. Molten salt roasting can potentially improve the efficiency of metal recovery from spent LIBs and its impact on sustainability, cost-effectiveness, and environmental friendliness by alleviating resource scarcity through the recovery of valuable materials (Li et al., 2023). On the other hand, the high energy requirements for maintaining elevated temperatures could pose challenges to its environmental and economic viability (Li et al., 2023). Moreover, the process must be carefully managed to avoid the release of toxic emissions and to ensure that it aligns with the principles of green and sustainable development (Li et al., 2023). In summary, molten salt roasting is an innovative approach for recycling spent LIBs and has the potential to improve the efficiency of material recovery. However, its broader impacts on sustainability, cost, and environmental protection require careful consideration and optimization to ensure that it contributes positively to the circular economy and ecological civilization (Li et al., 2023). Further research and development are necessary to fully assess and enhance the sustainability and environmental friendliness of this recycling method.
4.5.3 Coupling redox flow desalination: Another exciting development involves coupling redox flow desalination with lithium recovery from spent lithium-ion batteries [102]. The spontaneous reaction between the battery cathode material (LiFePO4) and ferricyanide enables continuous regeneration of the redox species essential for desalination. This dual-purpose system not only addresses desalination challenges but also contributes to sustainable lithium recovery.
[102] developed a novel approach that couples redox flow desalination with lithium recovery from spent lithium-ion batteries, particularly focusing on using spent LiFePO4 to regenerate redox species for desalination. By optimizing critical parameters, such as the current density and concentrations of redox species, this method enables continuous desalination and achieves a high lithium recovery rate. The redox flow system utilizes spent LiFePO4 from lithium-ion batteries to regenerate the redox species required for desalination and lithium recovery. The critical parameters optimized in the study to enhance the efficiency of the redox flow system included the concentration of redox species, such as \[Fe(CN)6\]4−, the NaCl concentration, and the molar ratio between \[Fe(CN)6\]4−and LiFePO4. Additionally, this study examined the effect of current density on the system's performance and energy consumption for desalination.
The EverBatt model [103, 104] was used to scale up a redox flow system from the laboratory scale to the large scale, considering costs such as equipment, raw materials, electricity, and personnel expenses. Each redox flow unit with a 1 m2 electrode incurs equipment costs and expenses for recyclable chemical consumables, yielding LiCl and freshwater from spent LFP powder. Optimizing for a higher discharge current can enhance the treatment capability and bring more benefits to the process. A large-scale redox flow processing line with 10,000 units can process approximately 2144.4 tons annually, using the EverBatt model for cost calculation. Redox flow processing lines for recycling LIBs are more efficient than pyrometallurgy and hydrometallurgy methods because of their lower chemical consumption, greenhouse gas emissions, and energy consumption [105]. The redox flow processing line incurs costs from ion exchange membranes but can generate revenue from LiCl production, making it potentially profitable [106].
Traditional recycling methods such as pyrometallurgy and hydrometallurgy [105-113] are not commercially viable for recovering cathode materials from spent LFP-based batteries due to economic losses.
The economic analysis shows that the redox flow system for lithium recovery from spent lithium-ion batteries offers significant advantages over traditional recycling methods. It requires less chemical consumption, has lower energy consumption, and generates less greenhouse gas emissions. Additionally, the redox flow system has the potential to be a profitable and environmentally friendly alternative for recycling spent lithium-ion batteries.
4.5.4 Biohydrometallurgy (bioleaching)
In a natural chemical process called bioleaching, insoluble materials are converted to soluble and extracted forms using microorganisms [114, 115]. Bioleaching is a promising biotechnological technology that can be utilized to recover secondary metal resources from used LIBs, thus contributing to the realization of an environmentally beneficial circular economy [115, 116]. Based on the types of interactions (direct or indirect) between microorganisms and pre-processed spent LIBs (crushed and sieved powder form of spent LIBs), bioleaching experiments are primarily carried out in three different ways: (1) one-step bioleaching, (2) two-step bioleaching, and (3) spent-medium bioleaching [117, 118]. The standard one-step procedure involves adding pre-growth microbes as an inoculum to the leaching media containing the powdered spent LIBs. Continuous bioacid production and simultaneous microbial development drive the leaching of metals from the complex spent LIB powder matrix [117]. The one-step approach works well with spent LIBs that have little to no toxic components, such as pre-processed LIBs (such as those that have undergone water washing and drying), as the toxicity effects slow the rate of microbe growth. For the purpose of producing bioacids, the leaching organism in the two-step process is cultivated in the leaching medium for a predetermined amount of time, often up to the logarithmic phase. The metal extraction process is subsequently started by adding the powered LIB [117]. In the absence of any pretreatment, such as washing and drying, this approach can be applied to spent LIBs that contain harmful compounds and could prevent the proliferation of microbes.
In the bioleaching process, biotic and abiotic variables influence the rate at which metals dissolve from spent LIBs [119, 120]. Abiotic variables include the variety of microbiological agents, such as fungi versus bacteria. Aeration, catalyst, pulp density, spent LIB particle size, aeration, and pH are among the additional abiotic elements. Abiotic factors also include environmental parameters such as temperature and the chemistry of the leaching solution (e.g., concentration of nutrients and energy/carbon source and pH).
4.5.5 Bacterial-based bioleaching: To bioleach precious metals from spent lithium-ion batteries, acidophilic sulfur-oxidizing bacteria (SOB) and iron-oxidizing bacteria (IOB) are often used. These bacteria use inorganic substances such as sulfur and ferrous ions as energy sources and carbon dioxide as a carbon source, respectively [121, 122]. They recover metals such as cobalt and lithium effectively and are resistant to metal toxicity. Research on the use of individual or groups of acidophilic bacteria in bioleaching procedures has demonstrated good rates of recovery for these metals [123, 124]. Liu et al. [125] reported that metallic stress decreased the bio-oxidative activity of microbiological agents, which in turn decreased the bioleaching efficiency. However, when exogenous glutathione (GSH), a ubiquitous intracellular peptide with a variety of uses (0.3 g/L), was added, the level of bacterial intracellular reactive oxygen species (ROS) decreased by 40%, and at 5% pulp density, 96.3% of the Co and 98.1% of the Li were recovered from a microbial consortium composed of L. ferriphilum and S. thermosul fidooxidans. The recoveries of Li and Co metals from spent lithium-ion batteries (LIBs) via bacterial-based bioleaching are presented in Table S2 (supplementary material) [36].
4.5.6 Fungal-based bioleaching: As heterotrophic microbes, fungi obtain carbon from organic carbon-based sources, which they use for growth and metabolism [126]. A number of fungal species, including Penicillium simplicissimum, Aspergillus niger, Aspergillus tubingensis, and Penicillium chrysogenum, are used to extract metals from electronic waste [126-128]. However, because Aspergillus niger grows and harvests more easily and yields more water, it is a widely preferred strain for spent LIB bioleaching [129]. Unlike bacteria, fungi can thrive in wastes that are both acidic and alkaline, and they have a shorter lag phase and a faster leaching rate, which allows them to be more tolerant to a wider range of hazardous metals [118].
The recoveries of Li and Co metals from spent lithium-ion batteries (LIBs) via bacterial-based bioleaching are presented in Table S3 (supplementary material) [36].
4.5.7 Mechanochemical Process: Emerging mechanochemical technology, which has the benefits of simplicity, adaptability, and rapid processing time, offers a new strategy for the highly efficient and environmentally friendly recycling of key metals from waste LIBs as an alternative to conventional pyrometallurgy and hydrometallurgy [130]. By applying mechanical driving forces to reactants, such as shear, crushing, friction, impact, and extrusion, this method modifies the physicochemical characteristics and structures of the reactants while also initiating mechanochemical reactions [131, 132]. Mechanochemical technology is currently widely utilized for the extraction of various important metals from a variety of environmental media because it can perform reactions at ambient temperature and normal pressure and only requires basic, inexpensive reaction equipment [133, 134].
The application of mechanical energy to solids, liquids, and other condensed substances through shear, friction, impact, extrusion, etc., to cause changes in their structure and physical and chemical properties and to initiate chemical reactions is known as mechanochemistry at the chemical reaction level [131, 135]. A mechanochemical reaction can be carried out without the need for extreme temperatures or pressures because, in contrast to typical thermochemical reactions, it is driven by mechanical energy rather than thermal energy.
The potential for recovering essential metals from waste LIBs by mechanochemical processes is mostly attributed to their favourable environmental and financial aspects. (1) Compared with pyrometallurgy, mechanochemical reactions require less energy when they are conducted at room temperature and normal pressure. In comparison to hydrometallurgy, it was also safer. Mechanical force-driven chemical reactions are solid-solid reactions that can utilize less water and have controlled exhaust gas discharges without posing a risk of secondary pollution to the environment. Thus, mechanochemistry might be regarded as an environmentally safe technology. (2) By combining alternative reagents while applying mechanical force, one can create a green and clean crucial metal extraction technique that uses fewer acid-base reagents. Moreover, metal extraction from a variety of cathode materials can be accomplished universally by mechanochemistry. Additionally, greater technical adaptability translates into additional opportunities for advancement [130].
The effects of conventional procedures (such as pyrometallurgy, hydrometallurgy, and biometallurgy) and mechanochemical processes of Li and Co recovery from LIBs on the environment and economy were compared for the first time by Wang et al. (2017) [136]. The comparatively low reaction temperature and sealed atmosphere of the mechanochemical process have less of an environmental impact than the high temperatures typically required for the pyrometallurgical process, which also releases HF gases. The primary leaching agent used in hydrometallurgy readily produces waste water and corrodes equipment. Li and Co leaching in biometallurgy is facilitated by a long reaction time. On the other hand, no acidic waste water was produced by the documented mechanochemical process, the LiCoO2/PVC/Fe solid-solid reaction, since it does not require the use of water as a medium. Furthermore, this mechanochemical approach is straightforward to scale up for commercial applications and has a much easier recovery process and far lower separation costs than existing methods. This study by Wang et al. [137] evaluated the economic and environmental impacts of the designed process and hydrometallurgy process (using HCl, H2SO4, formic acid, succinic acid, and citric acid as leaching agents) for the recovery of Li and Co from spent LiCoO2 batteries via mechanical activation combined with acetic acid leaching. Comparative analysis revealed that the designed method recovered metal at room temperature more quickly. Additionally, the economic viability of recovering 1 kg of used LIBs at laboratory size was validated by an economic assessment [138]. Moreover, room temperature mechanochemistry decreased the energy consumption of the reaction in the mechanochemical extraction and thermal reduction routes for Li and Co recovery, and the entire metal recovery process did not discharge wastewater or residue, which is in accordance with No. 6 and No. 1 of the 12 Principles of Green Chemistry, respectively [130]. However, there are a few obstacles to the application of mechanochemistry: (1) the activity of the reactants determines the effectiveness of mechanochemical processes. A lengthy reaction period and a large energy input are needed if the reactants are inert. Selecting ligand reagents with high activity can minimize energy use; however, the cost of these ligands is typically greater, which diminishes the process's economic advantages. Thus, even for the selective extraction of key metals from solid waste, the right choice of ligands is essential for the waste LIB process. (2) Only 25% of the energy input into the reaction system is utilized in mechanochemical reactions; the remaining energy is wasted as heat, and high energy input will result in high industrial costs, which will decrease recycling profit. This position may change in the future due to the growth of the new energy industry, economies of scale, and additional advancements in new energy technology [130]. Literature-based methods involving mechanochemistry are presented in Table 6.
Table 6: A summary of literature-based approaches involving mechanochemistry
|
Material |
Ball milling parameters |
Leaching parameters |
Leaching efficiency (%) |
References |
|
LiCoO2 |
Time: 30-min |
90% of Co and Li |
[43] |
|
|
LCO |
Speed:1650rpm Time:300min |
4M NH3·H2O; 1.5M NH4Cl; |
98.22% Co and 89.86% Li |
[44] |
|
0.5M Na2SO3;240min; 353K |
||||
|
LCO |
98% of Co and 99% of Li |
[45] |
||
|
LCO |
95% of Co and 98% of Li |
[46] |
||
|
LCO |
100% of Li |
[47] |
||
|
LCO |
Speed:650rpm Time:250min |
1M HNO3; |
99.9% Ni, 91.25% Co, |
[48] |
|
Co grinding reagent: Fe powder |
120min; 298K |
100% Mn and 77.15% Li |
||
|
LFP |
Speed:550rpm Time:120min |
0.6M H3PO4; |
97.67% Fe and 94.29% Li |
[49] |
|
Co grinding reagent: EDTA-2Na |
20min; 298K; 50g/L |
|||
|
LCO |
Speed:500rpm Time:60min |
1.0M L-ascorbic acid; |
99% Co and 100% Li |
[50] |
|
20min; 298K; 10g/L |
||||
|
LCO |
20vol% acetic acid; 5vol% H2O2; |
99.7% Co and 99.8% Li |
[22] |
|
|
Co grinding reagent: C |
15min; 298K |
|||
|
LFP |
Speed:500rpm Time:120min |
H2O |
94% Fe and 99% Li |
[51] |
|
Co grinding reagent: oxalic acid |
||||
|
ball milling medium:1 ml H2O |
||||
|
NCM |
Speed:550rpm Time:120min |
1.5M H2SO4; |
96.2% Ni, 94.3% Co, |
[52] |
|
Co grinding reagent: Zn powder |
15min; 323K; 20g/L |
91.0% Mn and 99.9% Li |
||
|
LCO |
Speed:500rpm Time:120min |
1M H2SO4; 0.03M NH4Cl; |
99.22% Co and 100% Li |
[53] |
|
60min; 353K; 20g/L |
||||
|
LCO |
Speed:400rpm Time:60min |
98.87% Co and 99.33% Li |
[54] |
|
|
Co grinding reagent: Grape skin |
||||
|
Wet ball milling medium:0.15M citric acid |
4.5.8 Electrochemical extraction: Electrowinning can be used to extract metal species from a solution and achieve the necessary reduction by supplying energy to two electrodes, resulting in the oxidation and reduction of ions in the solution. This method requires metals to have differing reductive and oxidative potentials. Once metals are separated into different solutions using solvent extraction, high-purity metals can be produced by electrochemical deposition. An electrolysis cell can be used where electrodes are separated by a membrane that allows only metal ions to pass through. Metal ions are concentrated at the cathode side until the solubility of the product is exceeded, and solid metal hydroxides are precipitated. In another technique, gaseous carbon dioxide is introduced into the electrolysis cell to initiate the precipitation of lithium carbonate at the cathode side. The electrochemical processes and productivity are slow [16]. During this process, an active material is immersed in an electrolyte solution, and a current is applied to the solution via an electrode. The lithium ions in the solution are attracted to the electrode and deposited as metallic lithium, which is essential for the function of lithium-ion batteries. This process also produces hydrogen gas as a byproduct, which can be used as a clean energy source. The Li+ ions can be separated through the oxygen evolution reaction during the charging process. [139] demonstrated the practicality of utilizing water and the contents of waste Li-ion batteries as electrodes in a Li-liquid battery system. Through electrochemical collection, Li metal was extracted from a discarded Li-ion battery that contained Li-ion source materials from the anode, cathode, and electrolyte. Using this method, the Li in the spent battery was successfully recycled at room temperature. Water was used as the cathode, and the captured Li metal was then released to generate energy. In comparison to fresh Li metal at the same current rate, which had a discharge voltage of 2.8 V, the water had a voltage of 2.7 V at 0.1 mA cm−2. This indicates that the battery system is highly efficient and can produce electricity at a lower cost. The proposed battery system is unique in that it utilizes water and waste Li-ion batteries as electrodes, which significantly reduces the cost of the battery. This makes it an attractive option for large-scale energy storage applications.
4.5.9 Polymer Inclusion Membranes (PIMs) Electrodialysis (PIMED): Polymer-based liquid membranes have been considered a substitute for traditional solvent extraction for 40 years. They have also been used to create sensing membranes. These liquid polymer membranes are now referred to as polymer inclusion membranes (PIMs) and are more stable and versatile than other types of liquid membranes, such as supported liquid membranes [140]. Polymer inclusion membranes (PIMs) are promising materials that use a polymer matrix and a carrier to selectively transport target species across a membrane. PIMs have many applications, including separating metal ions, organic compounds, and gases. Compared to other types of membranes, such as supported liquid membranes, PIMs offer benefits such as self-support, versatile fabrication, a longer lifespan, and resistance to fouling and degradation. Using an extractant, base polymer, or plasticizer or modifier, PIMs rely on a concentration gradient of species, carrier complex, or ion pair to facilitate transport across the membrane [141]. [142] reported that Co (II) can be effectively transported across PIMs that contain CTA, triisooctylamine, and 2-nitrophenyl pentyl ether. This was achieved in aqueous solutions that contained both Co (II) and Li (I). The initial fluxes for Co (II) and Li (I) were 14.4 and 4.4 μmol m−2 s−1, respectively. After 24 hours, the recovery rates for Co (II) and Li (I) reached 74.5% and 5.3%, respectively. PIMs containing specific components can effectively separate and recover Co (II) and Li (I) from water. However, metal ions have low permeability through PIMs, which limits their widespread use [140]. Recently, a new technology has emerged that combines PIM with electrodialysis (ED) to enhance PIM permeability. This innovative approach has proven to be highly effective, as evidenced by recent studies [143, 144]. Even at a low working voltage of only 2.6 V, the PIMED process has been shown to increase the initial flux of Cr (VI) by an impressive 86.4 times compared to the initial flux without the application of an electric field. Additionally, the transport efficiency of PIMED far surpasses that of PIM-EME processes. A study was conducted by [145] to improve the selectivity of the PIMED system for separating mixed metals. The study analysed various parameters and revealed that three different membranes were effective at separating Co (II) and Li (I) in an ED process, with the PVDF-HFP-Aliquat 336 membrane being the best at extracting Co (II). Using Aliquat 336 as a carrier in PIM resulted in high purity levels for both Li (I) and Co (II), with a separation factor Co/Li exceeding 164, showing that Aliquat 336 is an effective carrier for achieving high levels of purity. The addition of Aliquat 336 enhanced the performance of metal ion extraction processes, increasing transportation and separation. The study also revealed that the application of an electric field significantly improved the permeability coefficient and extraction degree of Co (II), highlighting the advantageous role of the PIMED system in enhancing PIM permeability. To create effective separation procedures, this study emphasized the significance of electric fields in transferring ions across polymer inclusion membranes (PIMs). High-purity precious metals can be extracted from spent lithium-ion batteries (LIBs) using a technique called Polymer Inclusion Membrane Electrodialysis (PIMED). Low energy usage and continual improvement are two benefits of this method. Therefore, PIMED can provide a sustainable solution for the recycling of LIBs.
4.6 Literature-sourced account of extraction methods, merits and demerits outlined
The spent LIBs are regarded as a secondary source of metals; occasionally, the metal concentration or quantity is higher than that of natural or concentrated ores [146]. Given the limited availability of Li and Co, it is critical to lower the high demand for natural metal resources, preserve the valuable metals found in spent LIBs, and lessen the environmental damage that the hazardous components of spent LIBs create. Thus, spent LIBs should be appropriately handled and recycled. Recycling could play a major role in the overall sustainability of future LIBs by recycling secondary metallic resources and contributing to a circular economy [147]. Recycling and reuse of valuable metals, namely, Co and Ni, from spent LIBs could save 51.3% of natural resources and reduce the mining of metals from virgin mineral sources [148]. Spent LIBs can be recycled to create new LIBs or other goods, such as supercapacitors, using the valuable metals that were recovered (Ratnam et al., 2022). Hydrometallurgy, biohydrometallurgy (bioleaching), direct recycling and pyrometallurgy are the recycling techniques most frequently utilized for spent LIBs [129, 147]. The major advantages and disadvantages of various types of recycling methods are given in Table 7. Among the various types of recycling methods, bleaching is cost-effective, environmentally friendly, simple in operation and less energy intensive [147, 149].
Table 7: Summary of extraction methods, advantages and disadvantages
|
Extraction Methods |
Advantages |
Disadvantages |
Reference |
|
|
Hydrometallurgy (acid/alkaline leaching) |
a) High efficiency |
a) Corrosive acid needs to be handled with care |
[55], [56] |
|
|
b) Low cost |
b) Acidic waste needs proper disposal |
|||
|
c) Simple process |
c) Possibility of metal contamination |
|||
|
Hydrometallurgy (DES) |
a) Greener |
a) Restricted recyclability, |
[57], [58] |
|
|
b) cost-effective than traditional |
b) high viscosity, |
|||
|
c) ability to selectively extract metals |
c) low thermal and chemical stability, |
|||
|
d) complex chemistry, |
||||
|
f) limited scalability |
||||
|
Pyrometallurgy |
a) High efficiency |
a) Energy-intensive process |
[58] |
|
|
b) Metals recovered are of high purity |
b) High temperatures required |
|||
|
c) The process can generate heat for other industrial processes. |
c) Expensive equipment needed |
|||
|
d) Possibility of airborne particle contamination |
||||
|
Electrochemical |
a) High efficiency |
a) Chemicals used can be expensive |
[58] |
|
|
b) Safe process |
b) Complex process |
|||
|
c)Low environmental impact |
c) The need for proper neutralization and disposal of waste after the recovery process. |
|||
|
Biohydrometallurgy |
a) Environmentally friendly |
a) Low efficiency |
[59] |
|
|
b) Low energy requirements |
b) Time-consuming process |
|||
|
c)Can use low-cost microorganisms |
c) Requires special conditions for microorganisms to thrive |
|||
|
d)Minimal use of chemical reagent |
d)Not feasible in high toxic environment |
|||
|
e) Achieve high efficiency at low metal concentration |
e) Low efficiency at higher pulp density |
|||
|
f) Less issue of toxic gas generation |
f) Hard process control measures |
|||
|
Mechanochemical |
a) Can be conducted at room temperature, b) minimize energy consumption |
a) May require extensive milling time. |
[60], [61]. |
|
|
Polymer Inclusion Membrane Electrodialysis (PIMED) |
a) high efficiency and purity. |
a) it requires the use of advanced materials and equipment |
[62] |
|
|
b) It avoids the use of chemicals and |
b) high initial investment and operating costs |
|||
|
c) generates little waste |
||||
|
Direct recycling |
a) Practically feasible to recover different components of spent LIBs |
a) Recovered materials may not perform like virgin material |
||
|
b) Active materials can be recovered with original chemical structures |
b) Mixing of cathode material could decrease the quality of the recycled material |
|||
|
c) Energy efficient |
c) Regeneration process is not developed yet |
|||
|
d) Economically feasible |
d) It is remains at laboratory-scale, not applied industrial-scale |
4.6.1 Cost-effective recycling technologies for spent LIBs: A number of technologies are used in the recycling of spent lithium-ion batteries (LIBs), each with a unique cost-effectiveness profile. On the market, the main methods used are hydrometallurgical, pyrometallurgical, and direct recycling techniques [151]. Studies indicate that the most commonly utilized methods are hydrometallurgical processes. They have the potential to be an economically feasible industrial application due to their high recycling efficiency, selectivity, and low energy consumption [152]. The recycling rate of spent LIBs, however, falls short of market growth due to the complexity of these technologies and their use [151]. Interestingly, new technologies such as bioleaching and redox flow systems for desalination combined with lithium recovery are being investigated, despite hydrometallurgical procedures being recognized for their potential cost-effectiveness [102, 153]. More specifically, a thorough cost study of the redox flow system indicates minimal energy usage and potential revenue production, hence demonstrating excellent economic and environmental benefits [102]. The use of deep eutectic solvents has also been demonstrated to be a viable and reusable recycling technique that could increase cost-effectiveness [154]. Although hydrometallurgical procedures are currently the most common and potentially economical way to recycle spent LIBs, novel technologies show promise in terms of their potential to improve both the environment and the economy. Innovative techniques that can further increase the cost-effectiveness of LIB recycling include redox flow systems and the use of deep eutectic solvents [102, 152, 154]. It is important to continue exploring and optimizing these technologies to enhance the recycling rate and cost efficiency of spent LIBs.
4.6.2 Scalability of recycling technologies for spent LIBs: The commercial viability of recycling techniques for spent lithium-ion batteries (LIBs) is influenced by various factors, including economic feasibility, environmental impact, and efficiency. Hydrometallurgical processes have gained widespread acceptance at the industrial level because of their notable advantages, including high recycling efficiency, superior selectivity towards metals, minimal energy consumption, and reduced capital expenditure [152]. At the industrial level, pyrometallurgical options have also been applied, with thermal pretreatment techniques being employed to recover active cathode materials [74] in companies such as Recupyl, Retriev Process, Accurec Process, and the Umicore Process, which combines both pyrometallurgy and hydrometallurgy processes [13]. These methods are commercially available due to their economic viability and ability to recover important metals. However, even though they have the potential to be sustainable, several innovative recycling technologies, such as green leaching and direct recycling, have not seen much industrial use. Because process optimization is difficult and high-purity input materials are needed, direct recycling, which attempts to maintain the structure of the cathode material, is still primarily performed at the laboratory or pilot scale [155]. Although organic acid-based "green leaching" technologies are more environmentally friendly than conventional techniques, they have limitations in terms of economic viability and scalability [156]. While pyrometallurgical and hydrometallurgical processes have been successfully scaled up for commercial use, developing technologies such as green leaching and direct recycling are still in the early stages of development, mainly because of technical and financial obstacles. Ensuring that these technologies are both commercially and environmentally feasible requires addressing these obstacles before they can be scaled up [74, 152, 155, 156].
4.6.3 Environmental sustainability of recycling technologies for spent LIBs: There are two common methods for recycling used lithium-ion batteries (LIBs): hydrometallurgy and pyrometallurgy. Each process has particular environmental consequences. In contrast to pyrometallurgy, hydrometallurgy is thought to be more environmentally benign since it uses chemical leaching to remove metals, which is well-known for its effectiveness in purifying and separating metals [157, 158]. Nonetheless, disposing of wastewater presents certain difficulties [158]. On the other hand, pyrometallurgy uses a lot of energy and produces air pollutants, both of which can harm the environment [158, 159]. A technique that is becoming increasingly popular is known as direct recycling, which can help to maintain the electrochemical properties of battery parts and prevent them from being converted into raw materials. This method has the potential to be a more environmentally friendly option [157, 159]. In order to lower operating costs and energy requirements, a biological process called bioleaching is also being investigated as an environmentally beneficial substitute for traditional techniques [129]. Furthermore, mechanochemical processes which use mechanical energy to trigger chemical reactions are being researched for their potential to offer a recycling option that is more environmentally friendly. [129] while traditional methods such as pyrometallurgy present significant environmental challenges, hydrometallurgy offers a cleaner alternative, albeit with its own drawbacks. Direct recycling, bioleaching, and mechanochemical methods show promise in terms of environmental sustainability, with the potential to minimize the ecological footprint of spent LIB recycling. These innovative approaches align with the principles of a circular economy and carbon neutrality, contributing to the development of green recycling technologies [129, 159]. Deep eutectic solvents (DESs) have emerged as promising green alternatives for recycling spent lithium-ion batteries (LIBs), thus promoting environmental sustainability. DESs are known for their low toxicity, biodegradability, and high efficiency in extracting valuable metals from spent LIBs. Several studies [32, 95, 160-163] have highlighted the potential of DESs in this regard [164]. The utilization of DESs aligns with the principles of green chemistry, offering a more environmentally friendly approach than traditional metallurgical methods. Furthermore, the ability to recover and regenerate DESs further enhances their environmental friendliness. Molten salt roasting is another technique that shows promise in spent LIB recycling, although specific details regarding its environmental impact are lacking. Nevertheless, these methods aim to improve recycling efficiency while minimizing environmental repercussions. Redox flow desalination, as demonstrated in Shan et al., [102] not only recovers lithium from spent LIBs but also addresses water purification. This dual functionality can contribute to sustainable development by reducing the environmental footprint of both battery recycling and water desalination processes. The method described in Shan et al. stands out for its low energy consumption and minimal greenhouse gas emissions, which are crucial factors in environmental sustainability. Deep eutectic solvents have gained recognition for their positive environmental impact in the realm of spent lithium-ion battery (LIB) recycling. They signify a shift towards more sustainable practices within the industry, holding the potential to decrease pollution and resource wastage. While molten salt roasting has been identified as an emerging technique, further clarification is needed regarding its specific environmental benefits. On the other hand, redox flow desalination is a unique approach that integrates lithium recovery with water purification, offering a comprehensive solution with significant environmental advantages. Together, these methods contribute to the overarching objective of establishing a circular economy and ecological civilization by fostering the green and sustainable advancement of battery recycling technologies [102, 154] as the demand for lithium-ion batteries continues to rise, it is crucial to prioritize sustainable and eco-friendly methods for their recycling. By embracing innovative techniques such as direct recycling, bioleaching, and mechanochemical methods, we can not only reduce the environmental impact of spent LIBs but also move towards a more circular economy model. These approaches not only help in minimizing waste but also contribute to the overall goal of achieving carbon neutrality in the recycling process. By investing in green recycling technologies, we can pave the way for a more sustainable future for the battery industry and beyond.
5. Performance of current recycling methods for the recovery of Li and Co
After a comprehensive analysis of the publication trend over the last 14 years (2010-2024) using a scientific database (Elsevier-ScienceDirect) (Supplementary Figure 1), this review confirms what can be found in the literature, namely, that hydrometallurgy, pyrometallurgy, and direct recycling are the most researched and commonly used extraction methods for recycling spent LIBs spanning 14 years (Figure1a.), with hydrometallurgy being the most common extraction method in the literature reviewed. Leaching, a hydrometallurgical method was found to be the most commonly used recycling method for spent LIBs, especially for the recovery of lithium and cobalt. In addition to hydrometallurgy, pyrometallurgy, and direct recycling, deep eutectic solvents and bioleaching are receiving much attention as emerging environmentally friendly methods, indicating researchers’ efforts towards developing sustainable and eco-friendly recycling technology for the recycling of spent LIBs. Further analysis of the efficiency of these extraction methods in the recovery of Li and Co, as shown in Figure 1b, revealed that mechanochemical methods are more efficient in the recovery of Li and Co, even though they are not the most commonly used methods in the literature, as they boast average recovery rates of almost 100% and 96.36%, respectively, based on the data analysed in this review. The mechanochemical process is also a viable option. However, due to its lengthy reaction period and high energy wasted as heat, which results in high industrial costs, this process might have led to its disadvantage, as researchers have pushed towards more sustainable and cost-effective recycling methods. After mechanochemical treatment, hydrometallurgy is still one of the most efficient methods for recovering lithium and cobalt, with average recovery rates of 89.38% and 88.49%, respectively. It is obviously one of the most common recycling methods for lithium and cobalt due to its simple process and low cost. Pyrometallurgy may not boast a high recovery rate for both Li and Co, but it is one of the extraction methods that has been scaled commercially and has seen major improvements over the years by companies such as Recupyl, Retriev Process, Accurec Process, and the Umicore Process, which combines both pyrometallurgy and hydrometallurgy processes [13]. Recently, biohydrometallurgy (bioleaching) has improved, with average recovery rates of 85.06% and 65.2% for Li and Co, respectively (Figure 3b). In their review of bioleaching, Biswal and Balasubramanian confirmed that both bacterial and fungal leaching are effective for metal dissolution from spent LIBs with a higher dissolution rate of Li than Co (Figure 2) [36]. In comparing the average recovery rates of cobalt and lithium across the different extraction methods, cobalt showed higher rates in the pyrometallurgy, hydrometallurgy, and mechanochemical methods, while lithium had higher rates in the biohydrometallurgy and electrochemical methods.
As shown in Figure 1c, leaching is the most commonly used hydrometallurgy method for lithium and cobalt recovery, with average recovery rates of 98.62% and 95.38%, respectively, based on the data analysed in this review. Deep eutectic solvents (DESs) are emerging as recycling methods with efficient recovery rates for both Li (89.22%) and Co (85.7%). The figure shows that the DES and leaching methods are slightly more effective for Li recovery than for Co recovery, and the precipitation is almost the same for both elements. Although leaching has proven to be the most effective method for extracting cobalt, it is important to note that this method also has disadvantages because it can be quite expensive, and the use of strong acids or strong alkaline agents can lead to harmful gas emissions and environmental pollution [63]. Nonetheless, due to its high recovery rate and efficiency, leaching has remained a popular choice for both cobalt and lithium extraction from spent LIBs.
The results of this study highlight the importance of selecting the appropriate extraction method for cobalt recovery. However, acid leaching, with its high recovery rate, may be the preferred method for industries that rely heavily on cobalt. However, it is not fully environmentally friendly because it emits some harmful gases. On the other hand, the pyrometallurgical process may be more suitable for applications where cost is a primary concern. By understanding the strengths and weaknesses of each extraction method, industries can make informed decisions that optimize their cobalt recovery rates.
Figure 1: (a)Comparison of recycling technologies per publications (source; Elsevier-ScienceDirect) (b) Comparison of the average recovery rates of lithium and cobalt from previous studies in the literature; (c) average recovery rates of lithium and cobalt from hydrometallurgical methods from previous studies in the literature
Hydrom-hydrometallurgy, Pyrom-pyrometallurgy, D.recycling-. Direct recycling, Biohydrom-biohydrometalurgy (bioleaching), DES-deep eutectic solvents
5.1 Best Practices for the Sustainable Recovery of Cobalt and Lithium
5.1.1 Recycling of anode materials in S-LIBs: Cathode materials in LIBs consisting of various oxides or phosphates, such as LiCoO2, LiNiO2, LiMn2O4, and LiFePO4, have recently received much attention at the laboratory level and commercially [165, 166]. Previous studies have been performed in the area of recycling spent LIBs to recover lithium and cobalt from cathode materials. There is no doubt that the literature substantiates the fact that cobalt is one of the most researched elements in terms of recovering elements from LIBs. Hydrometallurgy and pyrometallurgy have been the major recovery methods for the extraction of cathode materials in LIBs and have been scaled up commercially by industry [100, 167]. Although these methods have shown high recovery efficiency, they are far from fully sustainable. Moreover, there is little literature supporting the recovery of anode materials from LIBs in contrast to that of cathode materials [168, 169].
Recent statistics indicate that there is a significant demand for graphite anode materials, which is approximately 10-20 times greater than the demand for lithium cathodes [170]. Graphite anodes are highly sought-after substances due to their numerous advantages over other materials. Its exceptional stability and high conductivity (comparable to the 0.4 eV band gap) make it a reliable choice for various applications. Additionally, its lower lithium insertion potential (0.01-0.2 V) and higher theoretical capacity (372 mAh g1) make it a more efficient option than other carbon materials.[171]
A novel technique for converting used graphite from an anode to a cathode for dual-ion batteries has been created [171]. This procedure used a two-step treatment to recover and return the crystal structure and morphology of the spent graphite to a typical layered structure. To stop electrode deterioration and improve cycle performance, an amorphous carbon layer is also included. The performance of recycled graphite is comparable to that of commercial graphite, and recycled graphite has a high reversible capacity. This novel method offers a long-term remedy for recycling exhausted batteries. The demand for graphite anode materials is on the rise, and for good reasons. Its superior properties make it a valuable asset in various industries, and its potential for further development is promising.
5.1.2 Reuse of S-LIBs: The reuse of spent lithium-ion batteries can prolong their lifespan, reduce the demand for new batteries and prevent the total overhauling of spent lithium-ion batteries (S-LIBs). Some of the batteries may have a reasonable capacity and can be utilized in a secondary application after their first usage rather than being disposed of for recycling. There are certain recycled batteries from electric cars that work just as well in grid applications as brand-new lithium batteries but at a lower cost [172-174]. This approach may be applicable to lithium-ion batteries (LIBs) in personal electronic devices (PEDs) that could have a second life in other electrical systems. Given the significant number of discarded PEDs, second-life LIBs may offer valuable capacity for small-scale renewable energy systems, such as solar systems with a capacity of less than 100 Wh. Second-life LIBs may also find use in off-grid low-energy-intensity applications such as charging mobile phones, providing home lighting, serving as flashlights, and powering radios [175].
Energy storage is a crucial aspect of modern life, and lithium-ion batteries have proven to be a reliable and efficient solution. These batteries have sufficient energy storage capacity to be used for off-grid energy storage applications even after they are no longer appropriate for use in electronic equipment. This makes them an ideal choice for battery backup systems for solar panels and wind turbines. Another popular way to repurpose lithium-ion batteries is by turning them into portable power banks. These power banks can recharge mobile phones, laptops, tablets, and other small devices, making them convenient and cost-effective solutions for people on the go. To reduce the cost of buying a new battery for e-bike power tools such as drill machines, saws, and sanders, lithium-ion batteries can be converted into electric bike batteries. This solution is a great way to save money while still enjoying the benefits of an electric bike and while still enjoying the convenience of cordless tools.
In two real-world scenarios in Spain, Rallo et al. investigated the costs associated with establishing an energy storage system (SESS) powered by second-life batteries. The results showed that the viability of an SESS depends on an aging model combined with an economic study, as the aging of batteries plays an important role in determining economic results. The energy arbitrage strategy was found to produce greater savings than peak shaving. The study indicated that the best economic return can be obtained by over-dividing the SESS but requires considerable space for installation. Although their results did not show significant economic savings, they suggested that a decrease in the price of batteries could make SESS projects with second-life batteries more attractive to investors and reduce the effective price of electric vehicles [176].
The IEA 2022 report stated that grid storage will play a significant role in net zero emissions by 2050. With a total capacity of approximately 160 GW as of 2021, pumped-storage hydropower is reported to be the most frequently employed large-scale energy storage technology, accounting for more than 8,500 GWh of capacity in 2020, representing more than 90% of global power storage. Nevertheless, it has been projected that lithium-ion batteries will soon take over in the coming years.
R. Xu et al. were able to recycle volatile organic solvents (DMC and DEC) from used electrolytes through vacuum distillation and reuse them for lithium-ion batteries (LIBs) with recovery efficiencies of almost 100% and 79.40%, respectively. The recovered solvents showed a high discharge capacity and good cycling performance for Li/graphite batteries, even after 400 cycles at 1C. Additionally, the lithium remaining in the non-volatile component (EC) was recovered and turned into lithium carbonate, with a recovery efficiency of 86.93%. This process demonstrates the potential for the comprehensive recycling of electrolytes from spent LIBs [177].
5.1.3 Designing LIBs for Better Recoverability: The electric vehicles available on the market come in a variety of physical configurations, cell types, and cell chemistries. It has also been noted that various vehicle manufacturers have chosen different approaches to powering their vehicles. This poses a problem for the recycling of batteries [76]. Different methods for disassembly are needed due to variations in physical configurations. For instance, current batteries used in electric vehicles present differing form factors and capacities that also restrict their reuse. The use of diverse cell chemistries by electric car battery makers, according to Harper et al., requires distinct strategies for material reclamation and has a significant impact on the overall economics of recycling. The cylindrical cells are tightly coiled, which makes it more difficult to separate the electrodes for direct recycling procedures than the flat electrodes of the pouch and prismatic cells [76].
Improving the sustainability of battery recovery can be achieved through the design of easily disassembled and recycled batteries. This can be accomplished by utilizing fewer materials and implementing modular and standardized designs[178], which in turn can enhance the efficiency of the pretreatment process, as the current pretreatment process for S-LIBs is cumbersome and time-consuming. The current process also results in considerable waste. By adopting these measures, we can reduce the environmental impact of battery disposal and promote a more sustainable future.
5.1.4 Enhancing S-LIB recycling through artificial intelligence (AI)/machine learning: Machine learning could support the development of circular economy models for S-LIBs. By analysing usage patterns and end-of-life disposal data, these technologies can identify opportunities for reuse, refurbishment, and recycling [179]. This approach will not only reduce waste but also create new business opportunities and revenue streams. AI-driven predictive maintenance systems may help prevent battery failures and extend lifespans. Furthermore, timely maintenance or replacement of batteries may be possible due to early detection of possible problems caused by real-time battery performance monitoring systems. This will obviously enhance the reliability and minimize the downtime for S-LIB users. Consequently, efficiency, sustainability, and profitability can all be increased by incorporating AI or machine learning into the S-LIB production process. The production of batteries may thus become more environmentally and financially sustainable through the application of best practices, innovative technology, and circular economy concepts.
5.1.5 Automation of S-LIB disassembly
Automation is expected to lower costs and may make recycling economically feasible. Robotic battery disassembly is expected to reduce the possibility of injury to human workers. There are several ongoing research studies that are piloting the automation of battery disassembly [180, 181]. Automation has the ability to improve mechanical material and component separation, which increases the efficiency of downstream separation and recycling operations and improves the purity of the separated materials. The automation of dismantling automotive batteries is challenging due to the need for robots to adapt to various objects and handle uncertainty, which is a current frontier in artificial intelligence research. The assembly and waste handling of batteries, including those in mobile phones, are mostly manual and lack automation, posing challenges for disassembly and waste management. On the other hand, the assembly and waste handling of batteries, including those on mobile phones, are mostly manual and lack automation, posing challenges for disassembly and waste management. Progress has been made in automating battery sorting with the Optisort system using computer vision for recognizing labels and pneumatic actuators for segregation, but it is currently limited to AA and AAA batteries[182, 183]. With respect to automated battery disassembly, Apple has a line dedicated to automating the process that disassembles 1.2 million iPhones each 6 s annually, breaking them down into eight components with the help of 29 robots. The process includes removing the LIB with thermal protection due to fire hazards. The current Apple disassembly line is inflexible for new phone models because it only addresses the iPhone 6 design [184]. A flexible and adaptable robot disassembly line can be cost-effective by focusing on control algorithms, software, and advanced sensors for intelligent robot behavior.
State-of-the-art robotics, computer vision, and AI technologies [185] are now being adapted for robotic battery disassembly, facing challenges such as identifying materials, forceful interactions, and grasping deformable materials. Computer-vision algorithms are being developed to identify diverse waste materials[186], track objects in cluttered scenes [187], and guide robot arms dynamically[188-190]. Challenges in robotic battery disassembly include forceful interactions, grasping deformable materials, and autonomous grasp planning. Recent advancements show promise in addressing the complex research challenge of automated battery disassembly.
5.1.6 Implementation of Circular Economy Principles for S-LIB Recovery: Furthermore, implementing circular economy principles in S-LIB recovery can also help reduce the reliance on mining for raw materials, which can have negative environmental and social impacts. By reusing and repurposing recovered materials, the demand for new raw resources can be reduced, leading to a more sustainable and responsible approach to battery production. In addition, circular economy principles can also create new business opportunities and revenue streams for companies involved in battery production. For example, repurposing recovered materials from used batteries could lead to the development of new products or services that meet market demand. This could comprise the manufacture of new energy storage solutions or the development of innovative recycling technologies. Overall, adopting circular economy principles in S-LIB recovery is essential for promoting a more sustainable and responsible approach to battery production. By reducing waste, reusing materials, and developing secondary applications for used batteries, we can create a more eco-friendly and economically viable industry that benefits both people and the planet.
6. Summary
Hydrometallurgical operations, which include leaching, purifying, and precipitation techniques, are traditional recycling methods for extracting lithium and cobalt from spent lithium-ion batteries (LIBs). These methods are favoured over pyrometallurgical processes due to their lower energy consumption and potential for higher selectivity and recovery rates [191]. Specifically, the use of organic acids in leaching has been highlighted as an eco-friendly alternative to inorganic acids, with oxalic acid-based recycling showing promise in terms of environmental benignity and economic viability [192-194]. Additionally, closed-loop processes have been developed to enhance the sustainability of recycling methods by minimizing waste and reusing leaching agents, which also contributes to cost reduction [195]. Contradictions arise in the efficiency and eco-friendliness of these methods. While hydrometallurgical processes are generally more environmentally friendly, the disposal of wastewater remains a challenge [158]. Moreover, the efficiency of metal recovery varies, with some methods achieving over 98% recovery for nickel, cobalt, and copper [195], while others focus on the recovery of cobalt with high purity levels suitable for battery fabrication [192]. The cost-effectiveness of these methods is influenced by the recovery rates of valuable metals and the reuse of solvents. In general, the traditional recycling methods for obtaining lithium and cobalt from spent LIBs are predominantly hydrometallurgical, with a focus on organic acid leaching because of its eco-friendly properties. The efficiency of these methods is generally high, with some achieving significant recovery rates of valuable metals. Cost-effectiveness is enhanced through closed-loop processes and the reuse of solvents. However, the environmental impact of wastewater disposal remains a concern that must be addressed to improve the overall sustainability of these recycling methods [154, 158, 191-195] Bioleaching, also known as biometallurgy, is increasingly recognized as a sustainable and environmentally friendly approach for recycling spent lithium-ion batteries (LIBs). This method leverages microorganisms to selectively leach valuable metals from waste, offering a green alternative to conventional pyrometallurgical and hydrometallurgical processes, which are often associated with environmental concerns due to their energy-intensive nature and generation of secondary waste [36, 129, 196]. Interestingly, studies have shown that bioleaching can achieve high recovery rates of metals such as lithium (Li), cobalt (Co), nickel (Ni), and manganese (Mn) from spent LIBs. The process is cost-effective and reduces operating costs and energy demands compared to traditional methods. Moreover, bioleaching is adaptable to various operational scales and can be integrated into a closed-loop recycling system, aligning with the principles of the circular economy [36, 129, 197]. However, there are challenges to be addressed, such as the optimization of operational parameters and the scaling up of the process for industrial applications [153, 196]. Bioleaching presents a promising avenue for recycling spent LIBs, contributing to sustainability and environmental protection. This finding aligns with the growing need for eco-friendly recycling technologies that minimize ecological footprints and support resource conservation. As research progresses, it is expected that bioleaching will become more efficient and widely adopted, fostering a more sustainable approach to managing the lifecycle of LIBs [36, 129]
Current literature suggests that emerging recycling techniques such as deep eutectic solvents, molten salt roasting, and direct regeneration hold promise for improving recycling efficiency while reducing environmental impact [160]. The "green" approach, which encompasses the "3 L" criteria—less energy consumption, less greenhouse gas emissions, and less operational cost—is also advocated to achieve these objectives [179] and is included in the 4H battery recycling strategy with the objectives of high safety, high economic return, high efficiency, and high environmental benefit [198]. In lieu of conventional solvents, deep eutectic solvents (DESs) have shown great promise in the recycling of spent lithium-ion batteries (LIBs), providing a more environmentally friendly and sustainable method. The use of DESs in the recycling process aligns with the principles of green chemistry, as they are typically biodegradable, nontoxic, and operate under mild conditions, which reduces energy consumption and minimizes the generation of hazardous waste [154, 162, 163, 199-201]. Interestingly, DESs have been shown to achieve high leaching efficiencies for valuable metals such as lithium and cobalt, which are critical for the production of new batteries. This not only helps mitigate resource scarcity but also reduces the environmental impact associated with mining and processing virgin materials. Moreover, the ability to regenerate and reuse DESs further enhances the sustainability of the recycling process, as demonstrated by several studies where DESs were successfully recycled for multiple cycles without significant loss of efficiency [154, 163, 199, 202]. The application of DESs in the recycling of spent LIBs represents a significant advancement toward sustainable and environmentally friendly practices. The high recovery rates of valuable metals, coupled with the potential for solvent regeneration and reuse, contribute to the reduction of environmental pollution and resource depletion, thereby supporting the transition to a more circular economy in the battery industry [154, 162, 163, 199-203]. Molten salt roasting can also improve the efficiency of metal recovery from spent LIBs, and its impact on sustainability, cost-effectiveness and environmental friendliness in terms of alleviating resource scarcity by recovering valuable materials has been recognized. By converting refractory metal oxides into salt solutions that dissolve in water, this method degrades the cathode powder structure faster at lower temperatures and increases the recovery rate of precious metals. Polymer inclusion membranes (PIMs) are a stable and versatile technology that selectively transport target species across a membrane, offering benefits such as self-support and resistance to fouling. They have many applications, including separating metal ions, organic compounds, and gases. Recent studies have shown that combining PIM with electrodialysis (ED) can greatly enhance permeability, increasing the efficiency of the process. By using specific components in PIMs, such as Aliquat 336, high purity levels can be achieved in metal ion extraction processes. The best practices for the future of spent lithium-ion batteries (LIBs) should focus on enhancing sustainability, efficiency, cost-effectiveness, and environmental friendliness, as with continuous improvements in the emerging recycling techniques reviewed, and establishing a multimodal closed-loop recycling roadmap with regulation, AI-assisted pretreatment, and targeted recycling is emphasized as a strategic direction [179]. Additionally, the integration of recycling with other processes, such as redox flow desalination, can offer additional economic and environmental benefits [102]. On the other hand, the future sustainability of spent lithium-ion batteries should include the development and design of lithium-ion batteries for better recoverability in the standardization of LIB designs for easy disassembly for efficient pretreatment of spent LIBs, as well as the automation of disassembly and improvement of the overall recycling of spent LIBS through machine learning to improve the mechanical separation of materials and components, thereby increasing the purity of separated materials and the efficiency of downstream separation and recycling operations, and for a real-time battery performance monitoring system that enables timely maintenance or replacement of batteries. The future of LIB recycling should integrate advanced recycling technologies with comprehensive strategies that prioritize environmental protection and resource efficiency. The adoption of reusable solvents, the implementation of green approaches, and the exploration of innovative integrated systems are key to achieving a sustainable, efficient, and cost-effective recycling ecosystem. The development of policies and regulations that support these practices will be crucial for promoting the green and sustainable development of the battery recycling industry [102, 154, 179].
Declaration of conflicting interests
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Shanxi Provincial Department of Education/Shanxi University under project name “Science and Technology Innovation Base” [grant number: YDZJSX20231B002].
Abbreviations
LIBs- Lithium-ion batteries
SLIB- spent lithium-ion batteries
Electrodialysis of PIM-ED- polymer inclusion membranes
SX- solvent extraction
EV- electric vehicle
NMC- nickel-manganese-cobalt
PVDF- polyvinylidene fluoride
NMP - N-Methyl pyrrolidone
DES- deep eutectic solvents
DMF - Dimethylformamide
DMSO- Dimethyl sulfoxide
DMAC- dimethylacetamide
CAM cathode anode material
OER: oxygen evolution reaction
ORR: oxygen reduction reaction
ICP- Inductively Coupled Plasma
EDTA- ethylenediaminetetraacetic acid
PLS- Pregnant Leach’s solution
Reference
- W Bernhart. ‘24 - The Lithium-Ion Battery Value Chain—Status, Trends and Implications’, in Lithium-Ion Batteries, G. Pistoia, Ed., Amsterdam: Elsevier (2014): 553-565.
- N Nitta, F Wu, J T. Lee, et al. ‘Li-ion battery materials: present and future’, Mater. Today 18 (2015): 252-264.
- Z Wendy. ‘Hydrometallurgical Treatment of Spent Lithium-Ion Batteries: A study of optimal conditions for the leaching’, (2018).
- A Chagnes and B Pospiech, ‘A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries’, J. Chem. Technol. Biotechnol 88 (2013): 1191-1199.
- X Zheng, Z Ahu, X Lin, et al., ‘A mini-review on metal recycling from spent lithium ion batteries’, Engineering 4 (2018): 361-370.
- Y Li, J Yang, and J Song. ‘Design structure model and renewable energy technology for rechargeable battery towards greener and more sustainable electric vehicle’, Renew. Sustain. Energy Rev 74 (2017): 19-25.
- K M Winslow, S J Laux, and T G Townsend. ‘A review on the growing concern and potential management strategies of waste lithium-ion batteries’, Resour. Conserv. Recycl 129 (2018): 263-277.
- A Vezzini. ‘Manufacturers, materials and recycling technologies’, in Lithium-Ion Batteries, Elsevier (2014): 529-551.
- C Ekberg and M Petranikova, ‘Lithium Process Chemistry’ (2015).
- D Larcher and J M Tarascon. ‘Towards greener and more sustainable batteries for electrical energy storage’, Nat. Chem 7 (2015): 19-29.
- L L Gaines and J B Dunn. ‘Lithium-ion battery environmental impacts’, in Lithium-Ion Batteries, Elsevier (2014): 483-508.
- J Y Mo and W Jeon. ‘The impact of electric vehicle demand and battery recycling on price dynamics of lithium-ion battery cathode materials: a vector error correction model (VECM) analysis’, Sustainability 10 (2018): 2870.
- A Sonoc, J Jeswiet, and V K Soo. ‘Opportunities to Improve Recycling of Automotive Lithium Ion Batteries’, Procedia CIRP 29 (2015): 752-757.
- T Georgi-Maschler, B Friedrich, R Weyhe, et al. ‘Development of a recycling process for Li-ion batteries’, J. Power Sources 207 (2012): 173-182.
- L Gaines. ‘Lithium-ion battery recycling processes: Research towards a sustainable course’, Sustain. Mater. Technol 17 (2018): e00068.
- F E ELIBAMA, ‘Li-ion batteries recycling - the batteries end of life’ (2015).
- J Xu, H R Thomas, R W Francis, et al. ‘A review of processes and technologies for the recycling of lithium-ion secondary batteries’, J. Power Sources 177 (2008512-527.
- X Zeng, J Li, and N Singh. ‘Recycling of Spent Lithium-Ion Battery: A Critical Review’, Crit. Rev. Environ. Sci. Technol 44 (2014): 1129-1165.
- X Fan, C Song, X Lu, et al. ‘Separation and recovery of valuable metals from spent lithium-ion batteries via concentrated sulfuric acid leaching and regeneration of LiNi1/3Co1/3Mn1/3O2’, J. Alloys Compd 863 (2021): 158775.
- A Verma, G H Johnson, D R Corbin, et al. ‘Separation of Lithium and Cobalt from LiCoO2: A Unique Critical Metals Recovery Process Utilizing Oxalate Chemistry’, ACS Sustain. Chem. Eng 8 (2020): 6100-6108.
- L Yun,D Linh, L Shui, et al. ‘Metallurgical and mechanical methods for recycling of lithium-ion battery pack for electric vehicles’, Resour. Conserv. Recycl 136 (2018): 198-208.
- Y Wang, A Ning, L Wen, et al. ‘Recent progress on the recycling technology of Li-ion batteries’, J. Energy Chem 55 (2021): 391-419.
- L Li, J B Dunn, X X Zhang, et al. ‘Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment’, J. Power Sources 233 (2013): 180-189.
- G P Nayaka, K V Pai, J Manjanna, et al. ‘Use of mild organic acid reagents to recover the Co and Li from spent Li-ion batteries’, Waste Manag 51 (2016): 234-238.
- Y Yao, M Zhu, Z Zhao, et al. ‘Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review’, ACS Sustain. Chem. Eng 6 (2018): 13611-13627.
- D Gong, K Zhou, C Peng, et al. ‘Sequential extraction of tungsten from scheelite through roasting and alkaline leaching’, Miner. Eng 132 (2019): 238-244.
- F Pena-Pereira and J Namiesnik. ‘Ionic Liquids and Deep Eutectic Mixtures: Sustainable Solvents for Extraction Processes’, ChemSusChem 7 (2014): 1784-1800.
- L Shen, X Li, Q Zhou, et al. ‘Sustainable and efficient leaching of tungsten in ammoniacal ammonium carbonate solution from the sulfuric acid converted product of scheelite’, J. Clean. Prod 197 (2018): 690-698.
- L Shen, X Li, D Lindberg, et al. ‘Tungsten extractive metallurgy: A review of processes and their challenges for sustainability’, Miner. Eng 142 (2019): 105934.
- Bensheng Li, Qingzhu Li, Qingwei W, et al. ‘Deep eutectic solvent for spent lithium-ion battery recycling: comparison with inorganic acid leaching’, Phys. Chem. Chem. Phys (2022).
- Fengyu Huang, Taibai L, X Yan, et al. ‘Ternary Deep Eutectic Solvent (DES) with a Regulated Rate-Determining Step for Efficient Recycling of Lithium Cobalt Oxide.’, ACS Omega 7 (2022): 11452-11459.
- M Jafari, Sied Ziaedin Shafaie, H Abdollahi, et al. ‘A Green Approach for Selective Ionometallurgical Separation of Lithium from Spent Li-Ion Batteries by Deep Eutectic Solvent (DES): Process Optimization and Kinetics Modeling’, Miner. Process. Extr. Metall. Rev (2022): 1-13.
- Mengshun Liu, W Ma, X Zhang, et al. ‘Recycling lithium and cobalt from LIBs using microwave-assisted deep eutectic solvent leaching technology at low-temperature’, Mater. Chem. Phys 289 (2022): 126466-126466.
- Taibai Li, Y Xiong, X Yan, et al. ‘Closed-loop cobalt recycling from spent lithium-ion batteries based on a deep eutectic solvent (DES) with easy solvent recovery’, J. Energy Chem 72 (2022): 532-538.
- Yurun Tian, W Chen, B Zhang, et al. ‘A Weak Acidic and Strong Coordinated Deep Eutectic Solvent for Recycling of Cathode from Spent Lithium-Ion Batteries’, Chemsuschem 15 (2022):
- B K Biswal and R Balasubramanian. ‘Recovery of valuable metals from spent lithium-ion batteries using microbial agents for bioleaching: a review’, Front. Microbiol 14 (2023): 1197081.
- J Mao, C Ye, S Zhang, et al. ‘Toward practical lithium-ion battery recycling: adding value, tackling circularity and recycling-oriented design’, Energy Environ. Sci 15 (2022): 2732-2752.
- L Gaines and R Cuenca. ‘Costs of lithium-ion batteries for vehicles’, Argonne National Lab., IL (US) (2000).
- ‘Cobalt facts - Properties, Cobalt Development’ (2024).
- ‘Mineral Commodity Summaries-Cobalt’, Professional Paper (2022).
- K B Shedd. ‘COBALT -U.S. Geological Survey, Mineral Commodity Summaries (2020).
- ‘Mineral Commodity Summaries-Lithium’, Professional Paper (2022).
- M Kaya. ‘State-of-the-art lithium-ion battery recycling technologies’, Circ. Econ 1 (2022): 100015.
- J Guan, Y Li, Y Guo, et al. ‘Mechanochemical Process Enhanced Cobalt and Lithium Recycling from Wasted Lithium-Ion Batteries’, ACS Sustain. Chem. Eng 5 (2017): 1026-1032.
- T Suzuki, T Nakamura, Y Inoue, et al. ‘A hydrometallurgical process for the separation of aluminum, cobalt, copper and lithium in acidic sulfate media’, Sep. Purif. Technol 98 (2012): 396-401.
- M Xue, J Li, and Z Xu. ‘Environmental Friendly Crush-Magnetic Separation Technology for Recycling Metal-Plated Plastics from End-of-Life Vehicles’, Environ. Sci. Technol 46 (2012): 2661-2667.
- H Ku, Y Jung, M Jo, et al. ‘Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching’, J. Hazard. Mater 313 (2016): 138-146.
- J Li, G Wang, and Z Xu. ‘Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries’, J. Hazard. Mater 302 (2016): 97-104.
- J-Y Wang, N Banthia, and M-H Zhang. ‘Effect of shrinkage reducing admixture on flexural behaviors of fiber reinforced cementitious composites’, Cem. Concr. Compos 34 (2012): 443-450.
- J Li, G Wang, and Z Xu. ‘Generation and detection of metal ions and volatile organic compounds (VOCs) emissions from the pretreatment processes for recycling spent lithium-ion batteries’, Waste Manag 52 (2016): 221-227.
- J Kang, G Senanayake, J Sohn, et al. ‘Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272’, Hydrometallurgy 100 (2010): 168-171.
- S Kim, D Yang, K Rhee, et al. ‘Recycling process of spent battery modules in used hybrid electric vehicles using physical/chemical treatments’, Res. Chem. Intermed 40 (2014): 2447-2456.
- G Chen, L Yan, H Luo, et al. ‘Nanoscale Engineering of Heterostructured Anode Materials for Boosting Lithium-Ion Storage’, Adv. Mater 28 (2016): 7580-7602.
- T Zhang, Y He, L Ge, et al. ‘Characteristics of wet and dry crushing methods in the recycling process of spent lithium-ion batteries’, J. Power Sources 240 (2013): 766-771.
- M Zhou, S Liaw, Q Sun, et al. ‘Pretreatment, Recycling, and Regeneration Strategies of Cathode Active Materials from Spent Lithium Ion Batteries’, J. Mater. Sci. Eng 10 (2021): 1-9.
- H Bi, H Zhu, H Li, et al. ‘Low-temperature thermal pretreatment process for recycling inner core of spent lithium iron phosphate batteries’, Waste Manag 39 (2021): 146-155.
- P Afreh, A Sidhoum, G Lizhen, et al. ‘Optimization of High-Temperature Thermal Pretreatment Conditions for Maximum Enrichment of Lithium and Cobalt from Spent Lithium-Ion Polymer Batteries’. Rochester, NY 26 (2024): 4773920.
- S Fröhlich and D Sewing. ‘The BATENUS process for recycling mixed battery waste’, J. Power Sources 57 (1995): 27-30.
- H Pinegar and Y R Smith. ‘Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes’, J. Sustain. Metall 5 (2019): 402-416.
- S Al-Thyabat, T Nakamura, E Shibata, et al. ‘Adaptation of minerals processing operations for lithium-ion (LiBs) and nickel metal hydride (NiMH) batteries recycling: Critical review’, Miner. Eng 45 (2013): 4-17.
- M K Jha, A Kumari, A K Jha, et al. ‘Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone’, Waste Manag 33 (2013): 1890-1897.
- L Chen, X Tang, Y Zhang, et al. ‘Process for the recovery of cobalt oxalate from spent lithium-ion batteries’, Hydrometallurgy 108 (2011): 80-86.
- L Yao, Y Feng, and G Xi. ‘A new method for the synthesis of LiNi1/3Co1/3Mn1/3O2 from waste lithium ion batteries’, RSC Adv 5 (2015): 44107-44114.
- L Li, J Lu, Y Ren, et al. ‘Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries’, J. Power Sources 218 (2012): 21-27.
- W Wang, Y Zhang, X Liu, et al. ‘A Simplified Process for Recovery of Li and Co from Spent LiCoO2 Cathode Using Al Foil As the in Situ Reductant’, ACS Sustain. Chem. Eng 7 (2019): 12222-12230.
- S Aktas, D J Fray, O Burheim, et al. ‘Recovery of metallic values from spent Li ion secondary batteries’, Miner. Process. Extr. Metall 115 (2006): 95-100.
- Y Zhang, W Wang, J Hu, et al. ‘Stepwise Recovery of Valuable Metals from Spent Lithium Ion Batteries by Controllable Reduction and Selective Leaching and Precipitation’, ACS Sustain. Chem. Eng 8 (2020): 15496-15506.
- H Bae and Y Kim. ‘Technologies of lithium recycling from waste lithium ion batteries: a review’, Mater. Adv 2 (2021): 3234-3250.
- H Wang, K Huang, Y Zhang, et al. ‘Recovery of Lithium, Nickel, and Cobalt from Spent Lithium-Ion Battery Powders by Selective Ammonia Leaching and an Adsorption Separation System’, ACS Sustain. Chem. Eng 5 (2017): 11489-11495.
- A Umeno, Y Miyai, N Takagi, et al. ‘Preparation and Adsorptive Properties of Membrane-Type Adsorbents for Lithium Recovery from Seawater’, Ind. Eng. Chem. Res 41 (2002): 4281-4287.
- J Xiao, X Nie, S Sun, et al. ‘Lithium ion adsorption-desorption properties on spinel Li4Mn5O12 and pH-dependent ion-exchange model’, Adv. Powder Technol 26 (2015): 589-594.
- Y Yang, S Song, F Jiang, et al. ‘Short process for regenerating Mn-rich cathode material with high voltage from mixed-type spent cathode materials via a facile approach’, J. Clean. Prod 186 (2018): 123-130.
- L Zhang and Z Xu. ‘A review of current progress of recycling technologies for metals from waste electrical and electronic equipment’, J. Clean. Prod 127 (2016): 19-36.
- B Makuza, Q Tian, X Guo, et al. ‘Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review’, J. Power Sources 491 (2021): 229622.
- J Cui and L Zhang. ‘Metallurgical recovery of metals from electronic waste: A review’, J. Hazard. Mater 158 (2008): 228-256.
- G Harper, R Sommerville, E Kendrick, et al. ‘Recycling lithium-ion batteries from electric vehicles’, Nature 575 (2019): 7781.
- J Mao, J Li, and Z Xu. ‘Coupling reactions and collapsing model in the roasting process of recycling metals from LiCoO2 batteries’, J. Clean. Prod 205 (2018): 923-929.
- Y Fu, Y He, Y Yang, et al. ‘Microwave reduction enhanced leaching of valuable metals from spent lithium-ion batteries’, J. Alloys Compd 832 (2020): 154920.
- J-W Choi, C-W Cho, and Y-S Yun. ‘Organic acid-based linear free energy relationship models for green leaching of strategic metals from spent lithium-ion batteries and improvement of leaching performance’, J. Hazard. Mater 423 (2022): 127214.
- N Peeters, K Binnemans, and S Riaño. ‘Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents’, Green Chem 22 (2020): 4210-4221.
- A P Abbott, G Capper, D L Davieset al. ‘Novel solvent properties of choline chloride/urea mixtures’, Chem. Commun 1 (2003): 70-71.
- A P Abbott, D Boothby, G Capper, et al. ‘Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids’, J. Am. Chem. Soc 126 (2004): 9142-9147.
- A P Abbott, S S Alabdullah, A Y Al-Murshedi, et al. ‘Brønsted acidity in deep eutectic solvents and ionic liquids’, Faraday Discuss 206 (2018): 365-377.
- M K Tran, M-T F Rodrigues, K Kato, et al. ‘Deep eutectic solvents for cathode recycling of Li-ion batteries’, Nat. Energy 4 (2019): 339-345.
- B B Hansen, S Spittle, B Chen, et al. ‘Deep Eutectic Solvents: A Review of Fundamentals and Applications’, Chem. Rev 121 (2021): 1232-1285.
- J Wu, Q Liang, X Yu, et al. ‘Deep eutectic solvents for boosting electrochemical energy storage and conversion: a review and perspective’, Adv. Funct. Mater 31 (2021): 2011102.
- M Joulié, R Laucournet, and E Billy. ‘Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries’, J. Power Sources 247 (2014): 551-555.
- C K Lee and K-I Rhee. ‘Reductive leaching of cathodic active materials from lithium ion battery wastes’, Hydrometallurgy 68 (2003): 5-10.
- P Zhang, T Yokoyama, O Itabashi, et al. ‘Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries’, Hydrometallurgy 47 (1998): 259-271.
- D L Thompson, I M Pateli, C Lei, et al. ‘Separation of nickel from cobalt and manganese in lithium ion batteries using deep eutectic solvents’, Green Chem 24 (2022): 4877-4886.
- A K Dwamena. ‘Recent advances in hydrophobic deep eutectic solvents for extraction’, Separations 6 (2019): 9.
- L I Tomé, V Baião, W da Silva, et al. ‘Deep eutectic solvents for the production and application of new materials’, Appl. Mater. Today 10 (2018): 30-50.
- Z Yuan, H Liu, W F Yong, et al. ‘Status and advances of deep eutectic solvents for metal separation and recovery’, Green Chem 24 (2022): 1895-1929.
- G Inman, I C Nlebedim, and D Prodius. ‘Application of ionic liquids for the recycling and recovery of technologically critical and valuable metals’, Energies15 (2022): 628.
- C Padwal, S Jadhav, J Nerkar, et al. ‘Deep Eutectic Solvents: Green Approach for Cathode Recycling of Li-Ion Batteries’, Adv. Energy Sustain. Res 3 (2022): 2100133.
- M Fan, X Chang, Q Meng, et al. ‘Progress in the sustainable recycling of spent lithium-ion batteries’, SusMat 1 (2021): 241-254.
- K Du, E H Ang, X Wu, et al. ‘Progresses in sustainable recycling technology of spent lithium-ion batteries’, Energy Environ. Mater., vol. 5, no. 4, pp. 1012-1036, 2022.
- Fan et al., ‘Selective Recovery of Li and Fe from Spent Lithium-Ion Batteries by an Environmentally Friendly Mechanochemical Approach’, ACS Sustain. Chem. Eng 6 (2018): 11029-11035.
- J Lin, L Li, E Fan, et al. ‘Conversion Mechanisms of Selective Extraction of Lithium from Spent Lithium-Ion Batteries by Sulfation Roasting’, ACS Appl. Mater. Interfaces 12 (2020): 18482-18489.
- M Zhou, B Li, J Li, et al. ‘Pyrometallurgical Technology in the Recycling of a Spent Lithium Ion Battery: Evolution and the Challenge’, ACS EST Eng 1 (2021): 1369-1382.
- Z Zhou, Y Lai, Q Peng, et al. ‘Comparative Life Cycle Assessment of Merging Recycling Methods for Spent Lithium Ion Batteries’, Energies 14 (2021): 14196263.
- W Shan, Y Zi, H Chen, et al. ‘Coupling redox flow desalination with lithium recovery from spent lithium-ion batteries’, Water Res 252 (2024): 121205.
- Q Dai, J Spangenberger, S Ahmed, et al. ‘EverBatt: A closed-loop battery recycling cost and environmental impacts model’, Argonne National Lab.(ANL), Argonne, IL (United States) (2019).
- M S Peters and K D Timmerhaus. Plant design and economics for chemical engineers, 3rd ed. in McGraw-Hill chemical engineering series. McGraw-Hill (1980).
- P Xu, Q Dai, H Gao, et al. ‘Efficient Direct Recycling of Lithium-Ion Battery Cathodes by Targeted Healing’, Joule 4 (2020): 2609-2626.
- X Li, F Zhou, S Gao, et al. ‘NaOH-assisted low-temperature roasting to recover spent LiFePO4 batteries’, Waste Manag 153 (2022): 347-354.
- Z Li, L He, Y Zhu, et al. ‘A Green and Cost-Effective Method for Production of LiOH from Spent LiFePO4’, ACS Sustain. Chem. Eng 8 (2020): 15915-15926.
- H Mahandra and A Ghahreman. ‘A sustainable process for selective recovery of lithium as lithium phosphate from spent LiFePO4 batteries’, Resour. Conserv. Recycl 175 (2021): 105883.
- X Qiu, B Zhang, Y Xu, et al. ‘Enabling the sustainable recycling of LiFePO4 from spent lithium-ion batteries’, Green Chem 24 (2022): 2506-2515.
- C Xu, X Hu, Y Yang, et al. ‘Integrated process of CO2 sequestration and recycling spent LiFePO4 batteries’, Energy Storage Mater 60 (2023): 102819.
- X-H Yue and F-S. Zhang. ‘Recycling spent LiFePO4 battery for fabricating visible-light photocatalyst with adsorption-photocatalytic synergistic performance and simultaneous recovery of lithium and phosphorus’, Chem. Eng. J 450 (2022): 138388.
- J Zhang, J Hu, Y Liu, et al. ‘Sustainable and Facile Method for the Selective Recovery of Lithium from Cathode Scrap of Spent LiFePO4 Batteries’, ACS Sustain. Chem. Eng 7 (2019): 5626-5631.
- J Zhang, W Hu, J Zou, et al. ‘Directional High-Value Regeneration of Lithium, Iron, and Phosphorus from Spent Lithium Iron Phosphate Batteries’, ACS Sustain. Chem. Eng 10 (2022): 13424-13434.
- A Heydarian, S Mousavi, F Vakilchap, et al. ‘Application of a mixed culture of adapted acidophilic bacteria in two-step bioleaching of spent lithium-ion laptop batteries’, J. Power Sources 378 (2018): 19-30.
- M Villares, A Isildar, A Mendoza Beltran, et al. ‘Applying an ex-ante life cycle perspective to metal recovery from e-waste using bioleaching’, J. Clean. Prod 129 (2016): 315-328.
- A Orell, C A Navarro, R Arancibia, et al. ‘Life in blue: Copper resistance mechanisms of bacteria and Archaea used in industrial biomining of minerals’, Biotechnol. Adv 28 (2010): 839-848.
- B K Biswal, U U Jadhav, D Patil, et al. ‘Physicochemical and biological methods for treatment of municipal solid waste incineration ash to reduce its potential adverse impacts on groundwater’, in Contaminants of Emerging Concerns and Reigning Removal Technologies, CRC Press (2022): 1-35.
- N B Horeh, S M Mousavi, and S A Shojaosadati. ‘Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger’, J. Power Sources 320 (2016): 257-266.
- P Moazzam, Y Boroumand, P Rabiei, et al. ‘Lithium bioleaching: An emerging approach for the recovery of Li from spent lithium ion batteries’, Chemosphere 277 (2021): 130196.
- M Sethurajan and S Gaydardzhiev. ‘Bioprocessing of spent lithium ion batteries for critical metals recovery - A review’, Resour. Conserv. Recycl 165 (2021): 105225.
- S Ghassa, A Farzanegan, M Gharabaghi, et al. ‘Novel bioleaching of waste lithium ion batteries by mixed moderate thermophilic microorganisms, using iron scrap as energy source and reducing agent’, Hydrometallurgy 197 (2020): 105465.
- F Noruzi, N Nasirpour, F Vakilchap, et al. ‘Complete bioleaching of Co and Ni from spent batteries by a novel silver ion catalyzed process’, Appl. Microbiol. Biotechnol 106 (2022): 5301-5316.
- A Hariyadi, U Sholikah, B Gotama, et al. ‘Biohydrometallurgy for cobalt recovery from spent li-ion batteries using acidophilic bacteria isolated from acid mine drainage’, Chem J Tek Kim 9 (2022): 88-96.
- R A Putra, I A Fajri, and A Hariyadi. ‘Metal Bioleaching of Used Lithium-Ion Battery Using Acidophilic ferrooxidans Isolated from Acid Mine Drainage’, Key Eng. Mater 937 (2022): 193-200.
- X Liu, H Liu, W Wu, et al. ‘Oxidative Stress Induced by Metal Ions in Bioleaching of LiCoO2 by an Acidophilic Microbial Consortium’, Front. Microbiol., vol. 10 (2020): 3058.
- N Bahaloo-Horeh, S M Mousavi, and M Baniasadi. ‘Use of adapted metal tolerant Aspergillus niger to enhance bioleaching efficiency of valuable metals from spent lithium-ion mobile phone batteries’, J. Clean. Prod 197 (2018): 1546-1557.
- A Isildar, et al. ‘Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE) - A review’, J. Hazard. Mater 362 (2019): 467-481.
- A Lobos, V J Harwood, K M Scott, et al. ‘Tolerance of three fungal species to lithium and cobalt: Implications for bioleaching of spent rechargeable Li-ion batteries’, J. Appl. Microbiol 131 (2021): 743-755.
- J J Roy, B Cao, and S Madhavi. ‘A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach’, Chemosphere 282 (2021): 130944.
- M Wang, K Liu, J Yu, et al. ‘Recycling spent lithium-ion batteries using a mechanochemical approach’, Circ. Econ 1 (2022): 100012.
- S L James, C J Adams, C Bolm, et al. ‘Mechanochemistry: opportunities for new and cleaner synthesis’, Chem. Soc. Rev 41 (2012): 413-447.
- L Takacs. ‘The historical development of mechanochemistry’, Chem. Soc. Rev 42 (2013): 7649-7659.
- X Guo, D Xiang, G Duan, et al. ‘A review of mechanochemistry applications in waste management’, Waste Manag 30 (2010): 4-10.
- Q Tan and J Li. ‘Recycling Metals from Wastes: A Novel Application of Mechanochemistry’, Environ. Sci. Technol 49 (2015): 5849-5861.
- A Nasser and U Mingelgrin. ‘Mechanochemistry: A review of surface reactions and environmental applications’, Appl. Clay Sci 67-68 (2012): 141-150.
- M-M Wang, C-C Zhang, and F-S Zhang. ‘Recycling of spent lithium-ion battery with polyvinyl chloride by mechanochemical process’, Waste Manag 67 (2017): 232-239.
- M Wang, Q Tan, and J Li. ‘Unveiling the Role and Mechanism of Mechanochemical Activation on Lithium Cobalt Oxide Powders from Spent Lithium-Ion Batteries’, Environ. Sci. Technol 52 (2018): 13136-13143.
- M Wang, Q Tan, L Liu, et al. ‘Selective regeneration of lithium from spent lithium-ion batteries using ionic substitution stimulated by mechanochemistry’, J. Clean. Prod 279 (2021): 123612.
- N M Asl, S S Cheah, J Salim, et al. ‘Lithium-liquid battery: harvesting lithium from waste Li-ion batteries and discharging with water’, RSC Adv 2 (2012): 6094-6100.
- M I G S Almeida, R W Cattrall, and S D Kolev. ‘Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs)’, J. Membr. Sci 415-416 (2012): 9-23.
- M R Yaftian, M I G S Almeida, R W Cattrall, et al. ‘Selective extraction of vanadium(V) from sulfate solutions into a polymer inclusion membrane composed of poly(vinylidenefluoride-co-hexafluoropropylene) and Cyphos® IL 101’, J. Membr. Sci 545 (2018): 57-65.
- B Pospiech. ‘Selective recovery of cobalt(II) towards lithium(I) from chloride media by transport across polymer inclusion membrane with triisooctylamine’, Pol. J. Chem. Technol 16 (2014): 15-20.
- A Kaya, C Onac, H K Alpoguz, et al. ‘Removal of Cr(VI) through calixarene based polymer inclusion membrane from chrome plating bath water’, Chem. Eng. J 283 (2016): 141-149.
- Li, Y Liu, B Wang, et al. ‘Insights into the facilitated transport mechanisms of Cr(VI) in ionic liquid-based polymer inclusion membrane - Electrodialysis (PIM-ED) process’, Chem. Eng. J 397 (2020): 125324.
- B Wang, F Liu, F Zhang, et al. ‘Efficient separation and recovery of cobalt(II) and lithium(I) from spent lithium ion batteries (LIBs) by polymer inclusion membrane electrodialysis (PIMED)’, Chem. Eng. J 430 (2022): 132924.
- G Xi, H Xu, and L Yao. ‘Study on preparation of NiCo ferrite using spent lithium-ion and nickel-metal hydride batteries’, Sep. Purif. Technol 145 (2015): 50-55.
- R Golmohammadzadeh, F Faraji, B Jong, et al. ‘Current challenges and future opportunities toward recycling of spent lithium-ion batteries’, Renew. Sustain. Energy Rev 159 (2022): 112202.
- J Dewulf, G V D Vrst, K Denturck, et al. ‘Recycling rechargeable lithium ion batteries: Critical analysis of natural resource savings’, Resour. Conserv. Recycl 54 (2010): 229-234.
- P Mokarian, I Bakhshayeshi, F Taghikhah, et al. ‘The advanced design of bioleaching process for metal recovery: A machine learning approach’, Sep. Purif. Technol 291 (2022): 120919.
- J J Roy, S Rarotra, V Krikstolaityte, et al. ‘Green Recycling Methods to Treat Lithium-Ion Batteries E-Waste: A Circular Approach to Sustainability’, Adv. Mater 34 (2022): 2103346.
- I Belharouak, Y Bai, L Yu, et al. ‘(Invited) Science and Technology Endeavors in Lithium Ion Battery Recycling’, ECS Meet. Abstr MA2023-02 (2023): 80.
- J Wang and Z Guo. ‘Hydrometallurgically Recycling Spent Lithium-Ion Batteries’, in Recycling of Spent Lithium-Ion Batteries: Processing Methods and Environmental Impacts, L. An, Ed., Cham: Springer International Publishing (2019): 27-55.
- J Peng. ‘Environment impacts and recycling methods of spent lithium-ion batteries’, Appl. Comput. Eng 23 (2023): 16-24.
- H Li, N Chen, W Liu, et al. ‘A reusable deep eutectic solvent for the regeneration of Li and Co metals from spent lithium-ion batteries’, J. Alloys Compd 966 (2023): 171517.
- B He, H Zheng, K Tang, et al. ‘A Comprehensive Review of Lithium-Ion Battery (LiB) Recycling Technologies and Industrial Market Trend Insights’, Recycling 9 (2024): 1.
- D Marchese, C Giosue, A Staffolani, et al. ‘An Overview of the Sustainable Recycling Processes Used for Lithium-Ion Batteries’, Batteries 10 (2024): 1.
- Y Sun. ‘Lithium-Ion Battery Recycling: Challenges and Opportunities’, Highlights Sci. Eng. Technol 58 (2023): 365-370.
- S Zheng, T Chen, Y Fang, et al. ‘A Review of Cathode and Electrolyte Recovery from Spent Lithium-Ion Batteries: Recent technologies, processes and policies’, Resour. Chem. Mater (2024).
- Y Dong, H Ji, X Wu, et al. ‘Trends of sustainable recycling technology for lithium-ion batteries: Metal recovery from conventional metallurgical processes to innovative direct recycling’, MetalMat (2023): 5.
- P Li, S Luo, L Zhang, et al. ‘Progress, challenges, and prospects of spent lithium-ion batteries recycling: A review’, J. Energy Chem 89 (2024): 144-171.
- C Liu, J Yu, J Hu, et al. ‘Ultra-low viscosity betaine hydrochloride-formic acid deep eutectic solvent for leaching critical metals from spent NCM lithium-ion batteries’, J. Environ. Chem. Eng 12 (2024): 112586.
- M K Tran, M-T F Rodrigues, K Kato, et al. ‘Deep eutectic solvents for cathode recycling of Li-ion batteries’, Nat. Energy 4 (2019): 4.
- Y Zhang, F Wang, W Zhang, et al. ‘High-Selectivity Recycling of Valuable Metals from Spent Lithium-Ion Batteries Using Recyclable Deep Eutectic Solvents’, ChemSusChem (2024): e202301774.
- B Socas-Rodríguez, Á Santana-Mayor, A V Herrera-Herrera, et al. ‘Chapter 5 - Deep eutectic solvents’, in Green Sustainable Process for Chemical and Environmental Engineering and Science, Inamuddin, A. M. Asiri, and S. Kanchi, Eds., Elsevier (2020): 123-177.
- K Yang, L Chen, J Ma, et al. ‘Progress and perspective of Li1 + xAlxTi2-x(PO4)3 ceramic electrolyte in lithium batteries’, InfoMat 3 (2021): 1195-1217.
- J-L Zhang, C-L Li, W-H Wang, et al. ‘Facile synthesis of hollow Cu3P for sodium-ion batteries anode’, Rare Met 40 (2021): 3460-3465.
- X Kang, G Fu, X Wang, et al. ‘Copper-cobalt-nickel oxide nanowire arrays on copper foams as self-standing anode materials for lithium ion batteries’, Chin. Chem. Lett 32 (2021): 938-942.
- S Rothermel, M Evertz, J Kasnatscheew, et al. ‘Graphite Recycling from Spent Lithium-Ion Batteries’, ChemSusChem 9 (2016): 3473-3484.
- L Zhang, Y Wang, Z Niu, et al. ‘Single Atoms on Graphene for Energy Storage and Conversion’, Small Methods 3 (2019): 1800443.
- S Natarajan and V Aravindan. ‘An Urgent Call to Spent LIB Recycling: Whys and Wherefores for Graphite Recovery’, Adv. Energy Mater 10 (2020): 2002238.
- J-L Yang, X-X Zhao, W-H Li, et al. ‘Advanced cathode for dual-ion batteries: Waste-to-wealth reuse of spent graphite from lithium-ion batteries’, eScience 2 (2022): 95-101.
- P Eleftheriadis, S Leva, M Gangi, et al. ‘Second Life Batteries: Current Regulatory Framework, Evaluation Methods, and Economic Assessment’, in 2022 IEEE International Conference on Environment and Electrical Engineering and 2022 IEEE Industrial and Commercial Power Systems Europe (EEEIC / I&CPS Europe) (2022): 1-6.
- S J Tong, A Same, M A Kootstra, et al. ‘Off-grid photovoltaic vehicle charge using second life lithium batteries: An experimental and numerical investigation’, Appl. Energy 104 (2013): 740-750.
- P Wolfs. ‘An economic assessment of “second use” lithium-ion batteries for grid support’, in 2010 20th Australasian Universities Power Engineering Conference, IEEE (2010): 1-6.
- O Amuta and J Kowal. ‘State of Health Assessment of Spent Lithium-Ion Batteries Based on Voltage Integral during the Constant Current Charge’, Batteries 9 (2023): 11.
- H Rallo, L Canals Casals, D De La Torre, et al. ‘Lithium-ion battery 2nd life used as a stationary energy storage system: Ageing and economic analysis in two real cases’, J. Clean. Prod 272 (2020): 122584.
- R Xu, S Lei, T Wang, et al. ‘Lithium recovery and solvent reuse from electrolyte of spent lithium-ion battery’, Waste Manag 167 (2023): 135-140.
- S Arora and A Kapoor. Behaviour of Lithium-Ion Batteries in Electric Vehicles (2024).
- Q Zhao, K Sun, X Wang, et al. ‘Examining green-sustainable approaches for recycling of lithium-ion batteries’, DeCarbon 3 (2024): 100034.
- D A Dornfeld and B S Linke. Leveraging Technology for a Sustainable World (2024).
- The Faraday Institution, ‘ReLiB. Gateway Testing & Dismantling’, ReLiB Project (2024).
- H Chen and J Shen. ‘A degradation-based sorting method for lithium-ion battery reuse’, PloS One 12 (2017): e0185922.
- B Scrosati, J Garche, and W Tillmetz, et al. ‘Advances in Battery Technologies for Electric Vehicles’, in Advances in Battery Technologies for Electric Vehicles, in Woodhead Publishing Series in Energy. , Woodhead Publishing (2015): 517-526.
- C Rujanavech, J Lessard, S Chandler, et al. ‘Liam-an innovation story’, Apple Inc (2016).
- J M Dodds and J Rawcliffe. ‘Radionuclide distribution during ytterbium doped fibre laser cutting for nuclear decommissioning’, Prog. Nucl. Energy 118 (2020): 103122.
- L Sun, C Zhao, Z Yan, et al. ‘A Novel Weakly-Supervised Approach for RGB-D-Based Nuclear Waste Object Detection’, IEEE Sens. J 19 (2019): 3487-3500.
- Y Wang, X Wei, H Shen, et al. ‘Robust fusion for RGB-D tracking using CNN features’, Appl. Soft Comput 92 (2020): 106302.
- M Adjigble, N Marturi, V Ortenzi, et al. ‘Model-free and learning-free grasping by Local Contact Moment matching’, in 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS) (2018): 2933-2940.
- N Marturi, M Kopicki, A Rastegarpanah, et al. ‘Dynamic grasp and trajectory planning for moving objects’, Auton. Robots 43 (2019): 1241-1256.
- V Ortenzi, R Stolkin, J Kuo, et al. ‘Hybrid motion/force control: a review’, Adv. Robot 31 (2017): 1102-1113.
- K Omelchuk, M Haddad, G Cote, et al. ‘Metals Recovery from Spent Lithium-Ion Batteries: Synthesis and Physicochemical Properties of New Extractants for Solvent Extraction Processes’, ECS Meet. Abstr MA2016-03 (2016): 856.
- S Anwani, R Methekar, and V Ramadesigan. ‘Resynthesizing of lithium cobalt oxide from spent lithium-ion batteries using an environmentally benign and economically viable recycling process’, Hydrometallurgy 197 (2020): 105430.
- E Gerold, C Schinnerl, and H Antrekowitsch. ‘Critical Evaluation of the Potential of Organic Acids for the Environmentally Friendly Recycling of Spent Lithium-Ion Batteries’, Recycling 7 (2022): 1.
- A Mohanty and N Devi. ‘A Review on Green Method of Extraction and Recovery of Energy Critical Element Cobalt from Spent Lithium-Ion Batteries (LIBs)’, Miner. Process. Extr. Metall. Rev 44 (2023): 52-63.
- J-L Yang, X-X Zhao, M-Y Ma, et al. ‘Progress and prospect on the recycling of spent lithium-ion batteries: Ending is beginning’, Carbon Neutralization 1 (2022): 247-266.
- N Bahaloo-Horeh, F Vakilchap, and S M Mousavi. ‘Bio-hydrometallurgical Methods For Recycling Spent Lithium-Ion Batteries’, in Recycling of Spent Lithium-Ion Batteries: Processing Methods and Environmental Impacts, L. An, Ed., Cham: Springer International Publishing (2019): 161-197.
- T Jiang, Q Shi, Z Wei, et al. ‘Leaching of valuable metals from cathode active materials in spent lithium-ion batteries by levulinic acid and biological approaches’, Heliyon 9 (2023): 5.
- L Li, E Fan, F Wu, et al. ‘(Invited, Digital Presentation) Sustainable Recycling of Spent Lithium Ion Batteries’, ECS Meet. Abstr MA2022-01 (2022): 614.
- Y Luo, L Ou, and C Yin. ‘High-efficiency recycling of spent lithium-ion batteries: A double closed-loop process’, Sci. Total Environ 875 (2023): 162567.
- Y Luo, C Yin, and L Ou. ‘A Novel Green Process for Recovery of Transition Metal Ions from Used Lithium-ion Batteries by Selective Coordination of Ethanol’, ACS Sustain. Chem. Eng 11 (2023): 11834-11842.
- L Yurramendi, J Hidalgo, and A Siriwardana. ‘A Sustainable Process for the Recovery of Valuable Metals from Spent Lithium Ion Batteries by Deep Eutectic Solvents Leaching’, Mater. Proc 5 (2022): 1.
- Y Zhang, F Wang, W Zhang, et al. ‘Recyclable deep eutectic solvents for recycling LiCoO2 from spent lithium-ion batteries with high selectivity’, Sep. Purif. Technol 330 (2024): 125498.
- Z Liang, C Cai, G Peng, et al. ‘Hydrometallurgical Recovery of Spent Lithium Ion Batteries: Environmental Strategies and Sustainability Evaluation’, ACS Sustain. Chem. Eng 9 (2021): 5750-5767.
Tables and figures reference
- L Li, J Ge, F Wu, et al. ‘Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant’, J. Hazard. Mater 176 (2010): 288-293.
- X Chen, B Fan, L Xu, et al. ‘An atom-economic process for the recovery of high value-added metals from spent lithium-ion batteries’, J. Clean. Prod 112 (2016): 3562-3570.
- J Li, G Wang, and Z Xu. ‘Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries’, J. Hazard. Mater 302 (2016): 97-104.
- L Sun and K Qiu. ‘Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries’, Waste Manag 32 (2012): 1575-1582.
- L Li, J Lu, Y Ren, et al. ‘Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries’, J. Power Sources 218 (2012): 21-27.
- J Li, P Shi, Z Wang, et al. ‘A combined recovery process of metals in spent lithium-ion batteries’, Chemosphere 77 (2009): 1132-1136.
- L Chen, X Tang, Y Zhang, et al. ‘Process for the recovery of cobalt oxalate from spent lithium-ion batteries’, Hydrometallurgy 108 (2011): 80-86.
- H Dang, B Wang, Z Chang, et al. ‘Recycled Lithium from Simulated Pyrometallurgical Slag by Chlorination Roasting’, ACS Sustain. Chem. Eng 6 (2018): 13160-13167.
- L Xu, C Chen, and M-L. Fu. ‘Separation of cobalt and lithium from spent lithium-ion battery leach liquors by ionic liquid extraction using Cyphos IL-101’, Hydrometallurgy 197 (2020): 105439.
- G Granata, E Moscardini, F Pagnanelli, et al. ‘Product recovery from Li-ion battery wastes coming from an industrial pre-treatment plant: Lab scale tests and process simulations’, J. Power Sources 206 (2012): 393-401.
- W-S Chen and H-J Ho. ‘Recovery of Valuable Metals from Lithium-Ion Batteries NMC Cathode Waste Materials by Hydrometallurgical Methods’, Metals 8 (2018): 5.
- T A Atia, G Elia, R Hahn, et al. ‘Closed-loop hydrometallurgical treatment of end-of-life lithium ion batteries: Towards zero-waste process and metal recycling in advanced batteries’, J. Energy Chem 35 (2019): 220-227.
- M Kaya. ‘State-of-the-art lithium-ion battery recycling technologies’, Circ. Econ 1 (2022): 100015.
- H Bae and Y Kim. ‘Technologies of lithium recycling from waste lithium ion batteries: a review’, Mater. Adv2 (2021): 3234-3250.
- S Maroufi, M Assefi, R Khayyam Nekouei, et al. ‘Recovery of lithium and cobalt from waste lithium-ion batteries through a selective isolation-suspension approach’, Sustain. Mater. Technol 23 (2020): e00139.
- M Assefi, S Maroufi, Y Yamauchi, et al. ‘Pyrometallurgical recycling of Li-ion, Ni-Cd and Ni-MH batteries: A minireview’, Curr. Opin. Green Sustain. Chem 24 (2020): 26-31.
- W Wang, Y Han, T Zhang, et al. ‘Alkali Metal Salt Catalyzed Carbothermic Reduction for Sustainable Recovery of LiCoO2: Accurately Controlled Reduction and Efficient Water Leaching’, ACS Sustain. Chem. Eng 7 (2019): 16729-16737.
- G Lombardo, B Ebin, M R St J Foreman, et al. ‘Chemical Transformations in Li-Ion Battery Electrode Materials by Carbothermic Reduction’, ACS Sustain. Chem. Eng 7 (2019): 13668-13679.
- J Xiao, J Li, and Z Xu. ‘Novel Approach for in Situ Recovery of Lithium Carbonate from Spent Lithium Ion Batteries Using Vacuum Metallurgy’, Environ. Sci. Technol 51 (2017): 11960-11966.
- J Xiao, J Li, and Z Xu. ‘Recycling metals from lithium ion battery by mechanical separation and vacuum metallurgy’, J. Hazard. Mater 338 (2017): 124-131.
- W Wang, Y Zhang, X Liu, et al. ‘A Simplified Process for Recovery of Li and Co from Spent LiCoO2 Cathode Using Al Foil As the in Situ Reductant’, ACS Sustain. Chem. Eng 7 (2019): 12222-12230.
- D Wang, X Zhang, H Chen, et al. ‘Separation of Li and Co from the active mass of spent Li-ion batteries by selective sulfating roasting with sodium bisulfate and water leaching’, Miner. Eng 126 (2018): 28-35.
- C Peng, F Liu, Z Wang, et al. ‘Selective extraction of lithium (Li) and preparation of battery grade lithium carbonate (Li2CO3) from spent Li-ion batteries in nitrate system’, J. Power Sources 415 (2019): 179-188.
- E Fan, L Li, J Lin, et al. ‘Low-Temperature Molten-Salt-Assisted Recovery of Valuable Metals from Spent Lithium-Ion Batteries’, ACS Sustain. Chem. Eng 7 (2019): 16144-16150.
- T Georgi-Maschler, B Friedrich, R Weyhe, et al. ‘Development of a recycling process for Li-ion batteries’, J. Power Sources 207 (2012): 173-182.
- X Hu et al. ‘Possibility of using modified fly ash and organic fertilizers for remediation of heavy-metal-contaminated soils’, J. Clean. Prod 284 (2021): 124713.
- S Pindar and N Dhawan. ‘Kinetics and thermodynamical evaluation of electrode material of discarded lithium-ion batteries and its impact on recycling’, J. Therm. Anal. Calorim 146 (2021): 1819-1831.
- M K Tran, M-T F Rodrigues, K Kato, et al. ‘Deep eutectic solvents for cathode recycling of Li-ion batteries’, Nat. Energy 4 (2019): 4.
- H Wang, M Li, S Garg, et al. ‘Cobalt Electrochemical Recovery from Lithium Cobalt Oxides in Deep Eutectic Choline Chloride+Urea Solvents’, ChemSusChem 14 (2021): 2972-2983.
- J S Coleman. ‘Chloride complexes of cobalt(II) in anion and cation exchangers’, J. Inorg. Nucl. Chem 28 (1966): 2371-2378.
- Honghao Yu, Shaomian Wang , Yin Li, et al. ‘Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent’, Green Process. Synth 11 (2022): 868-874.
- N Peeters, K Binnemans, and S Riaño. ‘Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents’, Green Chem 22 (2020): 4210-4221.
- Y Zhang, P Cui, G Luo, et al. ‘One-step selective separation and efficient recovery of valuable metals from spent lithium batteries by phosphoric acid-based deep eutectic solvent’, Green Chem. Eng (2023): 002.
- Y Luo, C Yin, and L Ou. ‘A Novel Green Process for Recovery of Transition Metal Ions from Used Lithium-ion Batteries by Selective Coordination of Ethanol’, ACS Sustain. Chem. Eng 11 (2023): 11834-11842.
- Y Tian, W Chen B Zhang, et al. ‘A Weak Acidic and Strong Coordinated Deep Eutectic Solvent for Recycling of Cathode from Spent Lithium-Ion Batteries’, ChemSusChem 15 (2022): e202200524.
- R Morina, D Callegari, D Merli, et al. ‘Cathode Active Material Recycling from Spent Lithium Batteries: A Green (Circular) Approach Based on Deep Eutectic Solvents’, ChemSusChem 15 (2022): e202102080.
- S Wang, Z Zhang, Z Lu, et al. ‘A novel method for screening deep eutectic solvent to recycle the cathode of Li-ion batteries’, Green Chem 22 (2020): 4473-4482.
- L Chen, Y Chao, X li, et al. ‘Engineering a tandem leaching system for the highly selective recycling of valuable metals from spent Li-ion batteries’, Green Chem 23 (2021): 2177-2184.
- Y Chen, Y Lu, Z Liu, et al. ‘Efficient Dissolution of Lithium-Ion Batteries Cathode LiCoO2 by Polyethylene Glycol-Based Deep Eutectic Solvents at Mild Temperature’, ACS Sustain. Chem. Eng 8 (2020): 11713-11720.
- S Tang, M Zhang, and M Guo. ‘A Novel Deep-Eutectic Solvent with Strong Coordination Ability and Low Viscosity for Efficient Extraction of Valuable Metals from Spent Lithium-Ion Batteries’, ACS Sustain. Chem. En 10 (2022): 975-985.
- X Chang, M Fan, C-F Gu, et al. ‘Selective Extraction of Transition Metals from Spent LiNixCoyMn1−x−yO2 Cathode via Regulation of Coordination Environment’, Angew. Chem. Int. Ed 61 (2022): e202202558.
- Y Luo, L Ou, and C Yin. ‘A green and efficient combination process for recycling spent lithium-ion batteries’, J. Clean. Prod 396 (2023): 136552.
- S Saeki, J Lee, Q Zhang, et al. ‘Co-grinding LiCoO2 with PVC and water leaching of metal chlorides formed in ground product’, Int. J. Miner. Process 74 (2004): S373-S378.
- X Zhang, H Cao, Y Xie, et al. ‘A closed-loop process for recycling LiNi1/3Co1/3Mn1/3O2 from the cathode scraps of lithium-ion batteries: Process optimization and kinetics analysis’, Sep. Purif. Technol 150 (2015): 186-195.
- M-M Wang, C-C Zhang, and F-S Zhang. ‘An environmental benign process for cobalt and lithium recovery from spent lithium-ion batteries by mechanochemical approach’, Waste Manag 51 (2016): 006.
- K Liu and F-S Zhang. ‘Innovative leaching of cobalt and lithium from spent lithium-ion batteries and simultaneous dechlorination of polyvinyl chloride in subcritical water’, J. Hazard. Mater 316 (2016): 19-25.
- H Wang, K Huang, Y Zhang, et al. ‘Recovery of Lithium, Nickel, and Cobalt from Spent Lithium-Ion Battery Powders by Selective Ammonia Leaching and an Adsorption Separation System’, ACS Sustain. Chem. Eng 5 (2017): 11489-11495.
- J Guan, Y Li, Y Guo, et al. ‘Mechanochemical Process Enhanced Cobalt and Lithium Recycling from Wasted Lithium-Ion Batteries’, ACS Sustain. Chem. Eng 5 (2017): 1026-1032.
- Y Yang, X Zheng, X Cao, et al. ‘A Closed-Loop Process for Selective Metal Recovery from Spent Lithium Iron Phosphate Batteries through Mechanochemical Activation’, ACS Sustain. Chem. Eng 5 (2017): 9972-9980.
- Y Guo, Y Li, X Lou, et al. ‘Improved extraction of cobalt and lithium by reductive acid from spent lithium-ion batteries via mechanical activation process’, J. Mater. Sci 53 (2018): 13790-13800.
- E Fan, L Li, X Zhang, et al. ‘Selective Recovery of Li and Fe from Spent Lithium-Ion Batteries by an Environmentally Friendly Mechanochemical Approach’, ACS Sustain. Chem. Eng 6 (2018): 11029-11035.
- J Xie, Huang, Z Nie, et al. ‘An effective process for the recovery of valuable metals from cathode material of lithium-ion batteries by mechanochemical reduction’, Resour. Conserv. Recycl 168 (2021): 105261.
- S Zhang, C Zhang, X Zhang, et al. ‘Recovery of Li and Co from Spent Li-Ion Batteries by Mechanochemical Integration with NH4Cl’, ACS Sustain. Chem. Eng 10 (2022): 5611-5620.
- S Zhang, C Zhang, X Zhang, et al. ‘A mechanochemical method for one-step leaching of metals from spent LIBs’, Waste Manag 161 (2023): 245-253.
- T Mendes, G Nano, and L Magagnin. ‘Electrochemical Recovery of Metals in Deep Eutectic Solvents’, ECS Trans 53 (2013): 63.
- F J Alguacil. ‘Utilizing Deep Eutectic Solvents in the Recycle, Recovery, Purification and Miscellaneous Uses of Rare Earth Elements’, Molecules 29 (2024): 1356.
- S Meles Neguse, S Yoon, H Lim, et al. ‘The Pitfalls of Deep Eutectic Solvents in the Recycling of Lithium-Ion Batteries’, Energy Technol., vol. 12 (2024): 2301213.
- I M Pateli. ‘Metal oxides processing using deep eutectic solvents’, PhD Thesis, University of Leicester (2020).
- J Wang and Z Guo. ‘Hydrometallurgically Recycling Spent Lithium-Ion Batteries’, in Recycling of Spent Lithium-Ion Batteries: Processing Methods and Environmental Impacts, L. An, Ed., Cham: Springer International Publishing (2019): 27-55.
- X Guo, D Xiang, G Duan, et al. ‘A review of mechanochemistry applications in waste management’, Waste Manag 30 (2010): 4-10.
- Q Tan and J Li. ‘Recycling Metals from Wastes: A Novel Application of Mechanochemistry’, Environ. Sci. Technol 49 (2015): 5849-5861.
- M I G S Almeida, R W Cattrall, and S D Kolev. ‘Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs)’, J. Membr. Sci 415-416 (2012): 9-23.
- B K Biswal and R Balasubramanian. ‘Recovery of valuable metals from spent lithium-ion batteries using microbial agents for bioleaching: a review’, Front. Microbiol 14 (2023): 1197081.
Supplementary material
Sustainable Lithium and Cobalt Recovery from spent lithium-ion batteries: Best Practices for the Future. A Review
1 Supplementary Figures and Tables
1.1 Supplementary Figures
Supplementary Tables
Table S1: Residual parameters and conditions of hydrometallurgy used for S-LIBs from literature.
|
Spent materials |
Method |
Residue |
Efficiency |
Purity |
Condition |
Ref. |
|
LiCoO2 |
Precipitation |
Li2SO4 |
90% |
— |
4M, H2SO4, H2O2[80°C/4 h] |
[1] |
|
Ethanol, LiOH |
||||||
|
LIB scraps |
Precipitation |
LiF |
50% |
>99wt% |
500°C/5h, KHSO4 |
[2] |
|
9 M H2SO4, 30 wt%H2O2(3 mLg−1) [90-100 °C] |
||||||
|
6 M NaOH |
||||||
|
Cathode materials |
Precipitation |
Li2CO3 |
80 ± 1% |
96.97% |
4MHCl, [80 °C] (20 g L−1) |
[3] |
|
Na2CO3[100°C] |
||||||
|
Cathode materials |
Precipitation |
Li2CO3 |
71% |
— |
2MH2SO4,2% H2O2(33gL−1) [60 °C] |
[4] |
|
Na2CO3[50 °C] |
||||||
|
Mixed cathode materials |
Precipitation |
Li2CO3 |
80% |
— |
4M, H2SO4, 30wt%H2O2(50gL−1) [70-80 °C] |
[5] |
|
NaOH, Na2CO3[40 °] |
||||||
|
LiNi0.3Mn0.3Co0.3O2 |
Precipitation |
Li2CO3 |
Leaching 99.7% |
— |
3.0 M trichloroacetic acid, 4.0 vol% H2O (50 g L−1) [70-80 °C] |
[6] |
|
Saturated Na2CO3solution |
||||||
|
LiNi0.3Mn0.3Co0.3O2 |
Precipitation |
Li2CO3 |
Leaching 99% |
— |
2 M H2SO4+ 4 vol% H202(50g L−1) [50 °C/2 h] |
[7] |
|
KMnO4, C4H8N2O2 |
||||||
|
Na2CO3[90 °C] |
||||||
|
LiNi0.5Mn0.3Co0.2O2 |
Precipitation |
Li2CO3 |
76% |
>99.5% |
1Mof oxalic acid (10gL−1) [95 °C/12 h] |
[8] |
|
5MK2CO3[80°C/4 h] |
||||||
|
LiFePO4 |
Precipitation |
Li2CO3 |
99.35% |
— |
Ball mill with citric acid, H2O2(20 g g−1) |
[8] |
|
SaturatedNa2CO3[95 °C] |
||||||
|
Cathode |
Precipitation |
Li2CO3 |
38% |
99.48% |
1MH2SO4,5vol% H2O2[60°C/1h], sonication |
[9] |
|
2M NaOH |
||||||
|
LiFePO4 |
Precipitation |
Li2CO3 |
80% |
— |
4M MSA acid (80g L−1) 18%, H2O2 |
[10] |
|
5%NaOH solution, 30%Na2CO3[96°C/30 min] |
||||||
|
LiNi0.5Co0.2Mn0.3O2 |
Precipitation |
Li2CO3 |
91.23% |
99% |
Sodium persulfate (400 g L−1) [85 °C] |
[11] |
|
Na2CO3 |
||||||
|
LiNixMnyCo1−x−yO2 |
Precipitation |
Li3PO4 |
Leaching 99.1% |
— |
750°C/3h,2.75MH3PO4[40°C/10 min] |
[12] |
|
LiNi0.3Mn0.3Co0.3O2 |
Precipitation |
Li2CO3 |
Leaching 99% |
— |
2 M H2SO4+ 4 vol% H202(50g L−1) [50 °C/2 h] |
[7] |
|
KMnO4, C4H8N2O2 |
||||||
|
Na2CO3[90 °C] |
||||||
|
LiNi0.5Mn0.3Co0.2O2 |
Precipitation |
Li2CO3 |
76% |
>99.5% |
1Mof oxalic acid (10gL−1) [95 °C/12 h] |
[8] |
|
5MK2CO3[80°C/4 h] |
||||||
|
LiFePO4 |
Precipitation |
Li2CO3 |
99.35% |
— |
Ball mill with citric acid, H2O2(20 g g−1) |
[8] |
|
SaturatedNa2CO3[95 °C] |
||||||
|
Cathode |
Precipitation |
Li2CO3 |
38% |
99.48% |
1MH2SO4,5vol% H2O2[60°C/1h], sonication |
[9] |
|
2M NaOH |
||||||
|
LiFePO4 |
Precipitation |
Li2CO3 |
80% |
— |
4M MSA acid (80g L−1) 18%, H2O2 |
[10] |
|
5%NaOH solution, 30%Na2CO3[96°C/30 min] |
||||||
|
LiNi0.5Co0.2Mn0.3O2 |
Precipitation |
Li2CO3 |
91.23% |
99% |
Sodium persulfate (400 g L−1) [85 °C] |
[11] |
|
Na2CO3 |
||||||
|
LiNixMnyCo1−x−yO2 |
Precipitation |
Li3PO4 |
Leaching 99.1% |
— |
750°C/3h,2.75MH3PO4[40°C/10 min] 10 M NaOH |
[12] |
|
Cathode materials |
Precipitation |
Li2CO3 |
>90% |
99.93% |
3.5Macetic acid (40 g L−1), H2O24vol% [60°C] |
[13] |
|
Saturated Na2CO3[20\60 °C] |
||||||
|
Cathode materials |
Precipitation |
Li3PO4 |
85.56% |
— |
Oxidation at 600 °C, 0.28 M H2SO4+ H2O2(16 g L−1) [85°C/2 h] |
[14] |
|
NaOH, Na3PO4 |
||||||
|
Anode |
Precipitation |
Li2CO3 |
Leaching ≈ 100% |
>99% |
1.5M HCl (100 g L−1) CO2 |
[15] |
Table S2: Bacterial-based bioleaching for recovery of valuable metals from spent Li-ion batteries (LIBs)[16].
|
Recovery efficiency |
|||||
|
Bacteria |
Key leaching condition |
Co |
Li |
Additional information |
References |
|
A. thiooxidans (80191) |
Pulp density: 0.25% (w/v), pH: 2.4 |
23% |
60% |
Co and Li dissolution were higher in two-step bioleaching |
[17] |
|
A. ferrooxidans (ATCC 19859) |
Solid-to-liquid ratio: 5 g/L, pH: 2.5 |
65% |
9.5% |
Higher solid/liquid ratios reduced leaching efficiency |
[18] |
|
A. ferrooxidans (DSMZ, 1927) |
Pulp density: 100 g/L |
94% |
60.30% |
For optimum metal extraction, replenishment of microbial culture was done every 24 h for 3 cycles |
[19] |
|
A. ferrooxidans (DSMZ 1927) |
Pulp density: 100 g/L |
82% |
89% |
Leaching efficiency was increased with increase of sulphuric and ferric ion in the leaching medium as well as by replenishing the culture for three cycles |
[19] |
|
A. ferrooxidans (DSMZ 1927) |
Pulp density: 100 g/L |
90.4% |
89.9% |
NMC (NMC111 and NMC622) were regenerated from the oxalate-based coprecipitated product. The electrochemical stability of the regenerated NMC was similar to the commercial NMC. |
[20] |
|
A. ferrooxidans (isolated) |
Pulp density: 1% (s/v), bacteria inoculation: 5% (v/v), pH: 1.5 |
47.60% |
NA |
Enhancement of cobalt dissolution was observed at higher redox potential |
[21] |
|
A. thiooxidans (PTCC 1717) |
Pulp density: 30 g/L, pH: 2.0 |
60% |
99% |
Bioleached spent LIB residue was safe to disposal as meets the TCLP limit |
[22] |
|
A. thiooxidans (PTCC 1647) |
Pulp density: 40 g/L, pH: 2.0 |
88% |
100% |
The shrinking core model predicted that the diffusion of ferric ions plays a key role in metal leaching. |
[23] |
|
A. ferrooxidans (isolated) |
Pulp density 1% (s/v) |
99.90% |
NA |
Enhancement of cobalt dissolution was noticed with addition of copper ions (0.75 g/L). |
[24] |
|
A. ferrooxidans (isolated) |
Pulp density 1% (s/v) |
98.40% |
NA |
Enhancement of cobalt dissolution was noticed with addition of silver ions (0.02 g/L). |
[25] |
|
A. ferrooxidans (PTCC 1647) |
Pulp density: 10 g/L |
19.0% |
67% |
Ultrasonic treatment (203.5 W for 30 min) enhanced metal leaching efficiency. |
[26] |
|
A. ferrooxidans (isolated) |
pH: 2.5, inoculum concentration: 20% (v/v) |
57.8% |
NA |
Highest Co recovery was found at an inoculum concentration of 20% (v/v) in 14 days of incubation time. |
[27] |
|
A. ferrooxidans (isolated) |
Pulp density: 10 g/L, pH: 2- 4, inoculum concentration: 20% (v/v) |
73.95% |
NA |
Bacterial strain isolated from the acid mine drainage has the potential as oxidizing agent for recovery of metals (Co and Li) from spent LIBs. |
[28] |
|
L. ferriphilum (isolated) |
Pulp density: 1% (w/v), pH: 1.0 |
NA |
49% |
Leaching tests were done using pyrite (FeS2, 16 g/L) as the energy source and LFP as the cathode material. |
[29] |
|
A. thiooxidans (isolated) |
Pulp density: 1% (w/v), pH: 1.0 |
NA |
98% |
Leaching tests were done using S0 (16 g/L) as the energy source and LFP as the cathode material. |
[29] |
|
A. thiooxidans (isolated) |
Pulp density: 1% (w/v), pH: 1.0, |
NA |
97% |
Leaching tests were done using S0 (16 g/L) as the energy source and LMO as the cathode material. |
[29] |
|
Mixed bacterial culture (isolated) |
Pulp density: 2 g/L, pH: 7.0 |
NA |
63.8% |
Adaptation of bacteria with LiCl solution (576 µM) enhanced leaching efficiency of bacteria to Li. |
[30] |
|
Mixed culture of IOB and SOB (isolated) |
Pulp density 10 g/L, pH: 1.5 (2.0 g/L sulfur + 2.0 g/L FeS2) |
90.00% |
80.0% |
Acidolysis was the main mechanism for Li dissolution, whereas both acidolysis and redoxolysis contributed for Co dissolution. |
[31] |
|
Mixed culture 1 A. ferrooxidans and A. thiooxidans |
Iron sulfate: 36.7 g/L; sulfur: 5.0 g/L, pH: 1.5 |
50.40% |
99.20% |
Metal contents in spent LIB residue reduced to below the regulatory standard (USEPA), thus the bioleached LIBs can be reused or disposed safely |
[32] |
|
Mixed culture 1 A. ferrooxidans and A. thiooxidans |
Pulp density: 1% (w/v), pH: 2.0 |
99.95% |
NA |
High metal extraction yield observed in short time (3 days) in two-step leaching with addition of silver ions (0.02 g/L) |
[33] |
|
Mixed culture 1 A. ferrooxidans and A. thiooxidans |
Pulp density: 10% (w/v), pH: 1.8 |
53.20% |
60.00% |
Biogenic ferric ion-based critical metal leaching yield was further improved with addition 100 mM H2SO4. |
[34] |
|
Alicyclobacillus sp. (sulfur-oxidizing) and Sulfobacillus sp. (iron-oxidizing) bacteria |
72% |
89% |
Thermodynamics analysis shows bioleaching has much greater potential to happen compared to chemical leaching. |
[35] |
|
Table S3: Fungal-based bioleaching for recovery of valuable metals from spent Li-ion batteries (LIBs) [16].
|
Recovery efficiency |
|||||
|
Fungal |
Key leaching condition |
Co |
Li |
Additional information |
References |
|
Aspergillus niger (PTCC 5210) |
Pulp density: 1% (w/v); pH: 6.0 |
45% |
95% |
Spent medium exhibited highest metal extraction yield. Also, bioacids yielded higher metal extraction than synthetic chemical acids. |
[36] |
|
A. niger MM1/SG1 (isolated) |
Pulp density: 0.25% (w/v); carbon source: sucrose; pH: 3.5 |
80-82% |
100% |
Leaching efficiency was higher in cell-free spent medium. Also, bioacids yielded higher metal extraction than the synthetic chemical acid (citric acid). |
[37] |
|
A. niger (PTCC 5210) |
Pulp density: 1-2% (w/v), carbon source: sucrose |
64% |
100% |
Co and Ni recovery were higher at 1% pulp density, while Li, Cu, Al and Mn recovery was higher at 2% pulp density. |
[38] |
|
A. niger (PTCC 5210) |
Pulp density: 1 % (w/v), carbon source: sucrose |
38% |
100% |
Adapted fungi showed higher metal leaching performance compared to unadopted fungi. |
[39] |
|
A.niger (PTCC 5010) |
Pulp density: 10% (w/v), carbon source: glucose; pH: 4.5, |
NA |
73.3% |
A. niger showed higher metal leaching performance than Penicillium chrysogenum. |
[40] |
|
P. chrysogenum (PTCC 5037) |
Pulp density: 10% (w/v), carbon source: glucose; pH: 4.5, |
NA |
54.6% |
A. niger showed higher metal leaching performance than P. chrysogenum. |
[40] |
|
A.niger (isolated) |
Carbon sources: glucose, incubation time: 21 days |
57% |
72% |
Highest valuable metal recovery obtained in the one step process |
[27] |
|
Mixed culture 1 A. niger and Aspergillus tubingensis |
Pulp density: 1% (w/v), carbon source: sucrose, impure sucrose or vinasse |
∼60% |
∼95% |
Spent medium leaching showed higher metal recovery efficiency with vanasse as the carbon source |
[41] |
Reference
- S. Aktas, D. J. Fray, O. Burheim, J. Fenstad, and E. Açma, ‘Recovery of metallic values from spent Li ion secondary batteries’, Miner. Process. Extr. Metall., vol. 115, no. 2, pp. 95-100, Jun. 2006, doi: 10.1179/174328506X109040.
- J. F. Paulino, N. G. Busnardo, and J. C. Afonso, ‘Recovery of valuable elements from spent Li-batteries’, J. Hazard. Mater., vol. 150, no. 3, pp. 843-849, Feb. 2008, doi: 10.1016/j.jhazmat.2007.10.048.
- R.-C. Wang, Y.-C. Lin, and S.-H. Wu, ‘A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries’, Hydrometallurgy, vol. 99, no. 3, pp. 194-201, Nov. 2009, doi: 10.1016/j.hydromet.2009.08.005.
- S. Zhu, W. He, G. Li, X. Zhou, X. Zhang, and J. Huang, ‘Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation’, Trans. Nonferrous Met. Soc. China, vol. 22, no. 9, pp. 2274-2281, Sep. 2012, doi: 10.1016/S1003-6326(11)61460-X.
- H. Zou, E. Gratz, D. Apelian, and Y. Wang, ‘A novel method to recycle mixed cathode materials for lithium ion batteries’, Green Chem., vol. 15, no. 5, pp. 1183-1191, Apr. 2013, doi: 10.1039/C3GC40182K.
- X. Zhang et al., ‘A closed-loop process for recycling LiNi1/3Co1/3Mn1/3O2 from the cathode scraps of lithium-ion batteries: Process optimization and kinetics analysis’, Sep. Purif. Technol., vol. 150, pp. 186-195, Aug. 2015, doi: 10.1016/j.seppur.2015.07.003.
- R. Sattar, S. Ilyas, H. N. Bhatti, and A. Ghaffar, ‘Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries’, Sep. Purif. Technol., vol. 209, pp. 725-733, Jan. 2019, doi: 10.1016/j.seppur.2018.09.019.
- Q. Li, K. Y. Fung, L. Xu, C. Wibowo, and K. M. Ng, ‘Process Synthesis: Selective Recovery of Lithium from Lithium-Ion Battery Cathode Materials’, Ind. Eng. Chem. Res., vol. 58, no. 8, pp. 3118-3130, Feb. 2019, doi: 10.1021/acs.iecr.8b04899.
- C.-H. Jo and S.-T. Myung, ‘Efficient recycling of valuable resources from discarded lithium-ion batteries’, J. Power Sources, vol. 426, pp. 259-265, Jun. 2019, doi: 10.1016/j.jpowsour.2019.04.048.
- P. Yadav, C. J. Jie, S. Tan, and M. Srinivasan, ‘Recycling of cathode from spent lithium iron phosphate batteries’, J. Hazard. Mater., vol. 399, p. 123068, Nov. 2020, doi: 10.1016/j.jhazmat.2020.123068.
- W. Lv et al., ‘Selective Recovery of Lithium from Spent Lithium-Ion Batteries by Coupling Advanced Oxidation Processes and Chemical Leaching Processes’, ACS Sustain. Chem. Eng., vol. 8, no. 13, pp. 5165-5174, Apr. 2020, doi: 10.1021/acssuschemeng.9b07515.
- Y. Zhang, W. Wang, J. Hu, T. Zhang, and S. Xu, ‘Stepwise Recovery of Valuable Metals from Spent Lithium Ion Batteries by Controllable Reduction and Selective Leaching and Precipitation’, ACS Sustain. Chem. Eng., vol. 8, no. 41, pp. 15496-15506, Oct. 2020, doi: 10.1021/acssuschemeng.0c04106.
- W. Gao et al., ‘Selective recovery of valuable metals from spent lithium-ion batteries - Process development and kinetics evaluation’, J. Clean. Prod., vol. 178, pp. 833-845, Mar. 2018, doi: 10.1016/j.jclepro.2018.01.040.
- S. Tao, J. Li, L. Wang, L. Hu, and H. Zhou, ‘A method for recovering Li3PO4 from spent lithium iron phosphate cathode material through high-temperature activation’, Ionics, vol. 25, no. 12, pp. 5643-5653, Dec. 2019, doi: 10.1007/s11581-019-03070-w.
- Y. Yang et al., ‘A process for combination of recycling lithium and regenerating graphite from spent lithium-ion battery’, Waste Manag., vol. 85, pp. 529-537, Feb. 2019, doi: 10.1016/j.wasman.2019.01.008.
- B. K. Biswal and R. Balasubramanian, ‘Recovery of valuable metals from spent lithium-ion batteries using microbial agents for bioleaching: a review’, Front. Microbiol., vol. 14, May 2023, doi: 10.3389/fmicb.2023.1197081.
- B. K. Biswal, U. U. Jadhav, D. Patil, and E.-H. Yang, ‘Physicochemical and biological methods for treatment of municipal solid waste incineration ash to reduce its potential adverse impacts on groundwater’, in Contaminants of Emerging Concerns and Reigning Removal Technologies, CRC Press, 2022.
- D. Mishra, D.-J. Kim, D. E. Ralph, J.-G. Ahn, and Y.-H. Rhee, ‘Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans’, Waste Manag., vol. 28, no. 2, pp. 333-338, Jan. 2008, doi: 10.1016/j.wasman.2007.01.010.
- J. J. Roy, B. Cao, and S. Madhavi, ‘A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach’, Chemosphere, vol. 282, p. 130944, Nov. 2021, doi: 10.1016/j.chemosphere.2021.130944.
- M. P. Do, J. Jegan Roy, B. Cao, and M. Srinivasan, ‘Green Closed-Loop Cathode Regeneration from Spent NMC-Based Lithium-Ion Batteries through Bioleaching’, ACS Sustain. Chem. Eng., vol. 10, no. 8, pp. 2634-2644, Feb. 2022, doi: 10.1021/acssuschemeng.1c06885.
- L. Li et al., ‘Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment’, J. Power Sources, vol. 233, pp. 180-189, Jul. 2013, doi: 10.1016/j.jpowsour.2012.12.089.
- T. Naseri, N. Bahaloo-Horeh, and S. M. Mousavi, ‘Environmentally friendly recovery of valuable metals from spent coin cells through two-step bioleaching using Acidithiobacillus thiooxidans’, J. Environ. Manage., vol. 235, pp. 357-367, Apr. 2019, doi: 10.1016/j.jenvman.2019.01.086.
- T. Naseri, N. Bahaloo-Horeh, and S. M. Mousavi, ‘Bacterial leaching as a green approach for typical metals recovery from end-of-life coin cells batteries’, J. Clean. Prod., vol. 220, pp. 483-492, May 2019, doi: 10.1016/j.jclepro.2019.02.177.
- G. Zeng, X. Deng, S. Luo, X. Luo, and J. Zou, ‘A copper-catalyzed bioleaching process for enhancement of cobalt dissolution from spent lithium-ion batteries’, J. Hazard. Mater., vol. 199-200, pp. 164-169, Jan. 2012, doi: 10.1016/j.jhazmat.2011.10.063.
- G. Zeng, S. Luo, X. Deng, L. Li, and C. Au, ‘Influence of silver ions on bioleaching of cobalt from spent lithium batteries’, Miner. Eng., vol. 49, pp. 40-44, Aug. 2013, doi: 10.1016/j.mineng.2013.04.021.
- M. Nazerian, N. Bahaloo-Horeh, and S. M. Mousavi, ‘Enhanced bioleaching of valuable metals from spent lithium-ion batteries using ultrasonic treatment’, Korean J. Chem. Eng., vol. 40, no. 3, pp. 584-593, Mar. 2023, doi: 10.1007/s11814-022-1257-2.
- A. Hariyadi, U. Sholikah, B. Gotama, and M. A. Ghony, ‘Biohydrometallurgy for cobalt recovery from spent li-ion batteries using acidophilic bacteria isolated from acid mine drainage’, Chem J Tek Kim, vol. 9, pp. 88-96, 2022.
- R. A. Putra, I. A. Fajri, and A. Hariyadi, ‘Metal Bioleaching of Used Lithium-Ion Battery Using Acidophilic ferrooxidans Isolated from Acid Mine Drainage’, Key Eng. Mater., vol. 937, pp. 193-200, 2022, doi: 10.4028/p-sd859o.
- Y. Xin, X. Guo, S. Chen, J. Wang, F. Wu, and B. Xin, ‘Bioleaching of valuable metals Li, Co, Ni and Mn from spent electric vehicle Li-ion batteries for the purpose of recovery’, J. Clean. Prod., vol. 116, pp. 249-258, Mar. 2016, doi: 10.1016/j.jclepro.2016.01.001.
- M. Hartono, M. A. Astrayudha, and H. T. B. M. Petrus, ‘LITHIUM RECOVERY OF SPENT LITHIUM-ION BATTERY USING BIOLEACHING FROM LOCAL SOURCES MICROORGANISM’, Rasayan J. Chem., 2017, doi: 10.7324/RJC.2017.1031767.
- B. Xin et al., ‘Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulfur-oxidizing and iron-oxidizing bacteria’, Bioresour. Technol., vol. 100, pp. 6163-9, Sep. 2009, doi: 10.1016/j.biortech.2009.06.086.
- A. Heydarian, S. Mousavi, F. Vakilchap, and M. Baniasadi, ‘Application of a mixed culture of adapted acidophilic bacteria in two-step bioleaching of spent lithium-ion laptop batteries’, J. Power Sources, vol. 378, pp. 19-30, Feb. 2018, doi: 10.1016/j.jpowsour.2017.12.009.
- F. Noruzi, N. Nasirpour, F. Vakilchap, and S. M. Mousavi, ‘Complete bioleaching of Co and Ni from spent batteries by a novel silver ion catalyzed process’, Appl. Microbiol. Biotechnol., vol. 106, no. 13, pp. 5301-5316, Aug. 2022, doi: 10.1007/s00253-022-12056-0.
- N. J. Boxall, K. Y. Cheng, W. Bruckard, and A. H. Kaksonen, ‘Application of indirect non-contact bioleaching for extracting metals from waste lithium-ion batteries’, J. Hazard. Mater., vol. 360, pp. 504-511, Oct. 2018, doi: 10.1016/j.jhazmat.2018.08.024.
- Z. Niu, Y. Zou, B. Xin, S. Chen, C. Liu, and Y. Li, ‘Process controls for improving bioleaching performance of both Li and Co from spent lithium ion batteries at high pulp density and its thermodynamics and kinetics exploration’, Chemosphere, vol. 109, pp. 92-98, Aug. 2014, doi: 10.1016/j.chemosphere.2014.02.059.
- N. B. Horeh, S. M. Mousavi, and S. A. Shojaosadati, ‘Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger’, J. Power Sources, vol. 320, pp. 257-266, Jul. 2016, doi: 10.1016/j.jpowsour.2016.04.104.
- B. K. Biswal, U. U. Jadhav, M. Madhaiyan, L. Ji, E.-H. Yang, and B. Cao, ‘Biological Leaching and Chemical Precipitation Methods for Recovery of Co and Li from Spent Lithium-Ion Batteries’, ACS Sustain. Chem. Eng., vol. 6, no. 9, pp. 12343-12352, Sep. 2018, doi: 10.1021/acssuschemeng.8b02810.
- N. Bahaloo-Horeh and S. M. Mousavi, ‘Enhanced recovery of valuable metals from spent lithium-ion batteries through optimization of organic acids produced by Aspergillus niger’, Waste Manag., vol. 60, pp. 666-679, Feb. 2017, doi: 10.1016/j.wasman.2016.10.034.
- N. Bahaloo-Horeh, S. M. Mousavi, and M. Baniasadi, ‘Use of adapted metal tolerant Aspergillus niger to enhance bioleaching efficiency of valuable metals from spent lithium-ion mobile phone batteries’, J. Clean. Prod., vol. 197, pp. 1546-1557, Oct. 2018, doi: 10.1016/j.jclepro.2018.06.299.
- Z. Kazemian, M. Larypoor, and R. Marandi, ‘Evaluation of myco-leaching potential of valuable metals from spent lithium battery by Penicillium chrysogenum and Aspergillus niger’, Int. J. Environ. Anal. Chem., vol. 103, no. 3, pp. 514-527, Feb. 2023, doi: 10.1080/03067319.2020.1861605.
- N. Alavi, K. Partovi, M. Majlessi, M. Rashidi, and M. Alimohammadi, ‘Bioleaching of metals from cellphones batteries by a co-fungus medium in presence of carbon materials’, Bioresour. Technol. Rep., vol. 15, p. 100768, Sep. 2021, doi: 10.1016/j.biteb.2021.100768.





 Impact Factor: * 2.8
Impact Factor: * 2.8 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 